Trimetallic Nanoalloy of NiFeCo Embedded in Phosphidated Nitrogen Doped Carbon Catalyst for Efficient Electro-Oxidation of Kraft Lignin
Abstract
:1. Introduction
2. Experimental Details
2.1. Chemical and Reagents
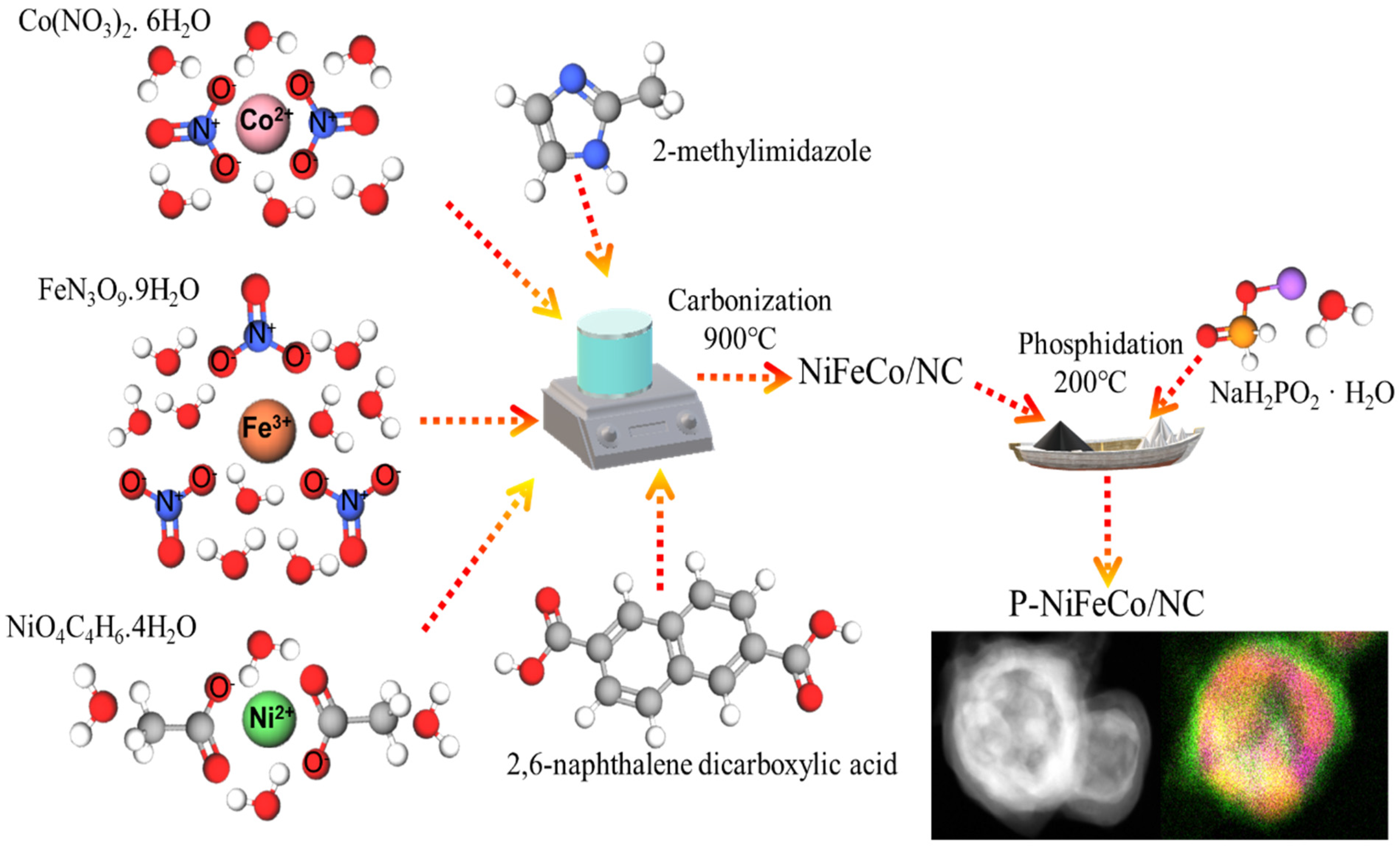
2.2. Synthesis of P-NiFeCo/NC Catalyst
2.3. Characterization
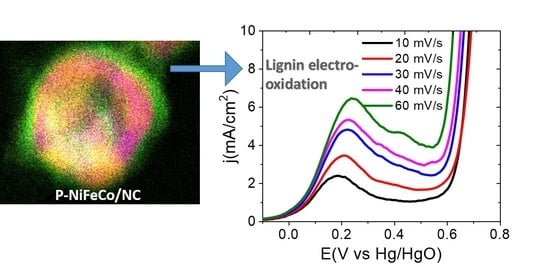
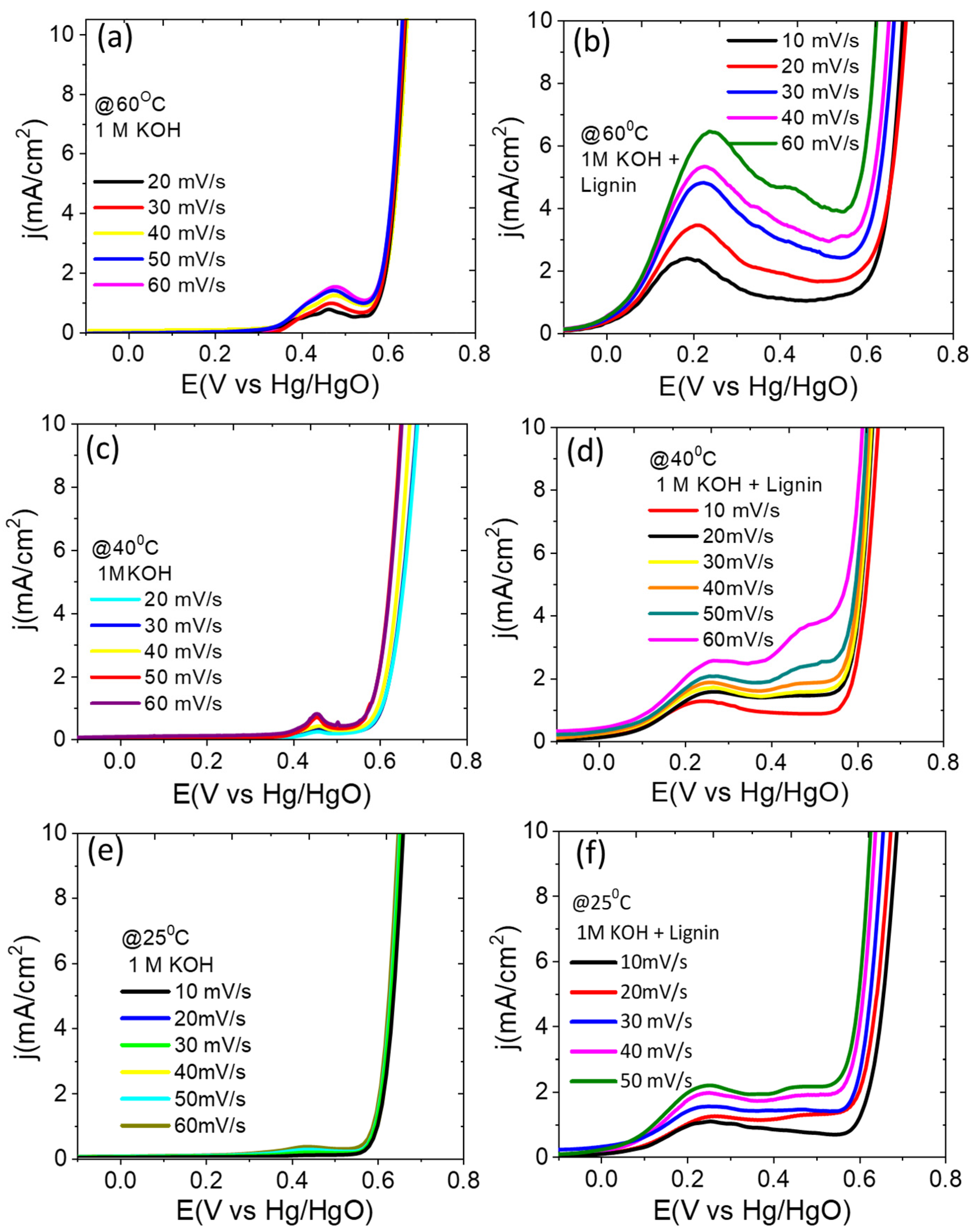
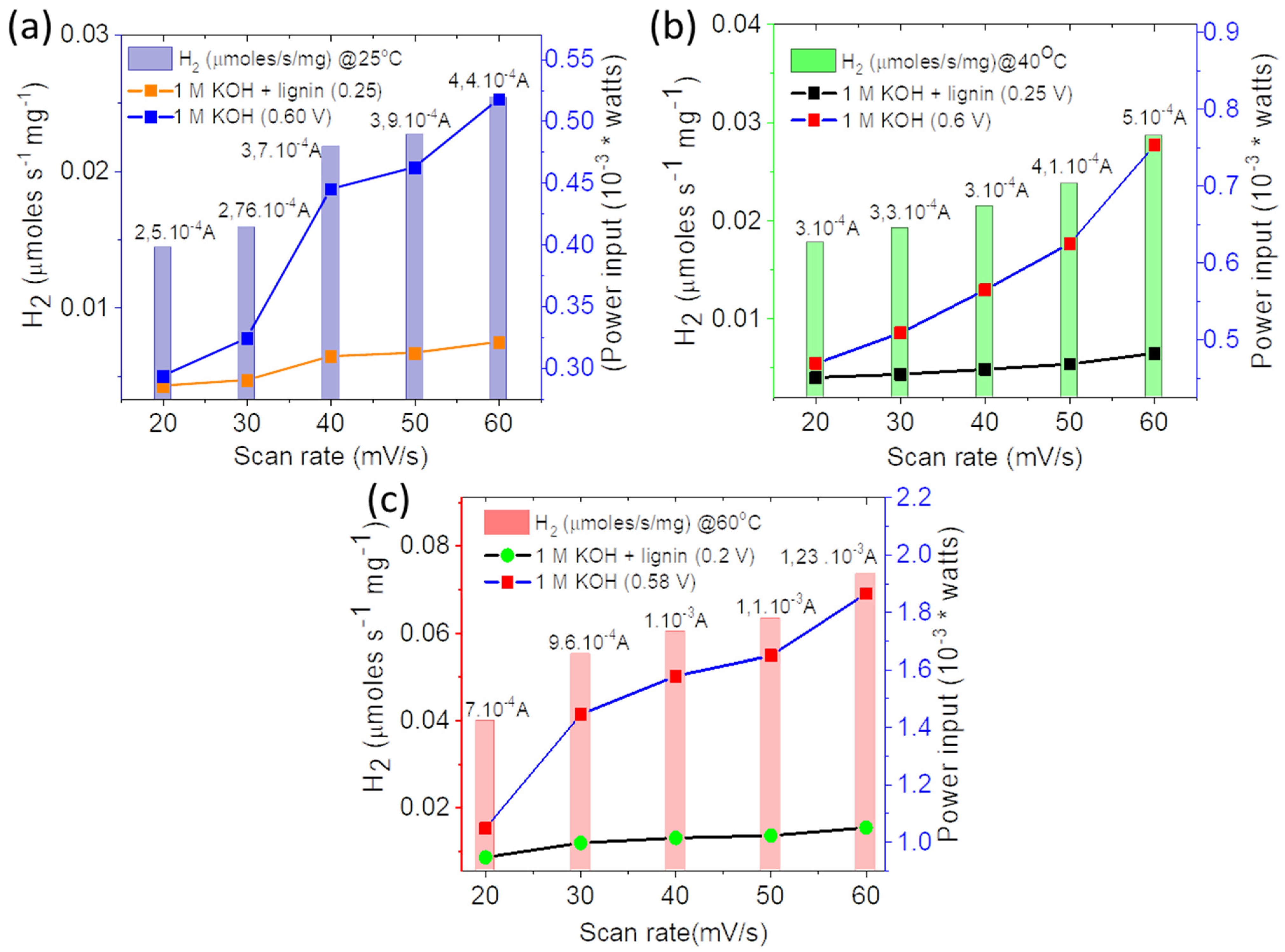
3. Results and Discussion
3.1. Synthesis of Catalyst
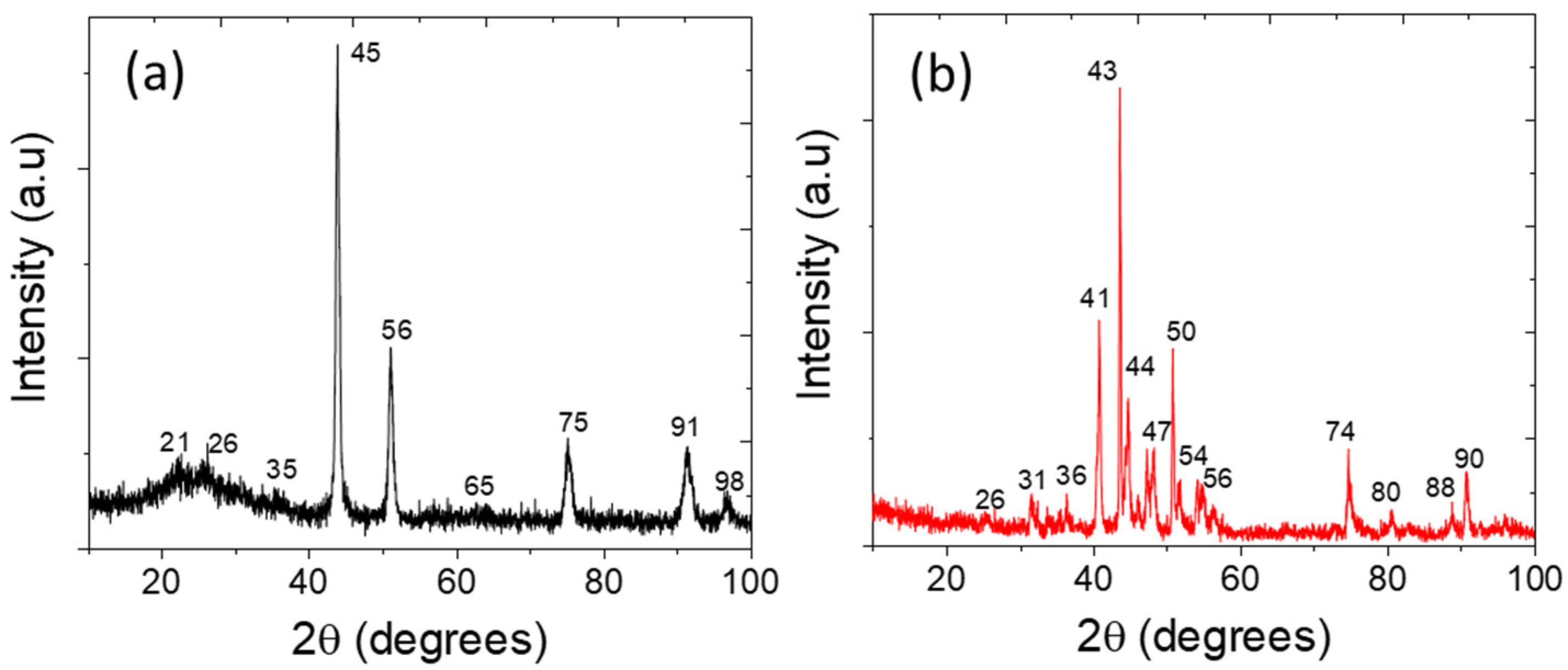
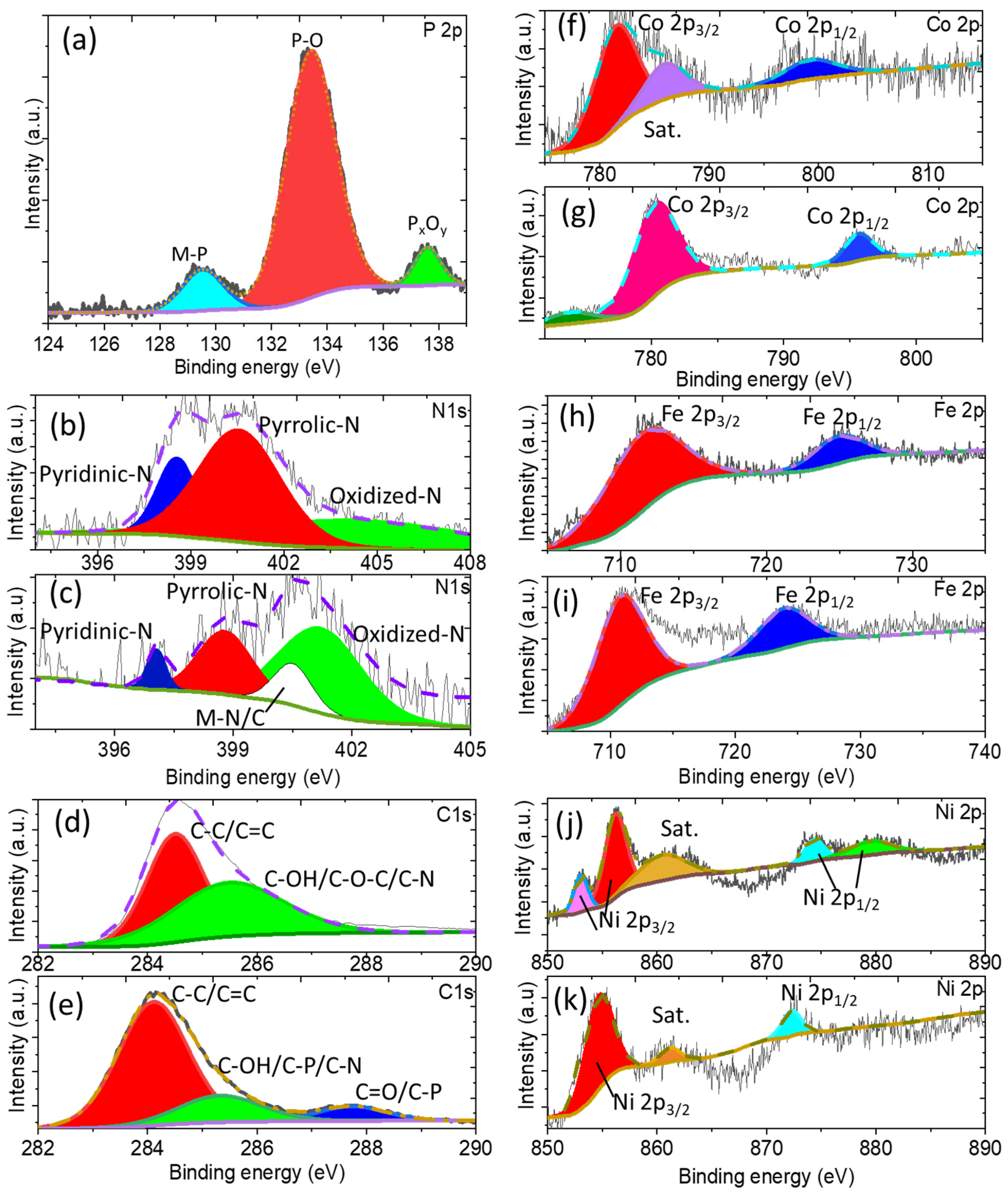
3.2. Physicochemical Characterizations
3.3. Electrochemical Characterizations
3.4. Theoretical Calculations for Hydrogen Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dou, Y.; Sun, L.; Ren, J.; Dong, L. Opportunities and Future Challenges in Hydrogen Economy for Sustainable Development, Hydrogen Economy; Academic Press: Cambridge, MA, USA, 2017; pp. 277–305. ISBN 9780128111321. [Google Scholar]
- Vincent, I.; Lee, E.C.; Kim, H.M. Comprehensive impedance investigation of low-cost anion exchange membrane electrolysis for large scale hydrogen production. Sci. Rep. 2021, 11, 293. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.; Choi, K.S. Combined biomass valorization and hydrogen production in a photoelectrochemical cell. Nat. Chem. 2015, 7, 328–333. [Google Scholar] [CrossRef]
- You, B.; Liu, X.; Sun, Y. Efficient H2 Evolution Coupled with Oxidative Refining of Alcohols via A Hierarchically Porous Nickel Bifunctional Electrocatalyst. ACS Catal. 2017, 7, 4564–4570. [Google Scholar] [CrossRef]
