High Molecular Weight AB-Polybenzimidazole and Its Solutions in a Complex Organic Solvent: Dissolution Kinetics and Rheology
Abstract
:1. Introduction
2. Materials and Methods
2.1. ABPBI Synthesis
2.2. Preparation of ABPBI Solutions
2.3. Rheology
- -
- cone-plate with a diameter of 20 mm and an angle between the cone and the plate of 1° (for solutions containing 9% of ABPBI);
- -
- cone-plate with a diameter of 60 mm and an angle between the cone and the plate of 1° (for solutions containing 7% of ABPBI);
- -
- a bicone with a diameter of 60 mm and an angle between the cone and the plates of 1° (for solutions with ABPBI content of 3%).
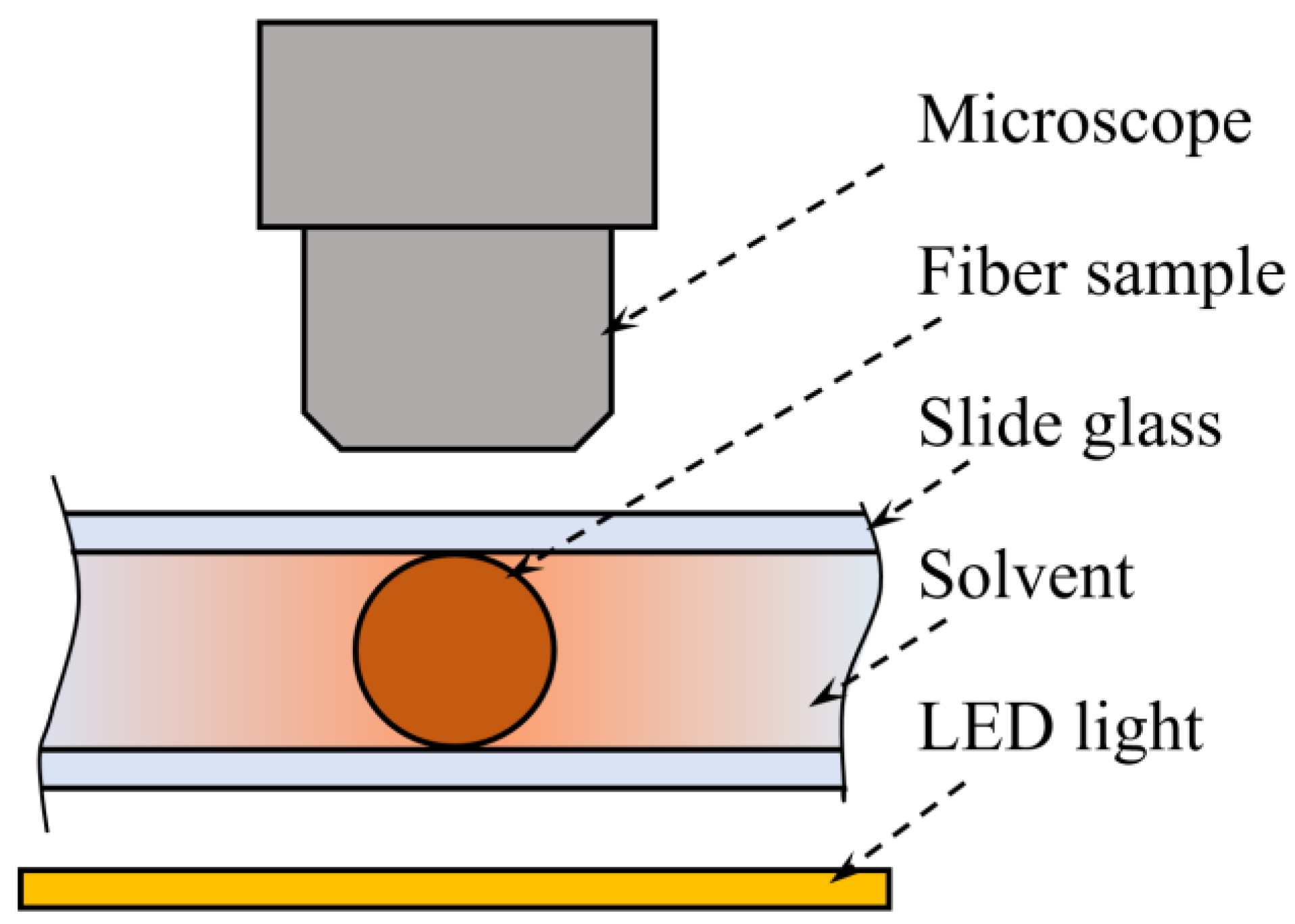
2.4. Polymer Dissolution Kinetics
2.5. Determination of Water Content
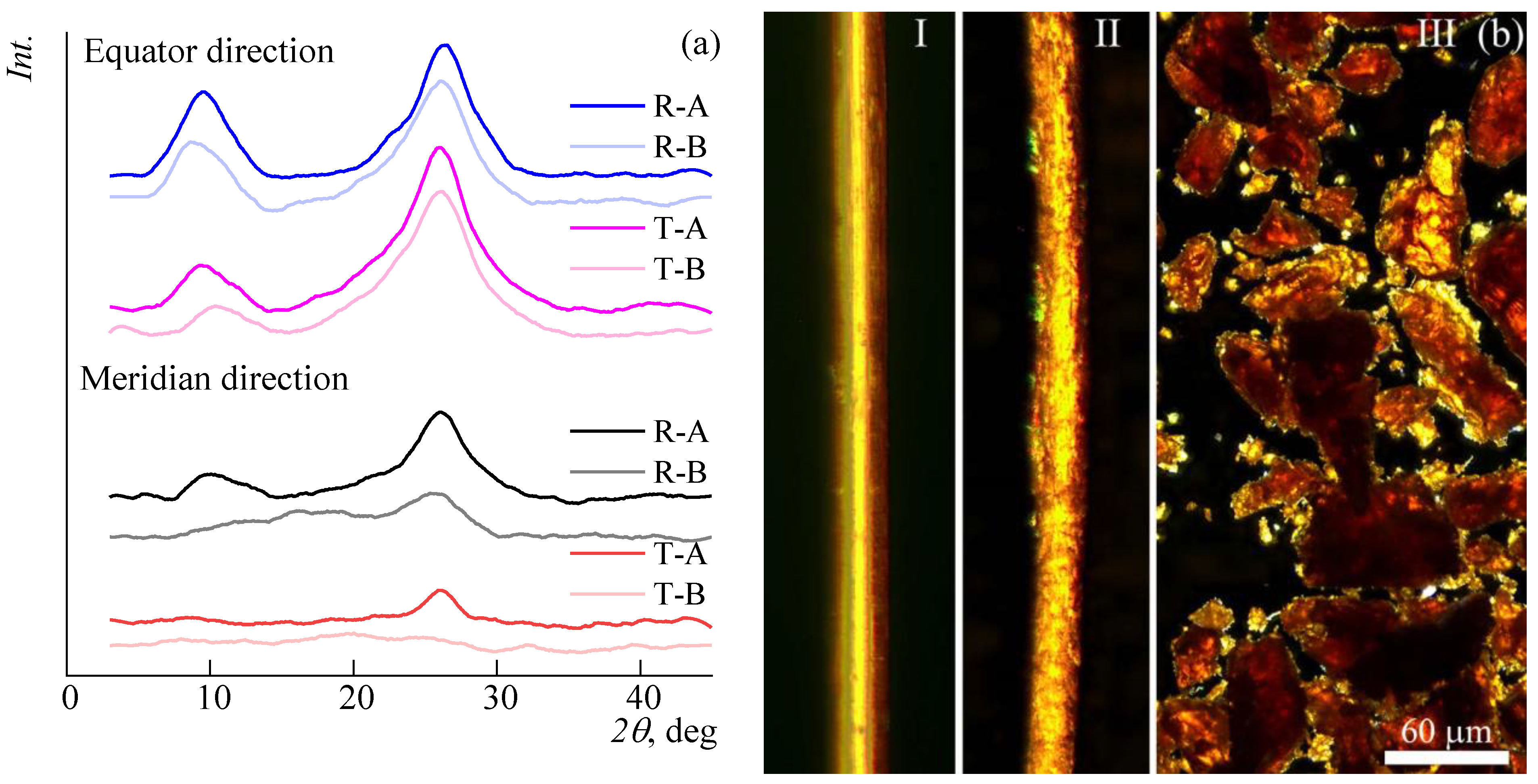
2.6. XRD Analysis
3. Results and Discussion
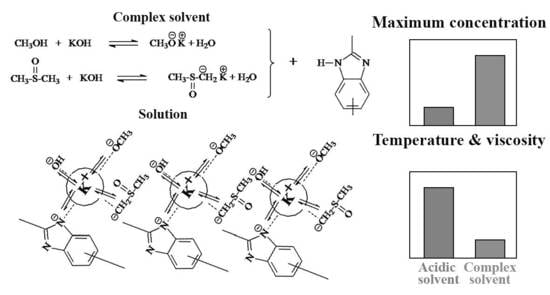
3.1. Composition of the Multicomponent Solvent
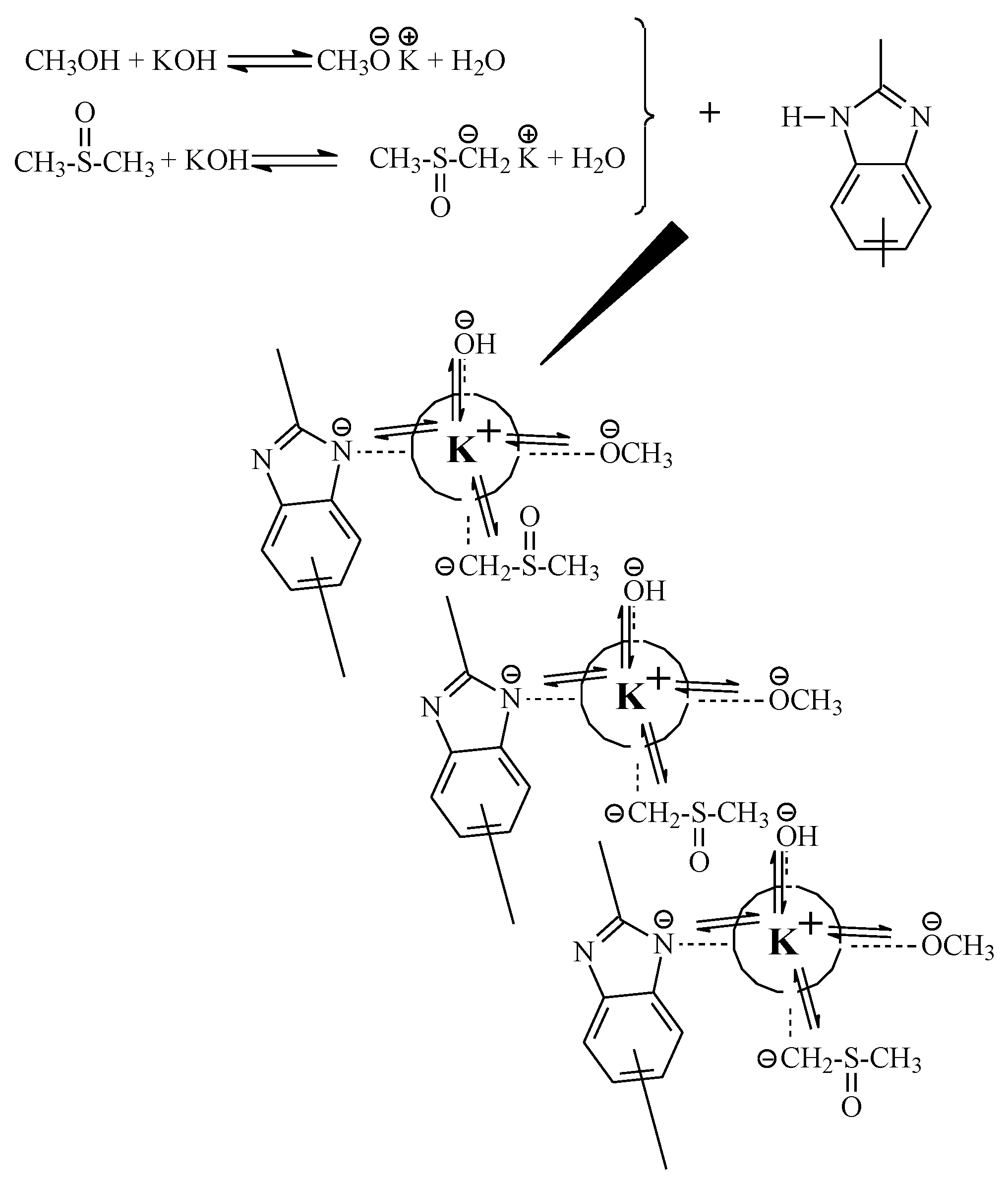
3.1.1. KOH
3.1.2. Methanol
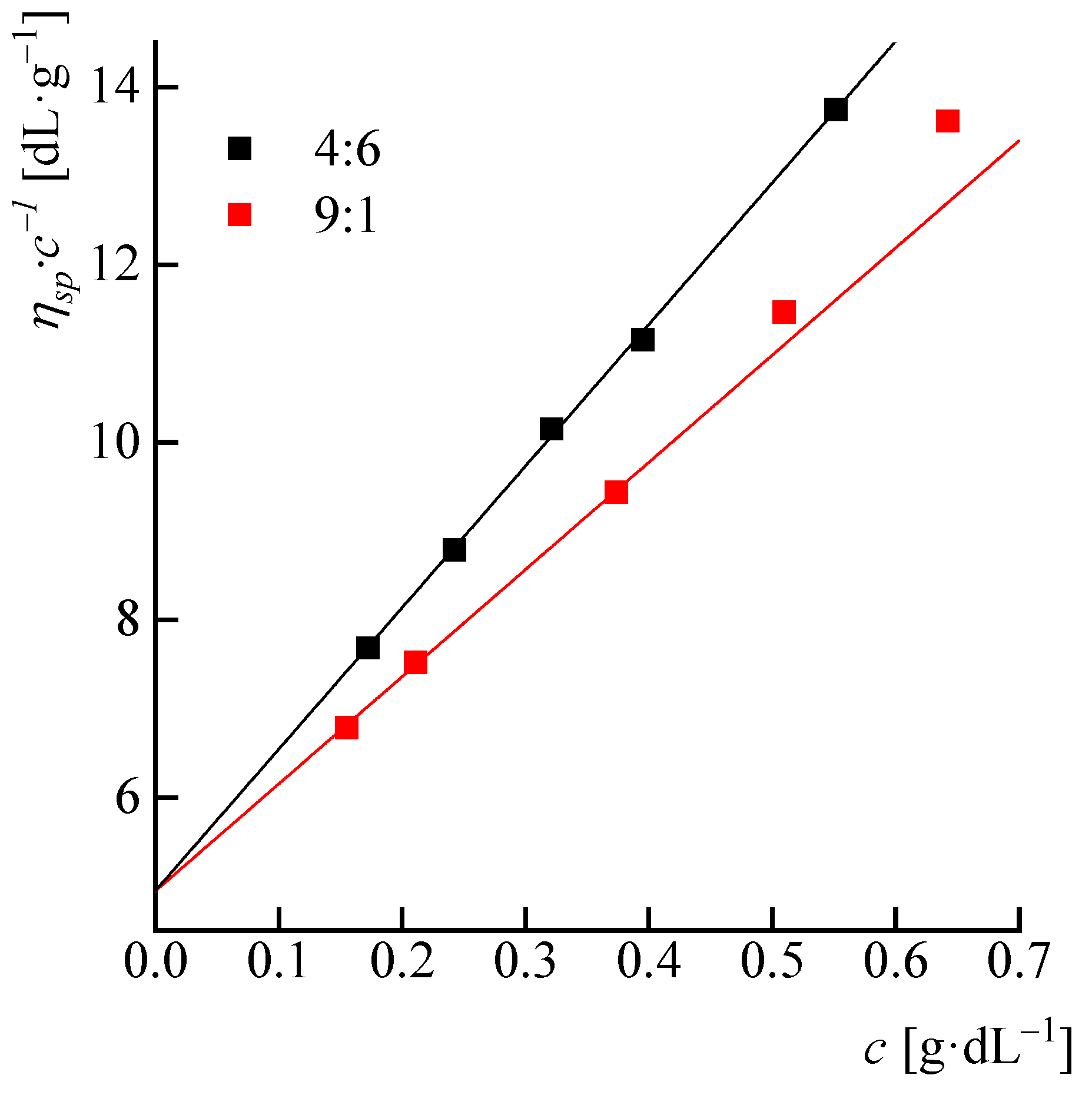
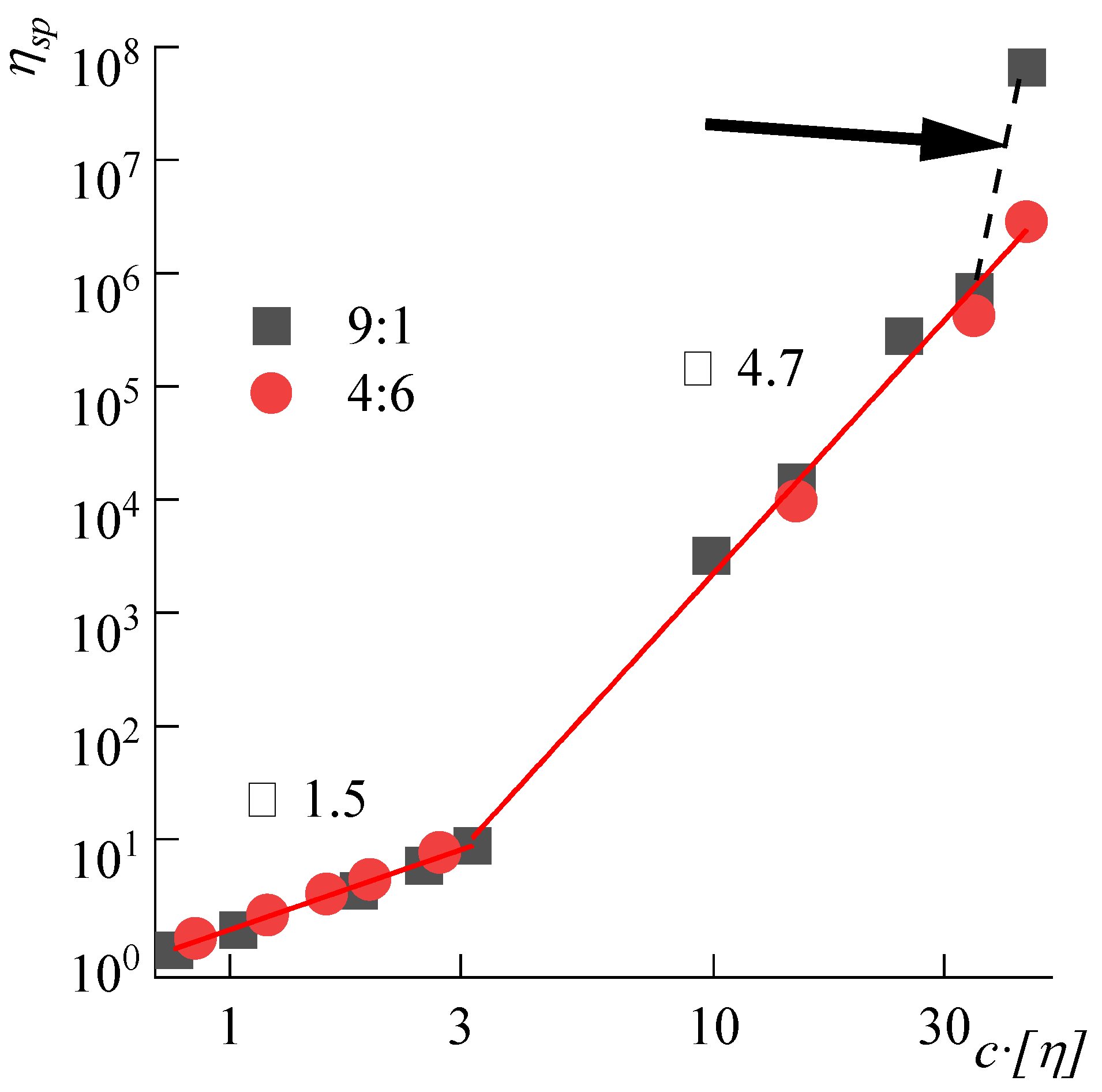
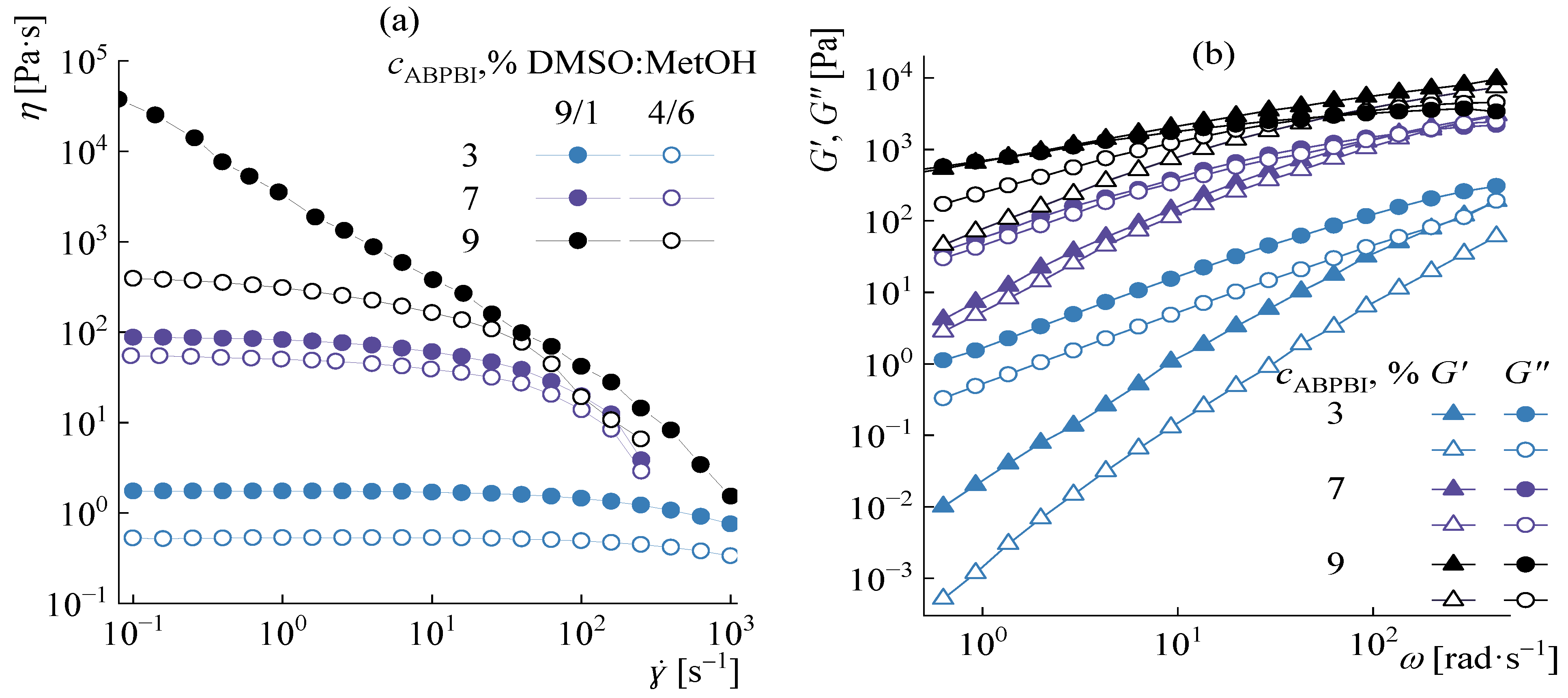
3.2. Viscometry of Solutions
