Bacterial Response to the Surface Aging of PLA Matrices Loaded with Active Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Reagents
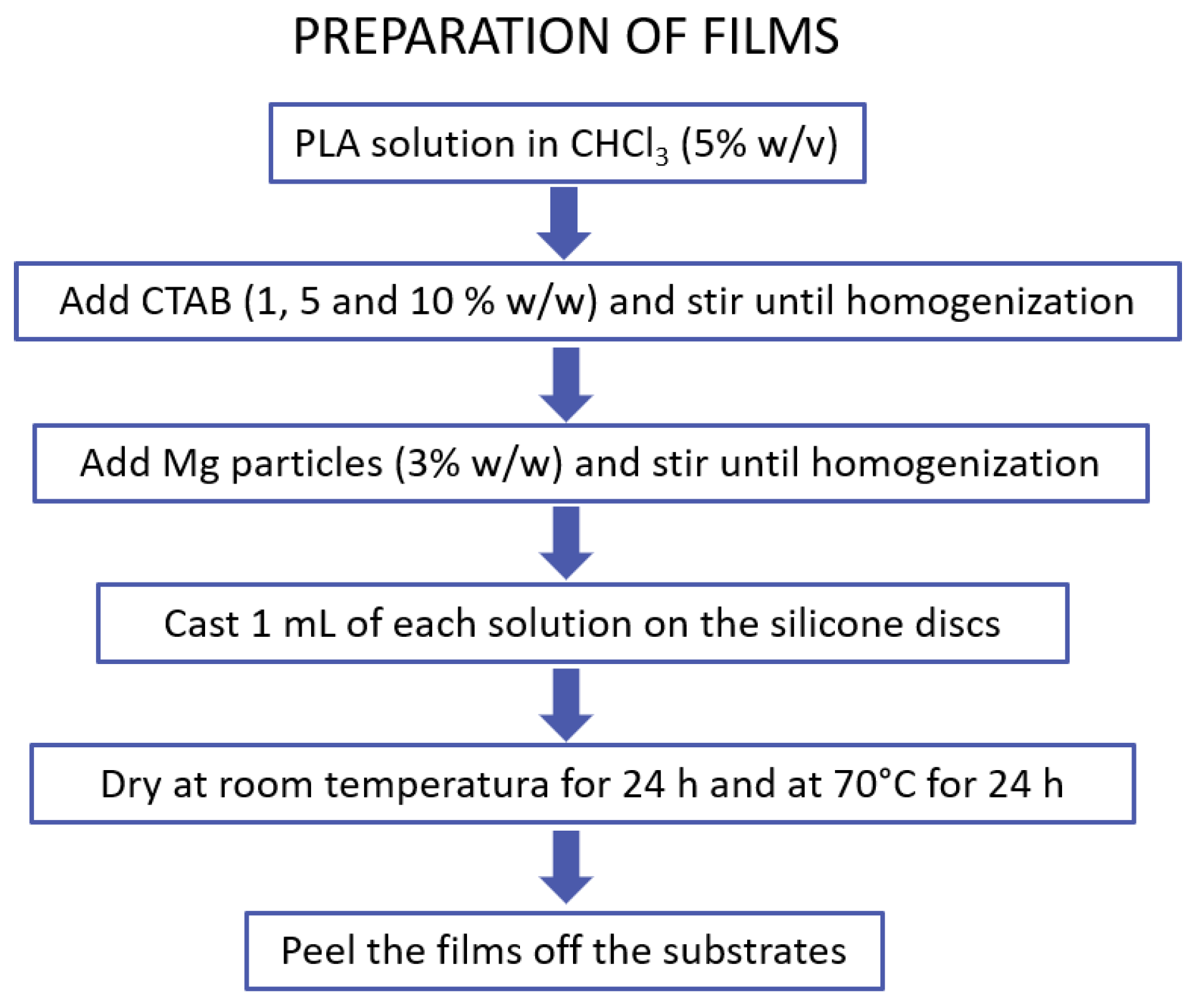
2.2. Preparation of Films
2.3. In Vitro Degradation
2.4. Contact Angle Measurements and Determination of Surface Tension
2.5. Surface Chemical Composition
2.6. Surface Topography Characterization
2.7. Bacterial Strains and Culture
2.7.1. Adhesion Experiments
2.7.2. Biofilms Formation
2.8. Static Analysis
3. Results
3.1. Films’ Surface Characterization
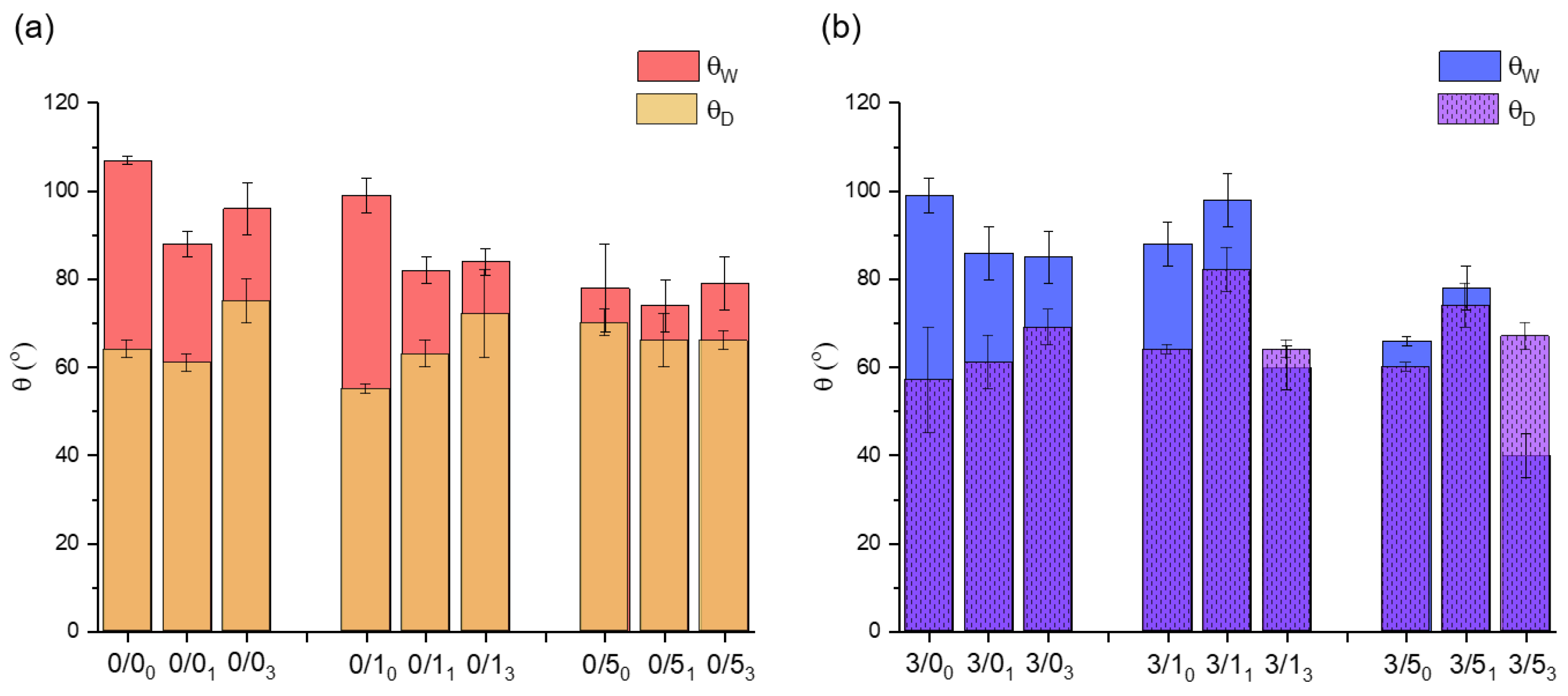
3.1.1. Contact Angle Measurements
- Magnesium effect
- In most cases, the addition of 3% (w/w) magnesium particles to PLA films results in a decrease of θW (Figure 2). Nevertheless, the samples degraded for 1 month do not follow the described trend (3/11 and 3/51). On the other hand, no clear tendency can be deduced with respect to θD, as in some cases, the value decreases with the addition of magnesium (0/0 vs. 3/0, whatever the degradation time and 0/50 vs. 3/50). In others cases, it increases slightly (0/1 vs. 3/1, except 0/13 vs. 3/13). Only for the case of samples degraded for 1 month is there a considerable increase in the value of the diiodomethane contact angle with Mg addition.
- CTAB effect
- With non-degraded films, regardless of whether the films contained magnesium particles or not, doping the films with CTAB results in a decrease in θW, with the films being more hydrophilic the higher the CTAB content (Figure 2). Thus, there was a decrease of about 29° and about 33° in θW with respect to the control for films doped with 5% (w/w) CTAB, without and with magnesium, respectively: 0/50 vs. 0/00 and 3/50 vs. 3/00. The opposite trend was observed for θD values. In these cases, there was a slight increase with respect to the controls (0/0 and 3/0) as the CTAB content in the films increased (0/50 vs. 0/00 and 3/50 vs. 3/00), with the exception of the 0/10 film, where θD decreased slightly.