- Arshad, F.; Haq, T.; Hussain, I.; Sher, F. Recent advances in electrocatalysts toward alcohol-assisted, energy-saving hydrogen production. ACS Appl. Energy Mater. 2021, 4, 8685–8701. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, D.; Wang, J.; Tu, W.; Wu, D.; Koh, S.W.; Gao, P.; Xu, Z.X.; Deng, S.; Zhou, Y.; et al. Raw biomass electro reforming coupled to green hydrogen generation. Nat. Commun. 2021, 12, 2008. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, H.; Sullivan, K.P.; Gogoi, P.; Deng, Y. Electrochemical lignin conversion. ChemSusChem 2020, 13, 4318–4343. [Google Scholar]
- Lalvani, S.B.; Rajagopal, R. Hydrogen production from lignin-water solution by electrolysis. Holzforschung 1993, 47, 283–286. [Google Scholar] [CrossRef]
- Caravaca, W.E.; Garcia-Lorefifice, S.; Gil, A.; de Lucas-Consuegra, P. Vernoux, Towards a sustainable technology for H2 production: Direct lignin electrolysis in a continuous-flow polymer electrolyte membrane reactor. Electrochem. Commun. 2019, 100, 43–47. [Google Scholar] [CrossRef]
- Hibino, T.; Kobayashi, K.; Nagao, M.; Teranish, S. Hydrogen production by direct lignin electrolysis at intermediate temperatures. ChemElectroChem 2017, 4, 3032–3036. [Google Scholar] [CrossRef]
- Beliaeva, K.; Elsheref, M.; Walden, D.; Dappozze, F.; Marquez, N.A.; Gil, S.; Guillard, C.; Vernoux, P.; Steinmann, S.N.; Caravaca, A. Towards understanding lignin electrolysis: Electro-oxidation of a b-O-4 linkage model on PtRu electrodes. J. Electrochem. Soc. 2020, 167, 134511. [Google Scholar] [CrossRef]
- Beliaeva, K.; Grimaldos-Osorio, N.; Ruiz-Lopez, E.; Burel, L.; Vernoux, P.; Caravaca, A. New insights into lignin electrolysis on nickel based electrocatalysts: Electrochemical performances before and after oxygen evolution. Int. J. Hydrogen Energy 2021, 46, 35752–35764. [Google Scholar] [CrossRef]