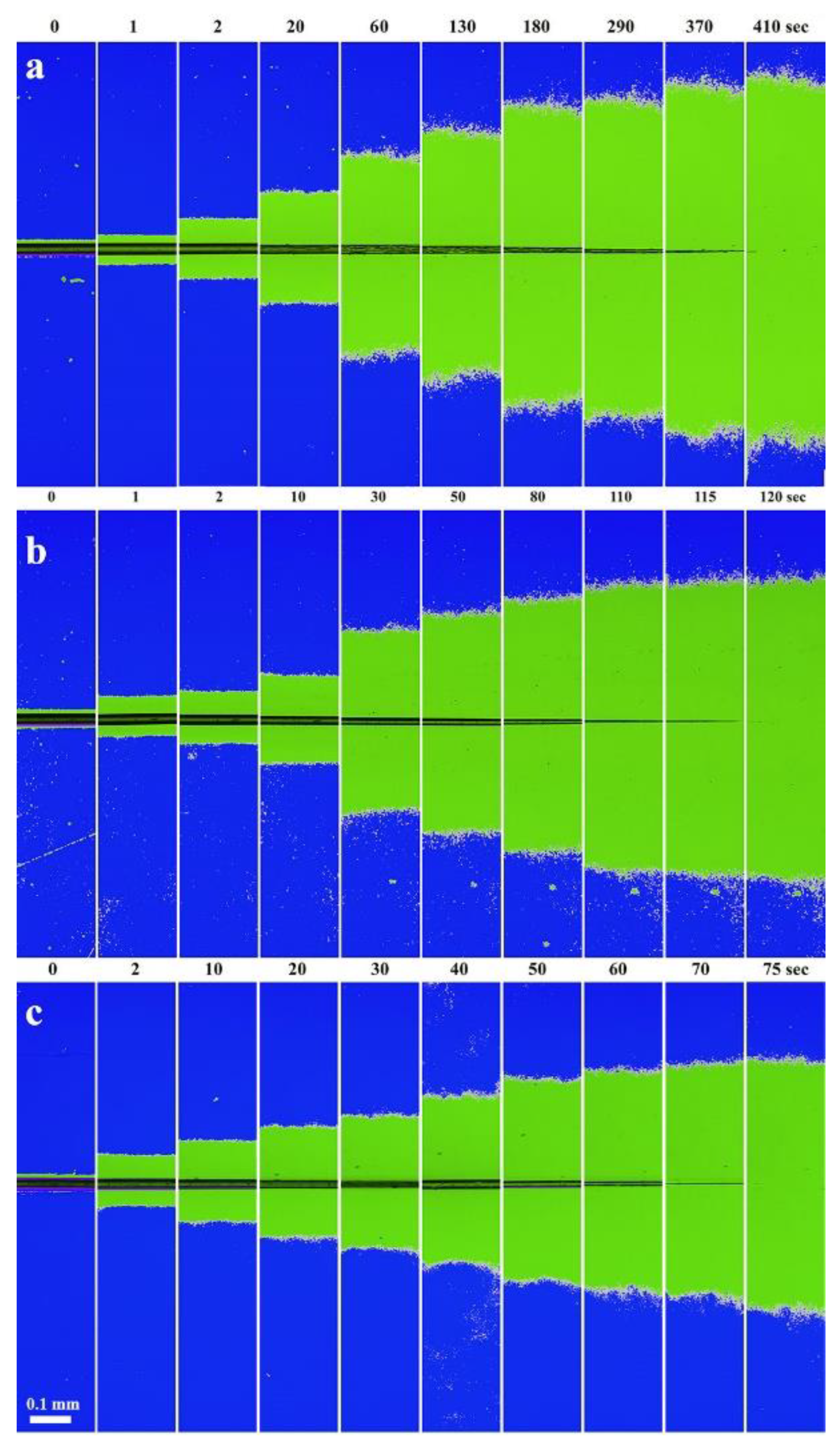
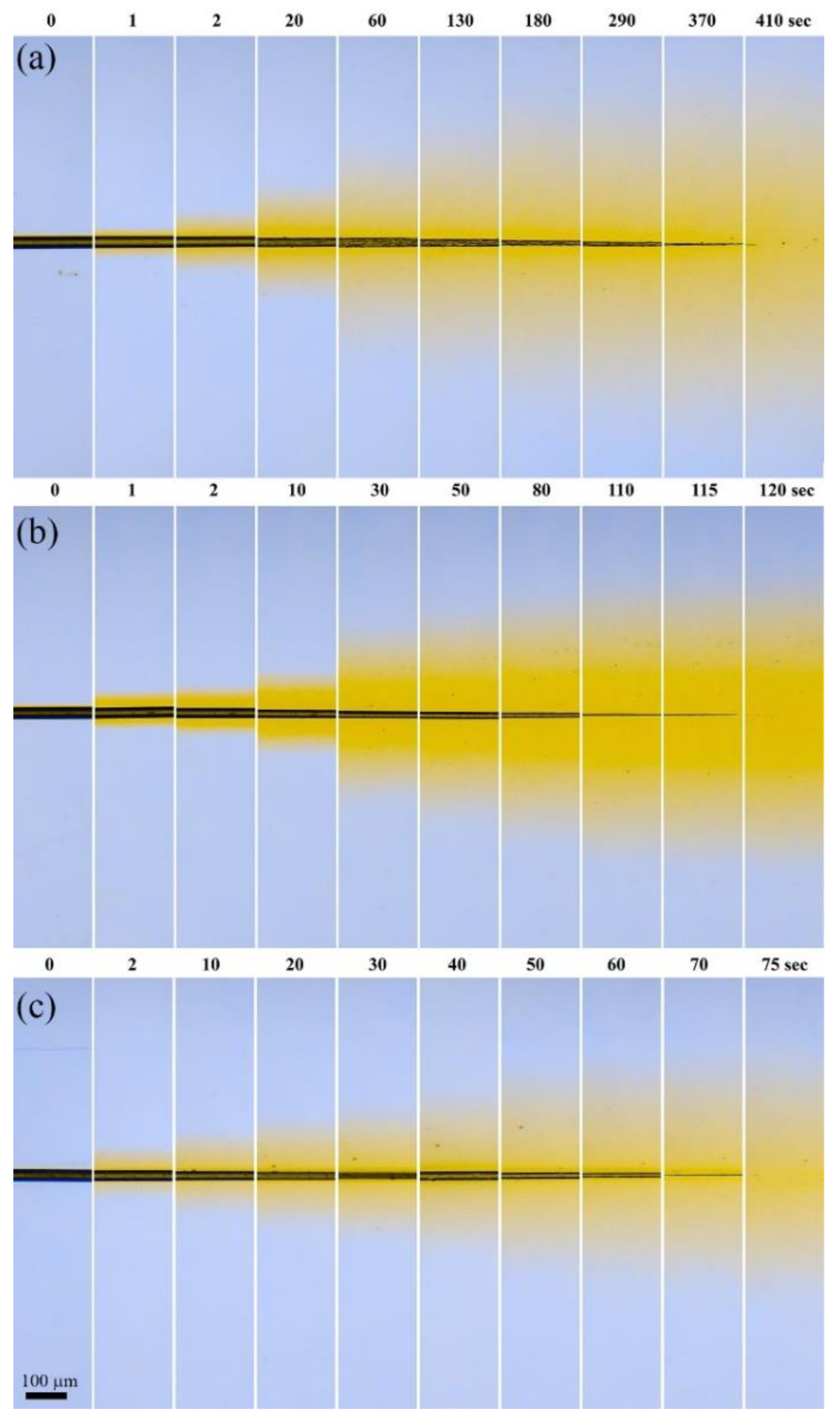
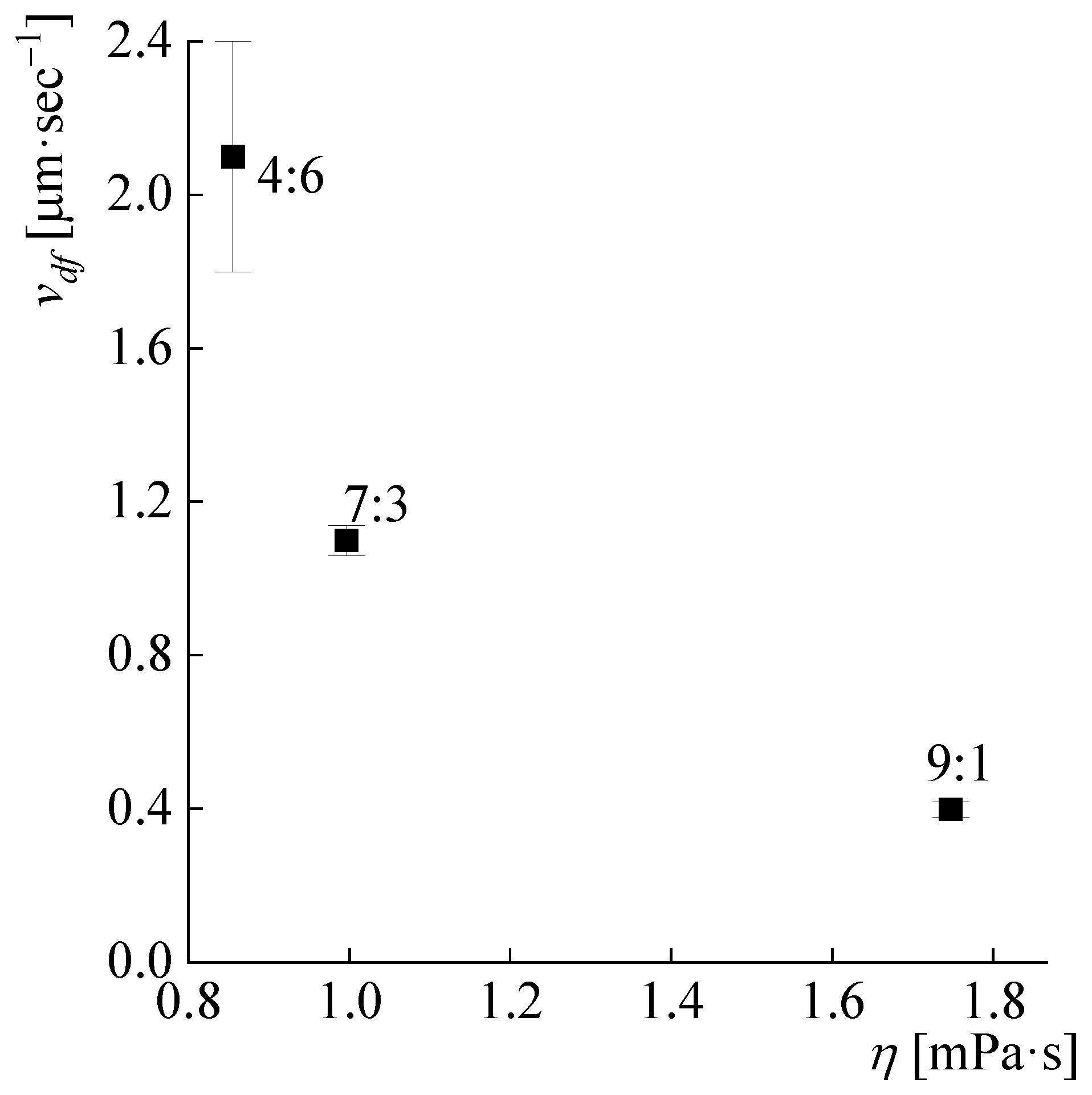
3.3. Polymer Dissolution Kinetics
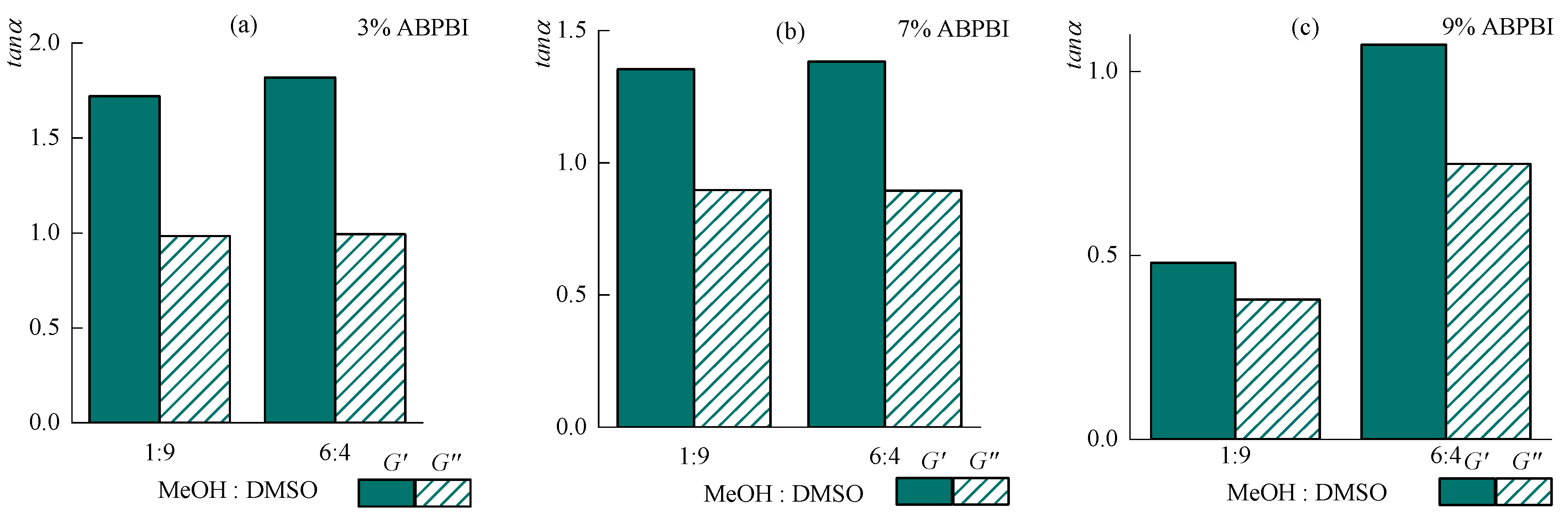
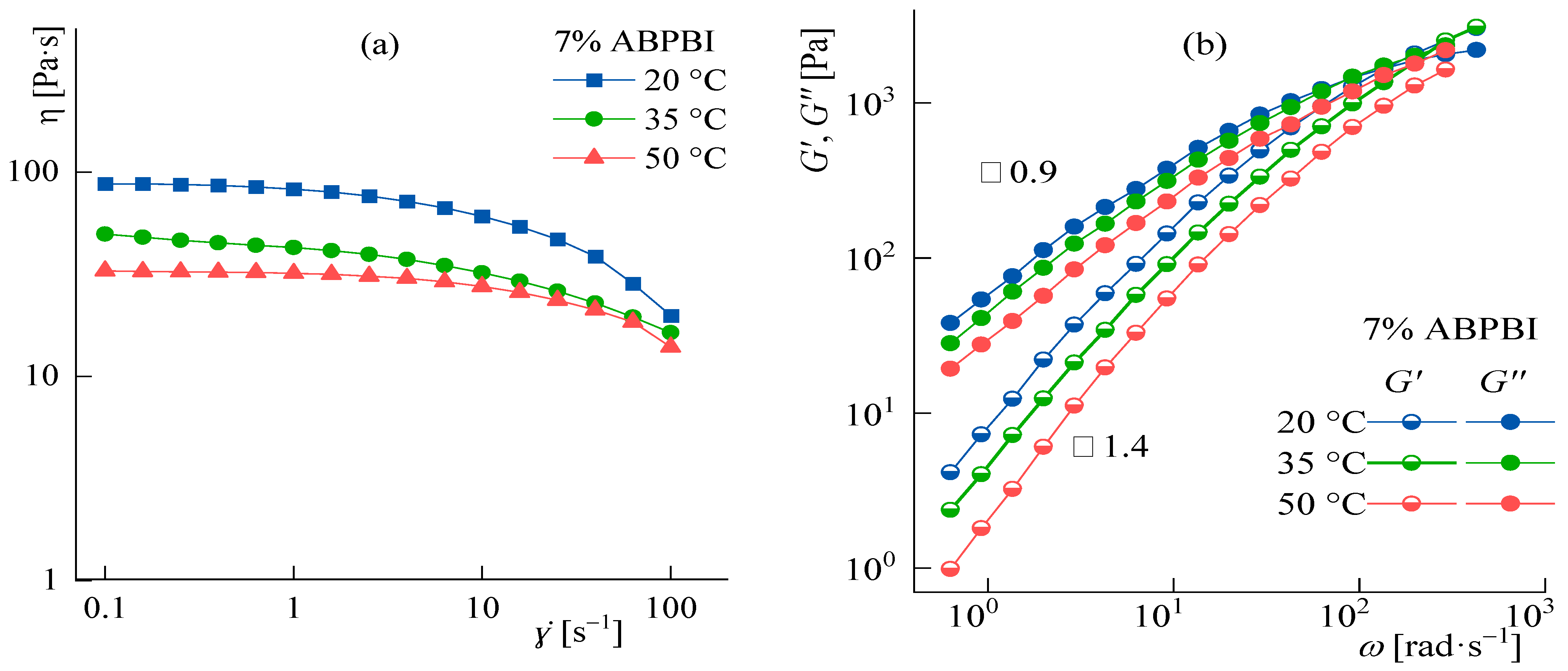
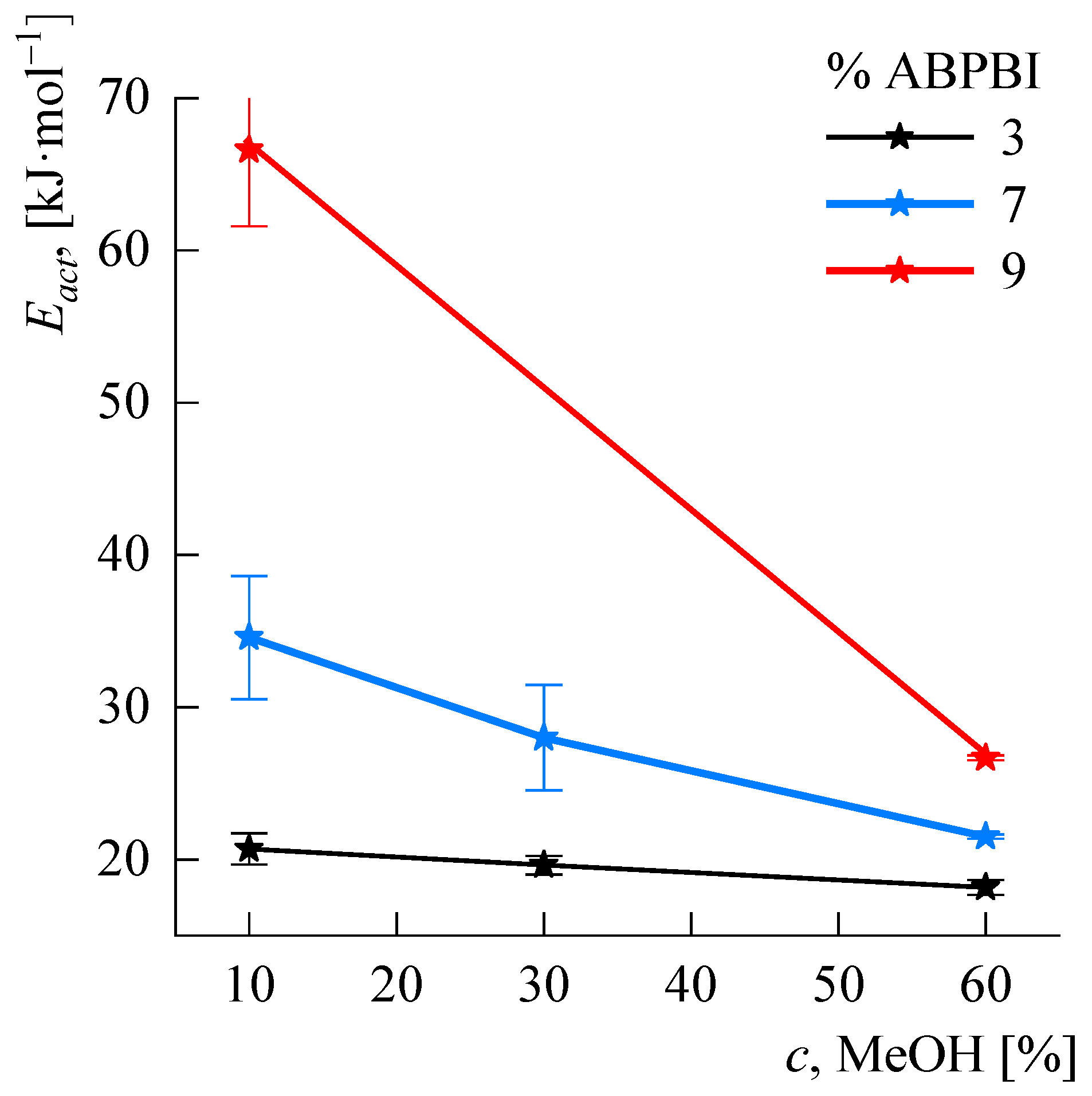
3.4. Rheology of Concentrated Solutions
Temperature Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Senchukova, A.S.; Lezov, A.A.; Ponomarev, I.I.; Tsvetkov, N.V. Characteristic and Conformational Parameters of Poly (2, 5 (6)-Benzimidazole) in a Ternary Organic Solvent. Nanobiotechnol. Rep. 2021, 16, 847–853. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Rybkin, Y.A.; Volkova, Y.A.; Razorenov, D.Y.; Skupov, K.M.; Ponomarev, I.I.; Senchukova, A.S.; Tsvetkov, N.V. New possibilities for the synthesis of high-molecular weight poly (2, 5 (6)-benzimidazole) and studies of its solutions in DMSO-based complex organic solvent. Russ. Chem. Bull. 2020, 69, 2320. [Google Scholar] [CrossRef]
- Sandor, R.B. PBI (Polybenzimidazole): Synthesis, properties and applications. High Perform. Polym. 1990, 2, 25–37. [Google Scholar] [CrossRef]
