Slow-Release Nitrogen Fertilizers with Biodegradable Poly(3-hydroxybutyrate) Coating: Their Effect on the Growth of Maize and the Dynamics of N Release in Soil
Abstract
:1. Introduction
2. Materials and Methods
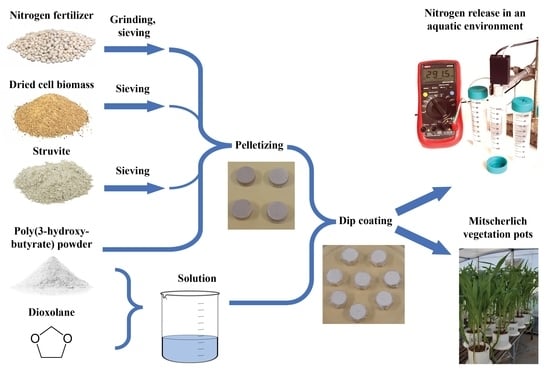
2.1. Preparation of Coated and Encapsulated Fertilizers
2.1.1. The Fertilizer
2.1.2. Filling Material in Pellets
2.1.3. Preparation of Fertilizer Pellets
2.1.4. The Material for the Coating Solution
2.1.5. Coating of Fertilizer Pellets and Encapsulation
2.2. Testing Nitrogen Release from Coated Fertilizer
- G…conductance (S)
- κ…specific conductivity (S · cm–1)
- A…probe electrode area (cm2)
- l…probe electrode distance (cm)
- Λm…molar conductivity (S · cm2 · mol−1)
- cIon…ion concentration (mol · cm−3) [35]
2.3. The Greenhouse Pot Experiment
2.3.1. Experimental Design
2.3.2. Soil and Plant Sampling and Analyses
2.3.3. Statistical Data Analysis
3. Results
3.1. Preliminary Tests of Coating Solutions
3.2. Slow-Release Fertilizers
3.3. Pellets Encapsulated in Foils
3.4. The Nitrogen Release from Coated Fertilizer in the Aquatic Environment
3.5. Efect of Coated Fertilizers in Greenhouse Experiment
3.5.1. The Nitrogen Release from the Fertilizers in the Soil
3.5.2. Effect of Coated and Encapsulated Fertilizers on Plant Biomass of Maize
Above-Ground Biomass Production and Root Size
Nitrogen Content in Plant, Chlorophyll Content, NDVI, and Quant Yield of PSII
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smil, V. Nitrogen Cycle and World Food Production. World Agric. 2011, 2, 9–13. [Google Scholar]
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2019: Highlights (ST/ESA/SER.A/423). 2019. Available online: https://population.un.org/wpp/publications/files/wpp2019_highlights.pdf (accessed on 1 July 2022).
- Adam, D. How Far Will Global Population Rise. Nature 2021, 597, 462–465. [Google Scholar] [CrossRef]
- Lawrencia, D.; Wong, S.K.; Low, D.Y.S.; Goh, B.H.; Goh, J.K.; Ruktanonchai, U.R.; Soottitantawat, A.; Lee, L.H.; Tang, S.Y. Controlled Release Fertilizers: A Review on Coating Materials and Mechanism of Release. Plants 2021, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Davidson, E.; Mauzerall, D.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisht, N.; Chauhan, P.S. Excessive and Disproportionate Use of Chemicals Cause Soil Contamination and Nutritional Stress. In Soil Contamination—Threats and Sustainable Solutions; Larramendy, M.L., Soloneski, S., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Fan, L.-T.; Singh, S.K. Introduction. In Controlled Release: A Quantitative Treatment, 1st ed.; Fan, L.-T., Singh, S.K., Eds.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 13, pp. 1–8. [Google Scholar]
- Gil-Ortiz, R.; Naranjo, M.Á.; Ruiz-Navarro, A.; Atares, S.; García, C.; Zotarelli, L.; San Bautista, A.; Vicente, O. Enhanced Agronomic Efficiency Using a New Controlled-Released, Polymeric-Coated Nitrogen Fertilizer in Rice. Plants 2020, 9, 1183. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, M.; Ni, B.; Xie, L. κ-Carrageenan–sodium alginate beads and superabsorbent coated nitrogen fertilizer with slow-release, water-retention, and anticompaction properties. Ind. Chem. Eng. Res. 2012, 51, 1413–1422. [Google Scholar] [CrossRef]