- NFidio, D.; Timmermans, J.W.; Antonetti, C.; Galleti, A.M.R.; Gosselink, R.J.A.; Bisselink, R.J.M.; Slaghek, T.M. Electro-oxidative depolymerisation of technical lignin in water using platinum, nickel oxide hydroxide and graphite electrodes. New J. Chem. 2021, 45, 9647–9657. [Google Scholar]
- Mahtab, N.; Bateni, F.; Chen, Z.; Harrington, P.; Staser, J. Biomass-depolarized electrolysis. J. Electrochem. Soc. 2019, 166, 317–322. [Google Scholar]
- Li, J.; Zhou, W.; Huang, Y.; Gao, J. Lignin-assisted water electrolysis for energy-saving hydrogen production with Ti/PbO2 as the anode. Front. Energy Res. 2021, 9, 762346. [Google Scholar] [CrossRef]
- Zhou, H.; Li, Z.; Xu, S.M.; Lu, L.; Xu, M.; Ji, K.; Ge, R.; Yan, Y.; Ma, L.; Kong, X.; et al. Selectively upgrading lignin derivatives to carboxylates through electrochemical oxidative C(OH)- C bond cleavage by a Mn-doped cobalt oxyhydroxide Catalyst. Angew. Chem. Int. Ed. 2021, 60, 8976–8982. [Google Scholar] [CrossRef]
- Rauber, D.; Dier, T.K.F.; Volmer, D.A.; Hempelmann, R. Electrochemical lignin degradation in ionic liquids on ternary mixed metal electrodes. Z. Phys. Chem. 2018, 232, 189–208. [Google Scholar] [CrossRef]
- Garedew, M.; Lin, F.; Song, B.; de Winter, T.M.; Jackson, J.E.; Saffron, C.M.; Lam, C.H.; Anastas, P.T. Greener routes to biomass waste valorization: Lignin transformation through electrocatalysis for renewable chemicals and fuels production. ChemSusChem 2020, 13, 4214–4237. [Google Scholar] [CrossRef]
- Coutanceau, C.; Stève, B. Electrochemical conversion of alcohols for hydrogen production: A short overview. Energy Environ. 2016, 5, 388–400. [Google Scholar] [CrossRef]