- Mader, J.; Xiao, L.; Schmidt, T.J.; Benicewicz, B.C. Polybenzimidazole/acid complexes as high-temperature membranes. In Advances in Polymer Science, Fuel Cell II; Scherer, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 216, pp. 63–124. [Google Scholar]
- Kulichikhin, V.G.; Skvortsov, I.Y.; Varfolomeeva, L.V. Compositions Based on PAN Solutions Containing Polydimethylsiloxane Additives: Morphology, Rheology, and Fiber Spinning. Polymers 2020, 12, 815. [Google Scholar] [CrossRef] [Green Version]
- Hwang, W.F.; Wiff, D.R.; Benner, C.L.; Helminiak, T.E. Composites on a molecular level: Phase relationships, processing, and properites. J. Macromol. Sci. Part B Phys. 1983, 22, 231–257. [Google Scholar] [CrossRef]
- Hwang, W.F.; Wiff, D.R.; Verschoore, C.; Price, G.E.; Helminiak, T.E.; Adams, W.W. Solution Processing and Properties of Molecular Composite Fibers and Films. Polym. Eng. Sci. 1983, 23, 784–788. [Google Scholar] [CrossRef]
- Nayak, R.; Sundarraman, M.; Ghosh, P.C.; Bhattacharyya, A.R. Doped poly (2,5-benzimidazole) membranes for high temperature polymer electrolyte fuel cell: Influence of various solvents during membrane casting on the fuel cell performance. Eur. Polym. J. 2018, 100, 111–120. [Google Scholar] [CrossRef]