- In the case of degraded films, the addition of CTAB also made the surfaces less hydrophobic on average, especially in films with 5% (w/w) CTAB.
- Degradation effect
- For magnesium-free films (Figure 2a), degradation of films immersed in PBS for 1 and 3 months resulted in a decrease of θW, compared to non-degraded films, in the cases of zero or a low amount of CTAB, i.e., 0/0 and 0/1. Moreover, this decrease in hydrophobicity was much more evident in the first 4 weeks than in the remaining 8 weeks. In the case of 0/5, the hydrophobicity of the films was similar before and after degradation. In addition, after 3 months degradation (0/03, 0/13 and 0/53), the average θW seemed to increase slightly with respect to the degraded films for 1 month.
- When magnesium was present in the films (Figure 2b), θW also decreased for samples degraded for 1 and 3 months, except for samples degraded for 1 month and containing 1% and 5% (w/w) CTAB. In this sense, the θW for the 3/11 sample was higher than for the 3/10 sample, and was also much higher than for the rest of the samples degraded for 1 month with different amounts of CTAB (3/01 and 3/51).
- In relation to the contact angle values of diiodomethane, degradation caused different trends depending on the presence or absence of magnesium and surfactant. In the samples without CTAB, 0/0 and 3/0, degradation after 1 month did not cause significant changes in θD, but it did after 3 months, at which time the θD increased significantly with respect to the non-degraded samples.
- In the samples without Mg and with small amounts of CTAB (0/1, Figure 2a), the average value of θD seemed to rise with the degradation time, while when more CTAB was added (0/5), the values of the angles were very similar with time, as was the case with θW.
- In the samples with Mg (Figure 2b), θD, irrespective of the amount of CTAB, increased after 1 month of degradation, relative to the non-degraded samples, and equaled the original θD value after 3 months of degradation.