- Jing, Y.; Dong, L.; Guo, Y.; Liu, X.; Wang, Y. Chemicals from Lignin: A review of catalytic conversion involving hydrogen. ChemSusChem 2020, 13, 4181. [Google Scholar] [CrossRef]
- Wang, T.; Huang, Z.; Liu, T.; Li, T.; Tian, J.; Gu, K.; Wei, X.; Zhou, P.; Gan, L.; Du, S.; et al. Transforming electrocatalytic biomass upgrading and hydrogen production from electricity input to electricity output. Angew. Chem. Int. Ed. 2022, 61, e202115636. [Google Scholar]
- Li, Z.; Yan, Y.; Xu, S.M.; Zhou, H.; Xu, M.; Ma, L.; Shao, M.; Kong, X.; Wang, B.; Zheng, L.; et al. Alcohols electro-oxidation coupled with H2 production at high current densities promoted by a cooperative catalyst. Nat. Commun. 2022, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Wang, Y.; Li, Z.; Xu, M.; Xu, S.M.; Zhou, H.; Ji, K.; Chen, F.; Zhou, J.; Duan, H. Selective electro-oxidation of biomass-derived alcohols to aldehydes in a neutral medium: Promoted water dissociation over a nickel-oxide-supported ruthenium single-atom catalyst. Angew. Chem. Int. Ed. 2022, 61, e202200211. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhou, H.; Tao, Y.; Ren, J.; Wu, C.; Wu, W. Recent development and perspectives of solvents and electrode materials for electrochemical oxidative degradation of lignin. Electroanalysis 2022. [Google Scholar] [CrossRef]
- Honorato, A.M.B.; Khalid, M.; Pessan, L.A.; Filho, G.T.; Varela, H. Trifunctional catalytic activities of trimetallic FeCoNi alloy nanoparticles embedded in a carbon shell for efficient overall water splitting. J. Mater. Chem. A 2020, 8, 9021. [Google Scholar]