- Berchtold, K.A.; Singh, R.P.; Young, J.S.; Dudeck, K.W. Polybenzimidazole composite membranes for high temperature synthesis gas separations. J. Membr. Sci. 2012, 415, 265–270. [Google Scholar] [CrossRef]
- Kalathil, A.; Raghavan, A.; Kandasubramanian, B. Polymer Fuel Cell Based on Polybenzimidazole Membrane: A Review. Polym.-Plast. Technol. Mater. 2019, 58, 465–498. [Google Scholar] [CrossRef]
- McNair, R.; Kumar, S.; Wonanke, A.D.; Addicoat, M.A.; Dryfe, R.A.; Szekely, G. Ionic covalent organic nanosheet (iCON)–quaternized polybenzimidazole nanocomposite anion-exchange membranes to enhance the performance of membrane capacitive deionization. Desalination 2022, 533, 115777. [Google Scholar] [CrossRef]
- Das, A.; Sana, B.; Bhattacharyya, R.; Chandra Ghosh, P.; Jana, T. Cross-Linked Alkaline Anion Exchange Membrane from N-Spirocyclic Quaternary Ammonium and Polybenzimidazole. ACS Appl. Polym. Mater. 2022, 4, 1523–1534. [Google Scholar] [CrossRef]
- Hu, J.; Hardian, R.; Gede, M.; Holtzl, T.; Szekely, G. Reversible crosslinking of polybenzimidazole-based organic solvent nanofiltration membranes using difunctional organic acids: Toward sustainable crosslinking approaches. J. Membr. Sci. 2022, 648, 120383. [Google Scholar] [CrossRef]