3.1.2. Surface Tension
- Magnesium effect
- Regardless of the sample selected, the total surface tension of the films was modulated by γd and γnd values. In the cases without Mg, the sum of both components gave γs values very similar to each other, taking into account experimental uncertainty. For the cases with Mg, there was more variability in γs. The lowest value of 3/11 and the highest value of 3/53 are noteworthy, and in turn correspond to the highest value of θD and the lowest value of θW, respectively.
- There were noticeable differences in the non-dispersive component, γnd. In the case of non-degraded samples, magnesium-containing samples without or with small amounts of CTAB (3/00 and 3/10) had a low, but non-zero γnd value, which infers a certain polarity to the surface, while those non-containing magnesium (0/00 and 0/10) were non-polar (γnd = 0 mJ/m2).
- Table 1 also shows the free energy of interaction between two surfaces (ΔGSWS), which provides the degree of hydrophobicity of a surface when immersed in water. Taking this parameter into account, a surface is considered hydrophobic when ΔGSWS < 0 mJ/m2 and hydrophilic when ΔGSWS > 0 mJ/m2 [45]. Thus, the more negative this value is, the more hydrophobic the material. In general, the 0/YZ films have lower ΔGSWS values than the 3/YZ films and therefore interaction with water is not favored in them, with the exception of 0/11 vs. 3/11.
- CTAB effect
- The addition of 1% w/w CTAB did not produce significant differences in most of the parameters presented in Table 1, bearing in mind the results uncertainty. An exception to this is the non-dispersive component, which increased in value when 1% w/w CTAB was added to PLA films, without Mg. Another exception occurred for sample 3/11 where the decrease in the dispersive surface tension component provoked the described decrease in γs and the increase in ΔGSWS.
- However, in most of the cases, and irrespective of the starting value, an important increase of the non-dispersive component was observed when 5% w/w CTAB was added, and this was especially noticeable in magnesium-containing samples.
- As described above, all the films examined had a hydrophobic character (ΔGSWS < 0 mJ/m2), but an appreciable decrease was noted as the amount of CTAB increased, especially when comparing 5% w/w CTAB with samples without CTAB.
- Degradation effect
- After degradation, higher values of the non-dispersive component seemed to be appreciated, although it is difficult to establish a time dependence. This was especially noticeable for the 3/Y3 (where Y is 0, 1 or 5) samples, whose value of the non-dispersive component is four times higher on average than for the non-degraded samples. These changes are reflected in the total surface tension of the solid, which, if taking into account the uncertainties associated with each value (as mentioned above), is generally higher for magnesium-containing films than for non-containing magnesium films, especially for the samples with the highest amount of CTAB.
- On comparing magnesium-containing samples before and after 3 months of degradation, the polarity of the samples increased by 350% for 3/13 and 153% for 3/53.
- Regarding ΔGSWS, a decrease in hydrophobic character was also observed after the samples were subjected to degradation, in particular for magnesium-containing samples. Again, it is worth noting the case of 3/53 with the lowest hydrophobicity found, according to its maximum surface energy (γs = 58 ± 6 mJ/m2).
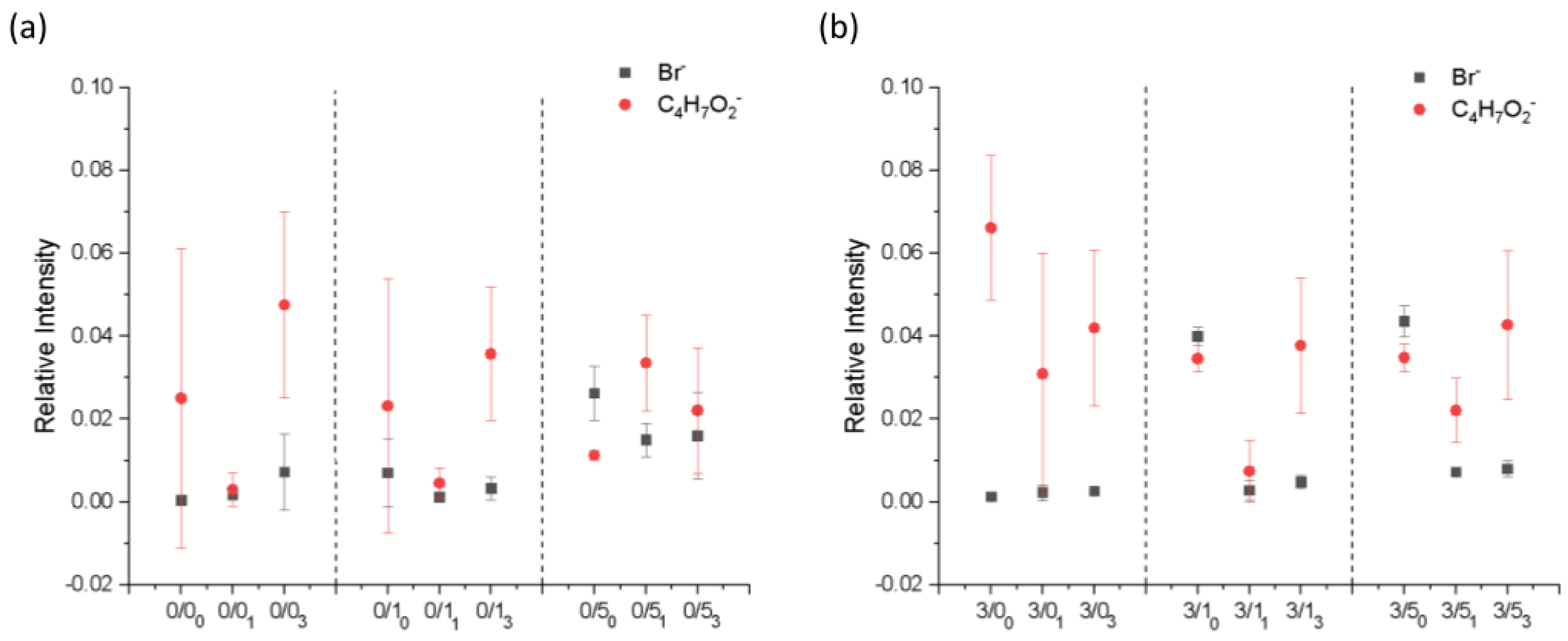
3.1.3. Compositional Changes after Film Degradation
- Magnesium effect
- In the case of non-degraded samples [34], the presence of Mg in PLA films made CTAB more significantly detectable on the surface of the samples, the change being more evident for lower concentrations of CTAB. The presence of magnesium in the films of PLA seemed to reduce the amount of surfactant on the surface of the degraded samples. This was more evident in samples with a higher amount of CTAB (0/5 vs. 3/5).