- Ni, S.; Li, S.; Du, Y.; Han, X.; Xu, P. How to reliably report the overpotential of an electrocatalyst. ACS Energy Lett. 2020, 5, 1083–1087. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.H.; Jeffery, A.A.; Min, J.; Jung, N.; Yoo, S.J. Emerging carbon shell-encapsulated metal nanocatalysts for fuel cells and water electrolysis. Nanoscale 2021, 13, 15116. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, G.; Khomyakov, P.A.; Brocks, G.; Karpan, V.V.; van den Brink, J.; Kelly, P. Doping graphene with metal contacts. J. Phys. Rev. Lett. 2008, 101, 026803. [Google Scholar] [CrossRef]
- Sagues, W.J.; Jain, A.; Brown, D.; Aggarwal, S.; Suarez, A.; Kollman, M.; Parka, S.; Argyropoulos, D.S. Are lignin-derived carbon fibers graphitic enough? Green Chem. 2019, 21, 4253–4265. [Google Scholar] [CrossRef]
- Honorato, A.M.B.; Khalid, M.; Pessan, L.A. Coral-like nitrogen doped carbon derived from polyaniline-silicon nitride hybrid for highly active oxygen reduction electrocatalysis. Electrochem. Sci. Adv. 2020, 1, e2000010. [Google Scholar]
- Liu, B.; Wang, R.; Yao, Y.; Ma, J.; Sun, Y.; Wan, J.; Zhang, Y.; Wang, S.; Zou, J. Hollow-structured CoP nanotubes wrapped by N-doped carbon layer with interfacial charges polarization for efficiently boosting oxygen reduction/evolution reactions. Chem. Eng. J. 2022, 431, 133238. [Google Scholar] [CrossRef]
- Ji, L.; Wei, Y.; Wu, P.; Xu, M.; Wang, T.; Wang, S.; Liang, Q.; Meyer, T.J. Heterointerface engineering of Ni2P-Co2P nanoframes for efficient water splitting. Chem. Chem. Mater. 2021, 33, 9165–9173. [Google Scholar] [CrossRef]
- Li, Z.; Deng, S.; Yu, H.; Yin, Z.; Qi, S.; Yang, L.; Lv, J.; Suna, Z.; Zhang, M. Fe-Co-Ni trimetallic organic framework chrysanthemum-like nanoflowers: Efficient and durable oxygen evolution electrocatalysts. J. Mater. Chem. A 2022, 10, 4230–4241. [Google Scholar] [CrossRef]