- Jin, Y.; Gao, B.; Bian, C.; Meng, X.; Meng, B.; Wong, S.I.; Yang, N.; Sunarso, J.; Tan, X.; Liu, S. Elevated-temperature H2 separation using a dense electron and proton mixed conducting polybenzimidazole-based membrane with 2D sulfonated graphene. Green Chem. 2021, 23, 3374–3385. [Google Scholar] [CrossRef]
- Kim, H.J.; Cho, Y.S.; An, S.J.; Eun, Y.C.; Kim, J.Y.; Yoon, H.K.; Kweon, H.J.; Yew, K.H. Synthesis of Poly(2,5-benzimidazole) for Use as a Fuel-Cell Membrane. Macromol. Rapid Commun. 2004, 25, 894–897. [Google Scholar] [CrossRef]
- Pingitore, A.T.; Molleo, M.; Schmidt, T.J.; Benicewicz, B.C. Polybenzimidazole Fuel Cell Technology: Theory, Performance, and Applications. In Fuel Cells and Hydrogen Production. Encyclopedia of Sustainability Science and Technology Series; Lipman, T., Weber, A., Eds.; Springer: New York, NY, USA, 2019; pp. 477–515. [Google Scholar]
- Eren, B.; Aydin, R.; Eren, E. Morphology and thermal characterization of montmorillonite/polybenzimidazole nanocomposite. J. Therm. Anal. Calorim. 2014, 115, 1525–1531. [Google Scholar] [CrossRef]