- Cole, J.C.; Smith, M.W.; Penn, C.J.; Cheary, B.S.; Conaghan, K.J. Nitrogen, phosphorus, calcium, and magnesium applied individually or as a slow release or controlled release fertilizer increase growth and yield and affect macronutrient and micronutrient concentration and content of field-grown tomato plants. Sci. Hortic. 2016, 211, 420–430. [Google Scholar] [CrossRef] [Green Version]
- Cong, Z.; Yazhen, S.; Changwen, D.; Jianmin, Z.; Huoyan, W.; Xiaoqin, C. Evaluation of waterborne coating for controlled-release fertilizer using Wurster fluidized bed. Ind. Eng. Chem. Res. 2010, 49, 9644–9647. [Google Scholar] [CrossRef]
- Trenkel, M.E. Slow- and Controlled-Release and Stabilized Fertilizers: An Option for Enhancing Nutrient Efficiency in Agriculture, 2nd ed.; IFA: Paris, France, 2010; p. 160. [Google Scholar]
- Shaviv, A. Controlled release fertilizers. In Proceedings of the IFA International Workshop on Enhanced-Efficiency Fertilizers, Frankfurt, Germany, 28–30 June 2005. [Google Scholar]
- AAPFCO. Official Publication No. 48; Association of American Plant Food Control Officials, Inc.: West Lafayette, IA, USA, 1995. [Google Scholar]
- Lubkowski, K.; Smorowska, A.; Grzmil, B.; Kozłowska, A. Controlled-release fertilizer prepared using a biodegradable aliphatic copolyester of poly (butylene succinate) and dimerized fatty acid. J. Agric. Food. Chem. 2015, 63, 2597–2605. [Google Scholar] [CrossRef] [PubMed]
- Fertilizers Europe. 2019/2020 Overview. Available online: https://www.fertilizerseurope.com/wp-content/uploads/2020/07/AR-2019_20_32-pager-screen.pdf (accessed on 16 July 2022).
- Obruca, S.; Benesova, P.; Oborna, J.; Marova, I. Application of protease-hydrolyzed whey as a complex nitrogen source to increase poly(3-hydroxybutyrate) production from oils by Cupriavidus necator. Biotechnol. Lett. 2014, 36, 775–781. [Google Scholar] [CrossRef]
- Silva, L.F.; Taciro, M.K.; Raicher, G.; Piccoli, R.A.M.; Mendonça, T.T.; Lopes, M.S.G.; Gomez, J.G.C. Perspectives on the production of polyhydroxyalkanoates in biorefineries associated with the production of sugar and ethanol. Int. J. Biol. Macromol. 2014, 71, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, K.; Dumont, M.-J.; Rio, L.F.D.; Orsat, V. Producing PHAs in the bioeconomy—Towards a sustainable bioplastic. Sustain. Prod. Consum. 2017, 9, 58–70. [Google Scholar] [CrossRef]
- Surendran, A.; Lakshmanan, M.; Chee, J.Y.; Sulaiman, A.M.; Thuoc, D.V.; Sudesh, K. Can Polyhydroxyalkanoates Be Produced Efficiently From Waste Plant and Animal Oils? Front. Bioeng. Biotechnol. 2020, 8, 169. [Google Scholar] [CrossRef]
- Nuttipon, Y.; Suchada, C.N. Toward non-toxical and simple recovery process of poly(3-hydroxybutyrate) using the green solvent 1,3-dioxolane. Process Biochem. 2018, 69, 197–207. [Google Scholar] [CrossRef]
- Boyandin, A.N.; Kazantseva, E.A.; Varygina, D.E.; Volova, T.G. Constructing Slow-Release Formulations of Ammonium Nitrate Fertilizer Based on Degradable Poly(3-hydroxybutyrate). J. Agric. Food Chem. 2017, 65, 6745–6752. [Google Scholar] [CrossRef] [PubMed]
- Lovochemie, a.s. Available online: https://www.lovochemie.cz/en (accessed on 26 July 2022).