- Zhang, W.; Han, N.; Luo, J.; Han, X.; Feng, S.; Guo, W.; Xie, S.; Zhou, Z.; Subramanian, P.; Wan, K.; et al. Critical role of phosphorus in hollow structures cobalt-based phosphides as bifunctional catalysts for water splitting. Small 2021, 18, 2103561. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, H.; Li, F.; Lu, L.; Li, W.; Feng, L.; Sun, L. Exploration of electrocatalytic water oxidation properties of NiFe catalysts doped with nonmetallic elements (P, S, Se). Int. J. Hydrogen Energy 2021, 46, 38992–39002. [Google Scholar] [CrossRef]
- Cui, M.Q.S.; Jiang, D.; Zhang, L.; Du, P. Highly efficient and stable water-oxidation electrocatalysis with a very low overpotential using FeNiP substitutional-solid-solution nanoplate Arrays. Adv. Mater. 2017, 29, 1704075. [Google Scholar]
- Lyu, F.; Wang, Q.; Mook, S.C.; Yin, Y. Noble-metal-free electrocatalysts for oxygen evolution. Small 2019, 15, 1804201. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Liu, W.; Lai, W.; Wang, M.; Li, Q.; Wang, Z.; Yuan, J.; Deng, Y.; Bao, J.; Ji, H. NiFe layered double hydroxide/FeOOH heterostructure nanosheets as an efficient and durable bifunctional electrocatalyst for overall seawater splitting. Inorg. Chem. 2021, 60, 17371–17378. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, Y.; He, H.; Peng, L.; Xiao, S.; Yao, S.; Xiao, P. Carbon-incorporated porous honeycomb NiCoFe phosphide nanospheres derived from a MOF precursor for overall water splitting. Chem. Commun. 2019, 55, 10896–10899. [Google Scholar] [CrossRef]
- Li, H.Z.Z.; Ma, L.; Duan, H. Electrocatalytic oxidative upgrading of biomass platform chemicals: From the aspect of reaction mechanism. Chem. Commun. 2020, 58, 897. [Google Scholar]