- Asensio, J.A.; Borrós, S.; Gómez-Romero, P.; Asensio, J.A.; Borrós, S.; Gómez-Romero, P. Proton-conducting membranes based on poly (2,5-benzimidazole)(ABPBI) and phosphoric acid prepared by direct acid casting. J. Membr. Sci. 2004, 241, 89–93. [Google Scholar] [CrossRef]
- Chaudhari, H.D.; Illathvalappil, R.; Kurungot, S.; Kharul, U.K. Preparation and investigations of ABPBI membrane for HT-PEMFC by immersion precipitation method. J. Membr. Sci. 2018, 564, 211–217. [Google Scholar] [CrossRef]
- Asensio, J.A.; Gómez-Romero, P. Recent Developments on Proton Conduc-ting Poly (2, 5-benzimidazole)(ABPBI) Membranes for High Temperature Polymer Electrolyte Membrane Fuel Cells. Fuel Cells 2005, 5, 336–343. [Google Scholar] [CrossRef]
- Luo, T.; Dreusicke, B.; Wessling, M. Tuning the ion selectivity of porous poly (2, 5-benzimidazole) membranes by phase separation for all vanadium redox flow batteries. J. Membr. Sci. 2018, 556, 164–177. [Google Scholar] [CrossRef]
- Nunes, R.W.; Martin, J.R.; Johnson, J.F. Influence of molecular weight and molecular weight distribution on mechanical properties of polymers. Polym. Eng. Sci. 1982, 22, 205–228. [Google Scholar] [CrossRef]