- TianAn Biopolymer. Available online: www.tianan-enmat.com (accessed on 26 July 2022).
- Nafigate Corporation. Available online: https://www.nafigate.com/ (accessed on 26 July 2022).
- Merck. Available online: https://www.sigmaaldrich.com/CZ/en (accessed on 26 July 2022).
- SPECAC. Available online: https://specac.com/ (accessed on 26 July 2022).
- Lach:ner. Available online: https://www.lach-ner.cz/en (accessed on 26 July 2022).
- PENTA Chemicals Unlimited. Available online: https://www.pentachemicals.eu/en/ (accessed on 26 July 2022).
- AvantorTM Delivered by VWRTM. Available online: https://www.vwr.com/ (accessed on 26 July 2022).
- Panara. Available online: https://panaraplast.com/ (accessed on 26 July 2022).
- STU. Available online: https://www.stuba.sk/english.html?page_id=132 (accessed on 26 July 2022).
- PubChem: Ammonium Nitrate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ammonium-nitrate (accessed on 26 July 2022).
- Miller, R.L.; Bradford, W.L.; Peters, N.E. Specific Conductance: Theoretical Considerations and Application to Analytical Quality Control; US Government Printing Office: Washington, DC, USA, 1988.
- Metex Corporation Limited. Available online: https://www.metexcorporation.com/hand-held-instruments.html (accessed on 26 July 2022).
- OriginLab. Available online: https://www.originlab.com/ (accessed on 26 July 2022).
- Oseva. Available online: https://oseva.com/about-oseva/ (accessed on 26 July 2022).
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis Part 1—Physical and Mineralogical Methods; Klute, A., Ed.; ASA and SSSA: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Schumacher, B.A. Methods for the Determination of Total Organic Carbon (TOC) in Soils and Sediments; United States Environmental Protection Agency, Environmental Sciences Division National, Exposure Research Laboratory: Las Vegas, NV, USA, 2002. [Google Scholar]
- Zbíral, J.; Malý, S.; Váňa, M. (Eds.) Soil Analysis III, 3rd ed.; Central Institute for Supervising and Testing in Agriculture: Brno, Czech Republic, 2011; pp. 18–52. (In Czech) [Google Scholar]
- Zbíral, J. Analysis of Soils I. Unified Techniques, 2nd ed.; Central Institute for Supervising and Testing in Agriculture: Brno, Czech Republic, 2002; p. 197. (In Czech) [Google Scholar]
- Netto, A.L.; Campostrini, E.; Goncalves de Oliverira, J.; Bressan-Smith, R.E. Photosynthetic pigments, nitrogen, chlorophyll a fluorescence and SPAD-502 readings in coffee leaves. Sci. Hortic. 2005, 104, 199–209. [Google Scholar] [CrossRef]
- Škarpa, P.; Klofáč, D.; Krčma, F.; Šimečková, J.; Kozáková, Z. Effect of Plasma Activated Water Foliar Application on Selected Growth Parameters of Maize (Zea mays L.). Water 2020, 12, 3545. [Google Scholar] [CrossRef]
- Zbíral, J. Plant Analysis: Integrated Work Procedures; Central Institute for Supervising and Testing in Agriculture: Brno, Czech Republic, 2005; p. 192. (In Czech) [Google Scholar]
- StatSoft, Inc. STATISTICA (Data Analysis Software System), Version 12. 2013. Available online: http://www.statsoft.com/ (accessed on 13 May 2021).