- CTAB effect
- As was the case for the non-degraded samples, in general, the bromide ion was barely detectable on the surface of films prepared with low concentrations of CTAB, and when the surfactant concentration increased, the presence of the surfactant became noticeable on the surface.
- Degradation effect
- Based on the results, after degradation, the amount of CTAB on the surface significantly decreased, regardless of whether the sample had been 1 month or 3 months under degradation.
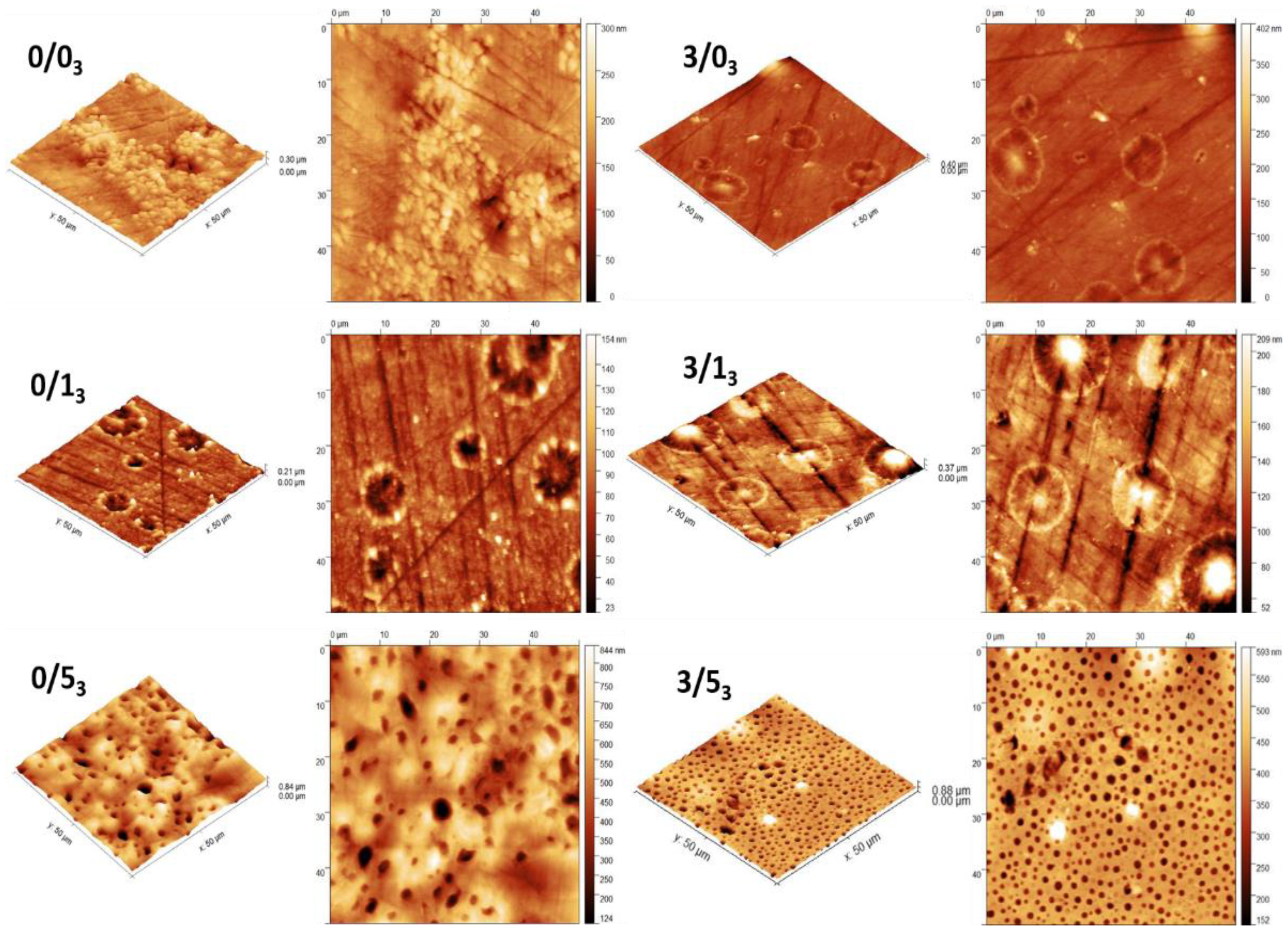
3.1.4. Topography of PLA Films
- Magnesium effect
- We observed in the previous work [41] that the topography of films fabricated on silicone was not affected by the single addition of Mg, that it was affected when this Mg was combined with CTAB, or the films were subjected to degradation. This observation was also evidenced when comparing 0/53 and 3/53 where a more uniform hole-like distribution appeared in the presence of Mg. In the cases of 0/13 vs. 3/13, images were similar with no change associated to Mg.