- Ghahremani, R.; Bateni, F.; Staser, J.A. Electrochemical oxidative valorization of lignin by the nanostructured PbO2/MWNTs electrocatalyst in a low-energy depolymerization process. J. Appl. Electrochem. 2021, 51, 65–78. [Google Scholar]
- Omar, M.C.; Allen, R.S.; Christian, A.T.; John, A.S. Electrochemical conversion of lignin to useful chemicals. Biomass Bioenergy 2016, 88, 89–96. [Google Scholar]
- Dupont, M.F.; Scott, W.D. Separating faradic and non-faradaic charge storage contribution in activated carbon electrochemical capacitors using electrochemical methods: 1. Step Potential Electrochemical Spectroscopy. J. Eletrochem. Soc. 2015, 162, A1246. [Google Scholar] [CrossRef]
- Movil, M.; Garlock, J.A. Staser, Non-precious metal nanoparticle electrocatalysts for electrochemical modification of lignin for low-energy and cost-effective production of hydrogen. Int. J. Hydrogen Energy 2015, 40, 4519–4530. [Google Scholar] [CrossRef]
- Ghahremani, R.; Farales, F.; Bateni, F.; Staser, J.A. Simultaneous hydrogen evolution and lignin depolymerization using NiSn electrocatalysts in a biomass-depolarized electrolyzer. J. Electrochem. Soc. 2020, 167, 043502. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Xiang, X.; Yan, D.; Lia, F. Engineering of Zn Co-layered double hydroxide nano walls toward high-efficiency electrochemical water oxidation. J. Mater. Chem. A 2014, 2, 13250–13258. [Google Scholar] [CrossRef]
- Anantharaj, S.; Kundu, S. Do the evaluation parameters reflect intrinsic activity of electrocatalysts in electrochemical water Splitting? ACS Energy Lett. 2019, 4, 1260–1264. [Google Scholar] [CrossRef]