- Skvortsov, I.Y.; Kulichikhin, V.G.; Ponomarev, I.I.; Varfolomeeva, L.V.; Kuzin, M.S.; Razorenov, D.Y.; Skupov, K.M. Some Specifics of Defect-Free Poly-(o-aminophenylene) naphthoylenimide Fibers Preparation by Wet Spinning. Materials 2022, 15, 808. [Google Scholar] [CrossRef] [PubMed]
- Makarov, I.S.; Golova, L.K.; Vinogradov, M.I.; Levin, I.S.; Sorokin, S.E. Structure of Polyacrylonitrile Fibers Produced from N-Methylmorpholine-N-Oxide Solutions. Fibre Chem. 2019, 50, 508–513. [Google Scholar] [CrossRef]
- Skvortsov, I.Y.; Kalugina, A.D.; Litvinova, E.G.; Malkin, A.Y.; Khotimskiy, V.S.; Kulichikhin, V.G. Fibers spinning from poly (trimethylsilylpropyne) solutions. J. Appl. Polym. Sci. 2020, 137, 48511. [Google Scholar] [CrossRef]
- Wei, H.; Suo, X.; Lu, C.; Liu, Y. A comparison of coagulation and gelation on the structures and stabilization behaviors of polyacrylonitrile fibers. J. Appl. Polym. Sci. 2020, 137, 48671. [Google Scholar] [CrossRef]
- ASTM D2857-16; Standard Practice for Dilute Solution Viscosity of Polymers. ASTM International: West Conshohocken, PA, USA, 2016.