- CTAB effect
- Samples without CTAB did not present holes on their surface, but the addition of 1% w/w CTAB provoked the appearance of small holes, without or with Mg, as described in the previous publication [41]. Moreover, the size of these microstructures increased with the CTAB content in the range of 1 to 5 µm [41]. In the cases under study (Figure 4), holes were only present in 0/53 and 3/53.
- Degradation effect
- Figure 4 shows the presence of holes on the surface of the films with the highest amount of CTAB after 3 months degradation (0/53 and 3/53) without or with Mg. However, for non-containing CTAB films (0/03 and 3/03) or with 1% (w/w) CTAB (0/13 and 3/13), circular-like deposited clusters appeared on the surface.
- Previous work [41] has shown that for non-containing-magnesium samples, the degradation after 1 month did not significantly affect the holes formed on the surface of the films when the amount of CTAB was higher than 5% (0/5), but in the case of samples with a lower amount of CTAB (0/1), these holes disappeared. Conversely, the presence of magnesium in the films degraded for 1 month seemed to affect the topography in the case of the high CTAB-content samples: some structures became wider and deeper than in the case of non-degraded film. In the case under study, after 3 months of degradation (Figure 4), the behavior was similar to 1 month: the surface of the films with low amounts of CTAB (0/13 and 3/13) did not show any holes after degradation, regardless of whether the films contained magnesium or not. For the samples with the highest amount of CTAB (0/5 and 3/5), those holes observed on the surface at 1 month degradation were still observed after 3 months of degradation, with the particularity that magnesium made the distribution of the structures more uniform, as we have already mentioned.
- Comparing these images with those already analyzed (0/53 vs. 0/50) [41], in the non-containing-magnesium samples with the highest amount of CTAB and degraded for 3 months (0/53), there was a considerable increase in the width and depth of these holes, becoming, in the most extreme cases, 170% wider and 250% deeper on average (holes were on average 5 ± 0.6 μm wide and 500 ± 30 nm deep). On the other hand, for samples containing magnesium and the highest amount of CTAB (3/53), there was no difference in the width and depth of the holes after 3 months of degradation compared to 1 month (holes were on average 1.9 ± 0.2 μm wide and 183 ± 40 nm deep).
3.2. Bacterial Adhesion and Viability of Adhered Bacteria
- Magnesium effect
- The single addition of Mg to PLA films showed no significant change (p-value > 0.05) in bacterial adhesion (comparisons between 0/00 and 3/00). However, when this addition was made in the presence of CTAB, the number of adhered bacteria decreased significantly (p-value < 0.05), regardless of the amount of CTAB: comparisons between 0/10 and 3/10 (from 51 ± 2 × 104 to 21 ± 1 × 104 n.° bacteria/cm2) and 0/50 and 3/50 (from 70 ± 6 × 104 to 26 ± 2 × 104 n.° bacteria/cm2). Additionally, it is important to point out that bacteria adhered to 0/10, 3/10, 0/50 and 3/50 were damaged or dead due to the red-like color present after staining.
- When Mg was present in degraded samples, it was not possible to describe a general behavior. After 1 month, Mg addition caused a significantly increase (p < 0.05) in the number of adhered bacteria (0/01 vs. 3/01 and 0/11 vs. 3/11), except for those with 5% (w/w) CTAB, where there was a significant reduction (p < 0.05). In the case of degradation for 3 months, the adhesion was maintained, without and with Mg, within the experimental error (0/03 vs. 3/03 and 0/13 vs. 3/13), the behavior of the samples again being different with 5% (w/w) CTAB, which generated significantly less p < 0.05) adhesion with degradation.
- CTAB effect
- The presence of 1% (w/w) CTAB in the samples without and with Mg caused a significant decrease (p < 0.05) in bacterial adhesion: between 0/00 and 0/10 (from 72 ± 7 × 104 to 50 ± 2 × 104 bacteria/cm2) and between 3/00 and 3/10 (from 68 ± 6 × 104 to 21 ± 1 × 104 bacteria/cm2). In addition, as mentioned before, with these amounts of CTAB, the films always presented bactericidal activity in the adhered microorganisms.
- When films had high concentrations of CTAB (0/50), the number of adhered bacteria was statistically equal to the “control” (0/00) in the cases without Mg, but the bactericidal surface activity was, again, only associated to CTAB presence. If magnesium was present, in addition to the bactericidal effect associated with CTAB, adhesion was significantly reduced (p < 0.05) (compare 3/00 with 3/50: from 68 ± 6 × 104 to 26 ± 2 × 104 bacteria/cm2).
- Another comparison was made within samples with both amounts of CTAB. Figure 4 shows that in the cases without Mg (0/10 and 0/50), the higher the CTAB concentration, the greater the adhesion (p < 0.05). In the cases with Mg (3/10 and 3/50), the average adhesion values were very similar, although adhesion also seemed to increase with concentration. However, what is most noticeable in these compared cases is the bactericidal power of the surface.