- Kozuch, S.; Martin, J.M.L. “Turning Over” definitions in catalytic cycles. ACS Catal. 2012, 2, 2787–27949. [Google Scholar] [CrossRef]
- Pintado, S.; Goberna, F.S.; Escudero, A.E.; Gala, C.J.R. Fast and persistent electrocatalytic water oxidation by Co-Fe prussian blue coordination polymers. J. Am. Chem. Soc. 2013, 135, 13270–13273. [Google Scholar] [CrossRef]
- Anantharaj, S.; Karthik, P.E.; Noda, S. The significance of properly reporting turnover frequency in electrocatalysis research. Angew. Chem. Int. Ed. 2021, 60, 23051–23067. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, W.; Chen, X.; Peng, Y.; Song, Y.; Lv, C.; Liu, H.; Sun, J.; Yuan, D.; Li, X.; et al. Defect-rich nitrogen doped Co3O4/C porous nanocubes enable high-efficiency bifunctional oxygen electrocatalysis. Adv. Funct. Mater. 2019, 29, 1902875. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honorato, A.M.B.; Khalid, M.; Curvelo, A.A.d.S.; Varela, H.; Shahgaldi, S. Trimetallic Nanoalloy of NiFeCo Embedded in Phosphidated Nitrogen Doped Carbon Catalyst for Efficient Electro-Oxidation of Kraft Lignin. Polymers 2022, 14, 3781. https://doi.org/10.3390/polym14183781
Honorato AMB, Khalid M, Curvelo AAdS, Varela H, Shahgaldi S. Trimetallic Nanoalloy of NiFeCo Embedded in Phosphidated Nitrogen Doped Carbon Catalyst for Efficient Electro-Oxidation of Kraft Lignin. Polymers. 2022; 14(18):3781. https://doi.org/10.3390/polym14183781
Chicago/Turabian StyleHonorato, Ana Maria Borges, Mohmmad Khalid, Antonio Aprigio da Silva Curvelo, Hamilton Varela, and Samaneh Shahgaldi. 2022. "Trimetallic Nanoalloy of NiFeCo Embedded in Phosphidated Nitrogen Doped Carbon Catalyst for Efficient Electro-Oxidation of Kraft Lignin" Polymers 14, no. 18: 3781. https://doi.org/10.3390/polym14183781