- Trofimov, B.A.; Amosova, S.V.; Mikhaleva, A.I.; Gusarova, N.K.; Vyalykh, Y.P. Fundamentalʹnyye Issledovaniya; Khimicheskiye nauki: Nauka, Novosibirsk, 1977; pp. 174–178. (In Russian) [Google Scholar]
- Vitkovskaya, N.M.; Larionova, E.Y.; Kaempf, N.V.; Kobychev, V.B.; Trofimov, B.A. Methanol interaction with potassium and rubidium hydroxides in dimethyl sulfoxide. J. Struct. Chem. 2011, 52, 652–658. [Google Scholar] [CrossRef]
- Huggins, M.L. Methods for the estimation of molecularweights of high polymersfrom data on properties of the irdilute solutions are critically discussed. Specificrecommendations are made regarding future procedure. J. Am. Chem. Soc. 1942, 64, 2716. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Semakov, A.V.; Skvortsov, I.Y.; Zatonskikh, P.V.; Kulichikhin, V.G.; Subbotin, A.V.; Semenov, A.N. Spinnability of dilute polymer solutions. Macromolecules 2017, 50, 8231–8244. [Google Scholar] [CrossRef]
- Clasen, C.; Kulicke, W.M. Determination of viscoelastic and rheo-optical material functions of water-soluble cellulose derivatives. Prog. Polym. Sci. 2001, 26, 1839–1919. [Google Scholar] [CrossRef]
- Ebagninin, K.W.; Benchabane, A.; Bekkour, K. Rheological characterization of poly (ethylene oxide) solutions of different molecular weights. J. Colloid Interface Sci. 2009, 336, 360–367. [Google Scholar] [CrossRef]
- Skvortsov, I.Y.; Kulichikhin, V.G.; Ponomarev, I.I.; Varfolomeeva, L.A.; Kuzin, M.S.; Skupov, K.M.; Volkova, Y.A.; Razorenov, D.Y.; Serenko, O.A. Solubility, Rheology, and Coagulation Kinetics of Poly-(O-Aminophenylene)Naphthoylenimide Solutions. Polymers 2020, 12, 2454–2472. [Google Scholar] [CrossRef]
- Makarova, V.V.; Kulichikhin, V.G. Interferometry. Research and Applications. In Science and Technology; Padron., I., Ed.; InTech: Rijeka, Croatia, 2012; Chapter 20; pp. 395–436. [Google Scholar]
- Palchikova, E.E.; Makarov, I.S.; Vinogradov, M.I.; Golova, L.K.; Shambilova, G.K.; Kulichikhin, V.G. Kinetics of Dissolution of Polyacrylonitrile in N-Methylmorpholine-N-oxide in the Absence and Presence of Cellulose. Polym. Sci. Ser. B 2021, 63, 833–842. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Askadsky, A.; Chalykh, K.V.; Kovriga, V. Experimental Methods of Polymer Physics; Mir-Prentice-Hall: Englewood Cliffs, NY, USA, 1983; pp. 260–288. [Google Scholar]
- Skvortsov, I.Y.; Malkin, A.Y.; Kuzin, M.S.; Bondarenko, G.N.; Gerasimenko, P.S.; Litmanovich, E.A. Rheology and molecular interactions in polyacrylonitrile solutions: Role of a solvent. J. Mol. Liq. 2022, 364, 119938–119954. [Google Scholar] [CrossRef]


| Solution Abbreviation | A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | A9 | A10 |
|---|---|---|---|---|---|---|---|---|---|---|
| c ABPBI, % | 3 | 3 | 3 | 3 | 7 | 7 | 7 | 7 | 9 | 9 |
| DMSO:MeOH | 9:1 | 7:3 | 4:6 | 3:7 | 9:1 | 7:3 | 4:6 | 3:7 | 9:1 | 4:6 |
| c KOH, % | 1.5 | 1.5 | 1.5 | 1.5 | 3.5 | 3.5 | 3.5 | 3.5 | 4.5 | 4.5 |
| DMSO:MeOH Ratio | Complete Dissolution Time, s | Diffusion Front Rate, µm∙s−1 | Fiber Thinning Rate, µm∙s−1 | ||
|---|---|---|---|---|---|
| Before the Bifurcation Point (I) | After the Bifurcation Point (III) | Before the Fiber Swelling (I) | After the Fiber Swelling (III) | ||
| 9:1 | 410 | 3.1 ± 0.3 | 0.4 ± 0.02 | 0.29 ± 0.08 | 0.06 ± 0.001 |
| 7:3 | 120 | 1.1 ± 0.04 | 0.55 ± 0.11 | 0.18 ± 0.02 | |
| 4:6 | 75 | 2.1 ± 0.3 | 0.44 ± 0.04 | 0.61 ± 0.04 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skvortsov, I.Y.; Varfolomeeva, L.A.; Ponomarev, I.I.; Skupov, K.M.; Maklakova, A.A.; Kulichikhin, V.G. High Molecular Weight AB-Polybenzimidazole and Its Solutions in a Complex Organic Solvent: Dissolution Kinetics and Rheology. Polymers 2022, 14, 4648. https://doi.org/10.3390/polym14214648
Skvortsov IY, Varfolomeeva LA, Ponomarev II, Skupov KM, Maklakova AA, Kulichikhin VG. High Molecular Weight AB-Polybenzimidazole and Its Solutions in a Complex Organic Solvent: Dissolution Kinetics and Rheology. Polymers. 2022; 14(21):4648. https://doi.org/10.3390/polym14214648
Chicago/Turabian StyleSkvortsov, Ivan Y., Lydia A. Varfolomeeva, Igor I. Ponomarev, Kirill M. Skupov, Aleksandra A. Maklakova, and Valery G. Kulichikhin. 2022. "High Molecular Weight AB-Polybenzimidazole and Its Solutions in a Complex Organic Solvent: Dissolution Kinetics and Rheology" Polymers 14, no. 21: 4648. https://doi.org/10.3390/polym14214648