- In the cases of degraded samples, the addition of 5% (w/w) surfactant always caused a significant increase (p < 0.05) in adhesion without Mg (0/01 vs. 0/51 and 0/03 vs. 0/53), whereas when Mg was present, it significantly decreased (p < 0.05) adhesion (3/01 vs. 3/51 and 3/03 vs. 3/53). In the case of 1% (w/w) CTAB, the coverage increased significantly p < 0.05, decreased or remained constant depending on the film additive and degradation time.
- Degradation effect
- Overall, degradation alters the bacterial surface coating, however, it is not possible to describe a generalized trend for all the systems studied. This means that the presence of magnesium and/or the presence of different amounts of CTAB are able to modulate bacterial adhesion and viability in different ways with time.
- Figure 6 shows the comparison with the non-degraded films, and that after 1 month degradation, the bacterial adhesion was significantly decreased (p < 0.05) in one case 0/01 (41 ± 3 × 104 bacteria/cm2), remained unchanged in 0/11 and 0/51 and increased (p-value < 0.05) in all Mg-containing films, 3/01 (111 ± 4 × 104 bacteria/cm2), 3/11 (66 ± 1 × 104 bacteria/cm2) and 3/51 (39 ± 3 × 104 bacteria/cm2). This degradation time was also crucial for the bactericidal effect initially shown in the 0/10, 0/50 and 3/10 systems to disappear. The bactericidal capacity of the surfaces was only maintained after 1 month of degradation time for films with 5% (w/w) CTAB, i.e., 3/51.
- After 3 months degradation, compared with non-degraded films, bacterial adhesion also decreased in the system 0/03 (from 53 ± 2 × 104 bacteria/cm2 to 72 ± 7 × 104 bacteria/cm2), while in the case 0/13, it increased (68 ± 5 × 104 bacteria/cm2), but not significantly (p-value > 0.05). The film 0/53 slightly decreased significantly (p < 0.05) the number of adhered bacteria, around 10 fewer bacteria/cm2. In the magnesium cases, for 3/03, despite the large increase after 1 month of degradation, after 3 months of degradation, the bacterial coverage was similar to 3/00. A different behavior was seen for 3/1 and 3/5, where 3/13 and 3/53 films had significantly (p-value < 0.05) more bacteria coverage than their non-degraded systems. Again, the bactericidal capacity of the 3/5 film surface even after 3 months degradation is noteworthy.
- In samples without Mg, there was an increase in bacterial coverage on the surface in 0/0 and 0/1 samples related to the significant increase (p < 0.05) in degradation time (0/01 vs. 0/03 and 0/11 vs. 0/13). However, in 0/5 films, degradation generated the opposite effect, where less degradation was associated with significantly higher (p < 0.05) bacterial adhesion (0/51 vs. 0/53). In the cases with Mg, for 3/1 and 3/5 films, a similar coverage was found after 1 and 3 months of degradation. In the case of sample 3/0, the longer the degradation time, the lower the bacterial adhesion.
3.3. Biofilms Formation
- Magnesium effect:
- The single addition of magnesium (without any CTAB) to the PLA films resulted in a decrease of biofilm on their surface when samples were not degraded (0/00 vs. 3/00). In the case of degraded samples, Mg made the biofilm significantly increase (p < 0.05), especially after 1 month degradation (0/01 vs. 3/01).
- In the case of the addition of magnesium together with CTAB (3/10 or 3/50), Figure 6 shows that no viable biofilm was found.
- Biofilm creation in degraded samples exhibited different behavior depending on the presence of Mg in the sample. In the samples containing 1% (w/w) CTAB, when films suffered degradation, Mg significantly decreased (p < 0.05) biofilm after 1 month (0/11 vs. 3/11) and it practically remained constant after 3 months (0/13 vs. 3/13). In the samples with 5% (w/w) CTAB, Mg presence was not relevant since biofilm was suppressed at this surfactant concentration.
- CTAB effect
- As already mentioned, the presence of CTAB in non-degraded films, whatever the amount, did not generate viable biofilm. This bactericidal effect was always maintained in 5% (w/w) regardless of further degradation undergone.
- In samples degraded for 1 month, the addition of 1% (w/w) CTAB caused a significant reduction (p < 0.05) in biofilm formation only in the presence of Mg: from 243 ± 30% RLU (3/01) to 52 ± 14% RLU (3/11).
- Degradation effect
- Degradation time did not affect biofilm formation in the same way for the different samples. A general observation is that degradation leads to an increase in biofilm formation, although this absolute increase is not a function of degradation time. Samples with 5% (w/w) CTAB, either with or without Mg, did not participate in any degradation trend as they did not form biofilm on their surface. Focusing on the samples with 1% (w/w) CTAB, it was found that all the samples showed a significant increase (p < 0.05) in biofilm formation with increased degradation time, especially with Mg doping. Likewise, samples containing neither CTAB nor Mg exhibited the same affinity for biofilm formation at both degradation times. In contrast, in the films 3/0, the maximum biofilm formation occurred after one month of degradation. In particular, the evolution was from 71 ± 8% RLU to 246 ± 30% RLU after 1 month. These changes were significantly larger for both sample 3/00 and sample 3/01. Specifically, samples subjected to a degradation of 3 months generated a similar biofilm coating on their surfaces, within the experimental error, although the highest average value was for 3/13.