- Rashidzadeh, A.; Olad, A. Slow-released NPK fertilizer encapsulated by NaAlg-g-poly(AA-co-AAm)/MMT superabsorbent nanocomposite. Carbohydr. Polym. 2014, 114, 269–278. [Google Scholar] [CrossRef]
- Grubbs, J.B., III; Locklin, J.J. PLA/PHA Biodegradable Coatings for Seeds and Fertilizers. U.S. Patent Application Number 16/880083, 26 November 2020. [Google Scholar]
- Liu, C.; Chen, F.; Li, Z.; Cocq, K.L.; Liu, Y.; Wu, L. Impact of nitrogen practices on yield, grain quality, and nitrogen-use efficiencyof crops and soil fertility in three paddy-upland cropping systems. J. Sci. Food Agric. 2021, 101, 2218–2226. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, L.; Yang, Y.; Shi, R. Application of controlled release urea improved grain yield and nitrogen use efficiency: Ameta-analysis. PLoS ONE 2020, 15, e0241481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, Z.; Yu, Q.; Liu, B.; Duan, M.; Wang, L. Effect of controlled-release urea fertilizers for oilseed rape (Brassica napus L.) on soil carbon storage and CO2 emission. Environ. Sci. Pollut. Res. 2020, 27, 31983–31994. [Google Scholar] [CrossRef]
- Liao, J.; Liu, X.; Song, H.; Chen, X.; Zhang, Z. Effects of biochar-based controlled release nitrogen fertilizer on nitrogen-use efficiency of oilseed rape (Brassica napus L.). Sci. Rep. 2020, 10, 11063. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.L. Loss of fertilizer N from plants-soil system and the strategies and techniques for its reduction. Soil Environ. Sci. 2000, 9, 1–6. [Google Scholar]
- Zvomuya, F.; Rosen, C.J.; Russelle, M.P.; Gupta, S.C. Nitrate leaching and nitrogen recovery following application of polyolefin-coated urea to potato. J. Environ. Qual. 2003, 32, 480–489. [Google Scholar] [CrossRef]
- Mikkelsen, R.L.; Williams, H.M.; Behel, A.D., Jr. Nitrogen leaching and plant uptake from controlled-release fertilizers. Fertil. Res. 1994, 37, 43–50. [Google Scholar] [CrossRef]
- Cabrera, R.I. Comparative evaluation of nitrogen release patterns from controlled-release fertilizers by nitrogen leaching analysis. Hort. Sci. 1997, 32, 669–673. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Wan, Y.; Li, Y.; Liu, Z.; Chen, J.; Zhou, H.; Gao, Y.; Chen, B.; Zhang, M. Developing water and nitrogen budgets of a wheat-maize rotation system using auto-weighing lysimeter: Effects of blended application of controlled-release an un-coated urea. Environ. Pollut. 2020, 263, 114383. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, M.; Liu, Z.; Zhou, H.; Lu, H.; Zhang, W.; Yang, Y.; Li, C.; Chen, B. Combining controlled-release urea and normal urea to improve the nitrogen use efficiency and yield under wheat-maize double cropping system. Field Crops Res. 2016, 197, 52–62. [Google Scholar] [CrossRef]