- Comparisons between both degradation times show that degradation processes did not affect the biofilms obtained in the samples without magnesium (0/01 vs. 0/03 and 0/11 vs. 0/13). However, a significant decrease (p < 0.05) in biofilm was observed in 3/0 with increased degradation time (about 70 units), and a significant increase (p < 0.05) in biofilm was observed in 3/1 with increased degradation time (about 150 units).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pahlevanzadeh, F.; Setayeshmehr, M.; Bakhsheshi-Rad, H.R.; Emadi, R.; Kharaziha, M.; Poursamar, S.A.; Ismail, A.F.; Sharif, S.; Chen, X.; Berto, F. A Review on Antibacterial Biomaterials in Biomedical Applications: From Materials Perspective to Bioinks Design. Polymers 2022, 14, 2238. [Google Scholar] [CrossRef]
- Cheeseman, S.; Christofferson, A.J.; Kariuki, R.; Cozzolino, D.; Daeneke, T.; Crawford, R.J.; Truong, V.K.; Chapman, J.; Elbourne, A. Antimicrobial Metal Nanomaterials: From Passive to Stimuli-Activated Applications. Adv. Sci. 2020, 7, 1902913. [Google Scholar] [CrossRef] [Green Version]
- Mah, T.-F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef] [Green Version]
- Bazaka, K.; Jacob, M.; Crawford, R.J.; Ivanova, E.P. Efficient surface modification of biomaterial to prevent biofilm formation and the attachment of microorganisms. Appl. Microbiol. Biotechnol. 2012, 95, 299–311. [Google Scholar] [CrossRef]
- Lopes, N.; Freitas, A.; Ramos, H.; Vasconcelos, C. S. epidermidis Isolates from a Tertiary Care Portuguese Hospital Show Very High Antibiotic Non-Susceptible Rates and Significant Ability to Form Biofilms. Appl. Microbiol. 2021, 1, 150–161. [Google Scholar] [CrossRef]
- Carcione, D.; Leccese, G.; Conte, G.; Rossi, E.; Intra, J.; Bonomi, A.; Sabella, S.; Moreo, M.; Landini, P.; Brilli, M.; et al. Lack of Direct Correlation between Biofilm Formation and Antimicrobial Resistance in Clinical Staphylococcus epidermidis Isolates from an Italian Hospital. Microorganisms 2022, 10, 1163. [Google Scholar] [CrossRef]
- Donlan, R.M. Special Issue Characteristics of Biofilms on Indwelling Medical Devices. Emerg. Infect. Dis. 2001, 7, 277–281. [Google Scholar] [CrossRef]
- Falde, E.J.; Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W. Superhydrophobic materials for biomedical applications. Biomaterials 2016, 104, 87–103. [Google Scholar] [CrossRef]
- Luque-Agudo, V.; Romero-Guzmán, D.; Fernández-Grajera, M.; González-Martín, M.L.; Gallardo-Moreno, A.M. Aging of Solvent-Casting PLA-Mg Hydrophobic Films: Impact on Bacterial Adhesion and Viability. Coatings 2019, 9, 814. [Google Scholar] [CrossRef] [Green Version]
- Przekora, A. Current Trends in Fabrication of Biomaterials for Bone and Cartilage Regeneration: Materials Modifications and Biophysical Stimulations. Int. J. Mol. Sci. 2019, 20, 435. [Google Scholar] [CrossRef] [Green Version]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef]
- Ferrandez-Montero, A.; Lieblich, M.; Benavente, R.; González-Carrasco, J.L.; Ferrari, B. New approach to improve polymer-Mg interface in biodegradable PLA/Mg composites through particle surface modification. Surf. Coatings Technol. 2020, 383, 125285. [Google Scholar] [CrossRef]
- Ferrández-Montero, A.; Lieblich, M.; Benavente, R.; González-Carrasco, J.; Ferrari, B. Study of the matrix-filler interface in PLA/Mg composites manufactured by Material Extrusion using a colloidal feedstock. Addit. Manuf. 2020, 33, 101142. [Google Scholar] [CrossRef]
- Wan, P.; Yuan, C.; Tan, L.; Li, Q.; Yang, K. Fabrication and evaluation of bioresorbable PLLA/magnesium and PLLA/magnesium fluoride hybrid composites for orthopedic implants. Compos. Sci. Technol. 2014, 98, 36–43. [Google Scholar] [CrossRef]
- Swaroop, C.; Shukla, M. Nano-magnesium oxide reinforced polylactic acid biofilms for food packaging applications. Int. J. Biol. Macromol. 2018, 113, 729–736. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Calderón, M.; Romero-Guzmán, D.; Ferrández-Montero, A.; Pérez-Giraldo, C.; González-Carrasco, J.L.; Lieblich, M.; Benavente, R.; Ferrari, B.; González-Martín, M.; Gallardo-Moreno, A.M. Impact of PLA/Mg films degradation on surface physical properties and biofilm survival. Colloids Surf. B Biointerfaces 2020, 185, 110617. [Google Scholar] [CrossRef]
- Demchenko, V.; Mamunya, Y.; Kobylinskyi, S.; Riabov, S.; Naumenko, K.; Zahorodnia, S.; Povnitsa, O.; Rybalchenko, N.; Iurzhenko, M.; Adamus, G.; et al. Structure-Morphology-Antimicrobial and Antiviral Activity Relationship in Silver-Containing Nanocomposites Based on Polylactide. Molecules 2022, 27, 3769. [Google Scholar] [CrossRef]