- Xiao, Y.; Peng, F.; Zhang, Y.; Wang, J.; Zhuge, Y.; Zhang, S.; Gao, H. Effect of bag-controlled release fertilizer on nitrogen loss, greenhouse gas emissions, and nitrogen applied amount in peach production. J. Clean. Prod. 2019, 234, 258–274. [Google Scholar] [CrossRef]
- Škarpa, P.; Mikušová, D.; Antošovský, J.; Kučera, M.; Ryant, P. Oil-Based Polymer Coatings on CAN Fertilizer in Oilseed Rape (Brassica napus L.) Nutrition. Plants 2021, 10, 1605. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Zhou, X.; Liu, O.; Peng, J.; Wang, W.; Zhang, Z.; Yang, Y.; Song, H.; Guan, C. Effects of a controlled-release fertilizer on yield, nutrient uptake, and fertilizer usage efficiency in early ripening rapeseed (Brassica napus L.). J. Zhejiang Univ. Sci. 2016, 17, 775–786. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Chen, Z.; Xing, Z.; Zhou, L.; Liu, Q.; Zhang, Z.; Jiang, Y.; Hu, Y.; Zhu, J.; Cui, P.; et al. Effects of slow or controlled release fertilizer types and fertilization modes on yield and quality of rice. J. Integr. Agric. 2018, 17, 2222–2234. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, M.; Zheng, G.; Yao, Y.; Tao, R.; Zhu, M.; Ding, J.; Li, C.; Guo, W.; Zhu, X. Twice-split application of controlled-release nitrogen fertilizer met the nitrogen demand of winter wheat. Field Crops Res. 2021, 267, 108163. [Google Scholar] [CrossRef]
- Ye, Y.; Liang, X.; Chen, Y.; Liu, J.; Gu, J.; Guo, R.; Li, L. Alternate wetting and drying irrigation and controlled-release nitrogen fertilizer in late-season rice. Effects on dry matter accumulation, yield, water and nitrogen use. Field Crops Res. 2013, 144, 212–224. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, Y.; Liao, Y.; Nie, J.; Yie, J.; Yang, Z.; Zhoiu, X. Effects of the application of controlled release nitrogen fertilizer on rapeseed yield, agronomic characters and soil fertility. Agric. Sci. Technol. 2015, 16, 1226. [Google Scholar]
- Murugan, P.; Ong, S.Y.; Hashim, R.; Kosugi, A.; Arai, T.; Sudesh, K. Development and evaluation of controlled release fertilizer using P(3HB-co-3HHx) on oil palm plants (nursery stage) and soil microbes. Biocatal. Agric. Biotechnol. 2020, 28, 101710. [Google Scholar] [CrossRef]
- Koning, L.A.; Veste, M.; Freese, D.; Lebzien, S. Effects of nitrogen and phosphate fertilization on leaf nutrient content, photosynthesis, and growth of the novel bioenergy crop Fallopia sachalinensis cv. ‘Igniscum Candy’. J. Appl. Bot. Food Qual. 2015, 88, 22–28. [Google Scholar]
- Gianquinto, G.; Goffart, J.P.; Olivier, M.; Guarda, G.; Colauzzi, M.; Dalla Costa, L.; Delle Vedove, G.; Vos, J.; Mackerron, D.K.L. The Use of Hand-held Chlorophyll Meters As a Tool to Assess the Nitrogen Status and to Guide Nitrogen Fertilization of Potato Crop. Potato Res. 2004, 47, 35–80. [Google Scholar] [CrossRef]






| Soil Parameters | Value | Ref. |
|---|---|---|
| Clay | 20% | [39] |
| Dust | 27% | |
| Sand | 53% | |
| Oxidizable C content (Cox) | 0.80% | [40] |
| pH (CaCl2) | 6.09 | [41] |
| Cation exchange capacity | 164 mmol/kg | |
| Noverall | 0.19% | |
| NH4+ (K2SO4) | 1.48 mg/kg | |
| NO3− (K2SO4) | 17.2 mg/kg | |
| P (Mehlich 3) | 36.4 mg/kg | |
| K (Mehlich 3) | 400 mg/kg | |
| Ca (Mehlich 3) | 2720 mg/kg | |
| Mg (Mehlich 3) | 214 mg/kg |
| Treatment | Composition of Pellets Used in Vegetation Tests | Number of Pellets (pcs/Pot) | N Dose (g/Pot) |
|---|---|---|---|
| CAN-c | 100% CAN with P3HB coating | 4 | 0.54 |
| CAN/P-c | 50% CAN + 50% P3HB with P3HB coating | 8 | 0.54 |
| CAN/P/S-c | 50% CAN + 25% P3HB + 25% struvite with P3HB coating | 8 | 0.54 |
| CAN/P/B-c | 50% CAN + 50% biomass with P3HB coating | 8 | 0.54 |
| CAN/P-bf | 50% CAN + 50% P3HB encapsulated in biodegradable film | 8 | 0.54 |
| CAN | 100% CAN (positive reference) | 4 | 0.54 |
| CON-c | without fertilizer (negative reference) | 0 | 0 |
| Plant Parameter | Device Used | Terms | Ref. |
|---|---|---|---|
| Chlorophyll content (N-tester value) | Yara N-Tester chlorophyll meter (Yara International ASA, Oslo, Norway) | t1–t3 | [43] |
| Vegetation index (NDVI) | PlanPen NDVI310 device (Photon Systems Instruments, Drásov, Czech Republic) | t1–t3 | |
| Quantum yield of the PSII (ΦPSII) | PAR-Fluorpen FP110-LM/D device (Photon Systems Instruments, Drásov, Czech Republic) | t1–t3 | [44] |
| Dry weight of AGB | Laboratory-scale PCB Kern (KERN & Sohn GmbH, Balingen, Germany) | t1–t3 | |
| N content in AGB | Kjeltec 2300 device (Foss Analytical, Hillerød, Denmark) | t1–t3 | [45] |
| Root electrical capacitance (CR) | VOLTCRAFT LCR 4080 (Conrad Electronic GmbH, Wels, Austria) | t3 | [44] |
| Coating Solution | Coating Weights of the Pellets (%) |
|---|---|
| 7% P3HB in chloroform with ethanol | 82.4 ± 4.6 |
| 6% P3HB in dioxolane | 19.9 ± 1.5 |
| 6% P3HB in chloroform with amylene | Failed to form an acceptable coating |
| Formulation | 6-Fold Additional Coating Applied (% of Original Pellet Weight, Average and Standard Deviation) |
|---|---|
| 100% CAN | 11.9 ± 1.6 |
| 50% CAN + 50% P3HB | 16.9 ± 2.5 |
| 50% CAN + 25% P3HB + 25% struvite | 13.8 ± 2.8 |
| 50% CAN + 50% biomass | 14.4 ± 2.4 |
| Composition of 6-Fold Manually Coated Fertilizer Pellets | |
|---|---|
| CAN/P/B-C | 50% CAN + 50% biomass + coating P3HB in dioxolane |
| CAN/P-C | 50% CAN + 50% P3HB + coating P3HB in dioxolane |
| CAN/P/S-C | 50% CAN + 25% P3HB + 25% struvite + coating P3HB in dioxolane |
| CAN-c | 100% CAN + coating P3HB in dioxolane |
| CAN | 100% CAN (positive reference) |
| CAN/P-bf | 50% CAN + 50% P3HB + biodegradable polymeric film (encapsulation) |
| Term of Measured | Treatments | N Content in AGB (% of DM ± SD) | N-Tester Value | NDVI | ΦPSII |
|---|---|---|---|---|---|
| t1 | CAN-c | 5.99 a ± 0.25 | 519 def ± 8 | 0.75 cdefgh ± 0.01 | 0.835 abc ± 0.006 |
| CAN/P-c | 5.81 ab ± 0.04 | 529 def ± 12 | 0.77 abcde ± 0.01 | 0.835 abc ± 0.006 | |
| CAN/P/S-c | 5.70 ab ± 0.20 | 518 def ± 8 | 0.75 defgh ± 0.02 | 0.838 abc ± 0.005 | |
| CAN/P/B-c | 5.58 bc ± 0.08 | 506 ef ± 7 | 0.76 abcdefg ± 0.02 | 0.825 abcd ± 0.006 | |
| CAN/P-bf | 5.57 bc ± 0.07 | 506 ef ± 13 | 0.76 abcdefg ± 0.02 | 0.825 abcd ± 0.006 | |
| CAN | 5.64 ab ± 0.06 | 565 abc ± 7 | 0.76 abcdefg ± 0.02 | 0.825 abcd ± 0.006 | |
| CON-nf | 5.25 c ± 0.08 | 498 f ± 10 | 0.77 abcdef ± 0.01 | 0.833 abc ± 0.005 | |
| t2 | CAN-c | 3.54 d ± 0.25 | 574 ab ± 20 | 0.79 abcd ± 0.01 | 0.833 abc ± 0.005 |
| CAN/P-c | 3.32 de ± 0.13 | 538 cd ± 9 | 0.80 abc ± 0.01 | 0.840 ab ± 0.000 | |
| CAN/P/S-c | 3.47 de ± 0.11 | 530 de ± 12 | 0.80 a ± 0.00 | 0.840 ab ± 0.008 | |
| CAN/P/B-c | 3.30 de ± 0.16 | 543 bcd ± 16 | 0.80 ab ± 0.01 | 0.835 abc ± 0.013 | |
| CAN/P-bf | 3.14 e ± 0.23 | 541 cd ± 9 | 0.79 abcd ± 0.01 | 0.843 a ± 0.005 | |
| CAN | 3.29 de ± 0.12 | 579 a ± 3 | 0.78 abcd ± 0.02 | 0.825 abcd ± 0.010 | |
| CON-nf | 1.48 f ± 0.20 | 397 g ± 13 | 0.75 bcdefg ± 0.02 | 0.830 abc ± 0.008 | |
| t3 | CAN-c | 1.57 f ± 0.04 | 366 hi ± 19 | 0.71 gh ± 0.02 | 0.823 abcd ± 0.010 |
| CAN/P-c | 1.50 f ± 0.04 | 405 g ± 7 | 0.71 gh ± 0.01 | 0.823 abcd ± 0.010 | |
| CAN/P/S-c | 1.58 f ± 0.06 | 399 g ± 3 | 0.70 h ± 0.01 | 0.820 bcd ± 0.000 | |
| CAN/P/B-c | 1.55 f ± 0.06 | 358 hi ± 23 | 0.73 efgh ± 0.02 | 0.818 cd ± 0.010 | |
| CAN/P-bf | 1.62 f ± 0.06 | 388 gh ± 8 | 0.72 gh ± 0.01 | 0.823 abcd ± 0.013 | |
| CAN | 1.52 f ± 0.06 | 350 i ± 7 | 0.72 fgh ± 0.00 | 0.808 de ± 0.010 | |
| CON-nf | 0.80 g ± 0.04 | 249 j ± 8 | 0.62 i ± 0.05 | 0.790 e ± 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontárová, S.; Přikryl, R.; Škarpa, P.; Kriška, T.; Antošovský, J.; Gregušková, Z.; Figalla, S.; Jašek, V.; Sedlmajer, M.; Menčík, P.; et al. Slow-Release Nitrogen Fertilizers with Biodegradable Poly(3-hydroxybutyrate) Coating: Their Effect on the Growth of Maize and the Dynamics of N Release in Soil. Polymers 2022, 14, 4323. https://doi.org/10.3390/polym14204323
Kontárová S, Přikryl R, Škarpa P, Kriška T, Antošovský J, Gregušková Z, Figalla S, Jašek V, Sedlmajer M, Menčík P, et al. Slow-Release Nitrogen Fertilizers with Biodegradable Poly(3-hydroxybutyrate) Coating: Their Effect on the Growth of Maize and the Dynamics of N Release in Soil. Polymers. 2022; 14(20):4323. https://doi.org/10.3390/polym14204323
Chicago/Turabian StyleKontárová, Soňa, Radek Přikryl, Petr Škarpa, Tomáš Kriška, Jiří Antošovský, Zuzana Gregušková, Silvestr Figalla, Vojtěch Jašek, Marek Sedlmajer, Přemysl Menčík, and et al. 2022. "Slow-Release Nitrogen Fertilizers with Biodegradable Poly(3-hydroxybutyrate) Coating: Their Effect on the Growth of Maize and the Dynamics of N Release in Soil" Polymers 14, no. 20: 4323. https://doi.org/10.3390/polym14204323