- McFarland, A.W.; Elumalai, A.; Miller, C.C.; Humayun, A.; Mills, D.K. Effectiveness and Applications of a Metal-Coated HNT/Polylactic Acid Antimicrobial Filtration System. Polymers 2022, 14, 1603. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Mrozińska, Z.; Kaczmarek, A.; Lisiak-Kucińska, A. Deposition of Copper on Poly(Lactide) Non-Woven Fabrics by Magnetron Sputtering—Fabrication of New Multi-Functional, Antimicrobial Composite Materials. Materials 2020, 13, 3971. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, J.; Rauf, A.; Zhang, S.; Wang, G.; Shi, S.; Ning, G. A flexible fibrous membrane based on copper(ii) metal–organic framework/poly(lactic acid) composites with superior antibacterial performance. Biomater. Sci. 2021, 9, 3851–3859. [Google Scholar] [CrossRef]
- Aggarwal, D.; Kumar, V.; Sharma, S. Drug-loaded biomaterials for orthopedic applications: A review. J. Control Release 2022, 344, 113–133. [Google Scholar] [CrossRef]
- Seo, K.H.; Lee, K.E.; Yanilmaz, M.; Kim, J. Exploring the Diverse Morphology of Porous Poly(Lactic Acid) Fibers for Developing Long-Term Controlled Antibiotic Delivery Systems. Pharmaceutics 2022, 14, 1272. [Google Scholar] [CrossRef]
- Suner, S.C.; Yildirim, Y.; Yurt, F.; Ozel, D.; Oral, A.; Ozturk, I. Antibiotic loaded electrospun poly (lactic acid) nanofiber mats for drug delivery system. J. Drug Deliv. Sci. Technol. 2022, 71, 103263. [Google Scholar] [CrossRef]
- Tezel, U.; Pavlostathis, S.G. Quaternary ammonium disinfectants: Microbial adaptation, degradation and ecology. Curr. Opin. Biotechnol. 2015, 33, 296–304. [Google Scholar] [CrossRef]
- Nakata, K.; Tsuchido, T.; Matsumura, Y. Antimicrobial cationic surfactant, cetyltrimethylammonium bromide, induces superoxide stress in Escherichia coli cells. J. Appl. Microbiol. 2011, 110, 568–579. [Google Scholar] [CrossRef]
- Chen, P.; Lang, J.; Franklin, T.; Yu, Z.; Yang, R. Reduced Biofilm Formation at the Air–Liquid–Solid Interface via Introduction of Surfactants. ACS Biomater. Sci. Eng. 2021. [Google Scholar] [CrossRef]
- Ahmed, J.; Arfat, Y.A.; Castro-Aguirre, E.; Auras, R. Thermal properties of ZnO and bimetallic Ag–Cu alloy reinforced poly(lactic acid) nanocomposite films. J. Therm. Anal. 2016, 125, 205–214. [Google Scholar] [CrossRef]
- Črešnar, K.P.; Aulova, A.; Bikiaris, D.; Lambropoulou, D.; Kuzmič, K.; Zemljič, L.F. Incorporation of Metal-Based Nanoadditives into the PLA Matrix: Effect of Surface Properties on Antibacterial Activity and Mechanical Performance of PLA Nanoadditive Films. Molecules 2021, 26, 4161. [Google Scholar] [CrossRef]
- Chong, W.J.; Shen, S.; Li, Y.; Trinchi, A.; Pejak, D.; Kyratzis, I.; Sola, A.; Wen, C. Additive manufacturing of antibacterial PLA-ZnO nanocomposites: Benefits, limitations and open challenges. J. Mater. Sci. Technol. 2022, 111, 120–151. [Google Scholar] [CrossRef]
- Karakurt, I.; Ozaltin, K.; Pištěková, H.; Vesela, D.; Michael-Lindhard, J.; Humpolícek, P.; Mozetič, M.; Lehocky, M. Effect of Saccharides Coating on Antibacterial Potential and Drug Loading and Releasing Capability of Plasma Treated Polylactic Acid Films. Int. J. Mol. Sci. 2022, 23, 8821. [Google Scholar] [CrossRef]
- Huang, X.; Ge, X.; Zhou, L.; Wang, Y. Eugenol embedded zein and poly(lactic acid) film as active food packaging: Formation, characterization, and antimicrobial effects. Food Chem. 2022, 384, 132482. [Google Scholar] [CrossRef]
- Hierro-Oliva, M.; Luque-Agudo, V.; Gallardo-Moreno, A.; González-Martín, M. Characterization of Magnesium-Polylactic Acid Films Casted on Different Substrates and Doped with Diverse Amounts of CTAB. Molecules 2021, 26, 4811. [Google Scholar] [CrossRef]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of Biomaterial–Cell Interactions by Adsorbed Proteins: A Review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef]
- Lu, N.; Zhang, W.; Weng, Y.; Chen, X.; Cheng, Y.; Zhou, P. Fabrication of PDMS surfaces with micro patterns and the effect of pattern sizes on bacteria adhesion. Food Control 2016, 68, 344–351. [Google Scholar] [CrossRef]
- Lee, S.W.; Phillips, K.S.; Gu, H.; Kazemzadeh-Narbat, M.; Ren, D. How microbes read the map: Effects of implant topography on bacterial adhesion and biofilm formation. Biomaterials 2021, 268, 120595. [Google Scholar] [CrossRef]
- Francone, A.; Merino, S.; Retolaza, A.; Ramiro, J.; Alves, S.A.; de Castro, J.V.; Neves, N.M.; Arana, A.; Marimon, J.M.; Torres, C.M.S.; et al. Impact of surface topography on the bacterial attachment to micro- and nano-patterned polymer films. Surfaces Interfaces 2021, 27, 101494. [Google Scholar] [CrossRef]
- Perera-Costa, D.; Bruque, J.M.; González-Martín, M.L.; Gómez-García, A.C.; Vadillo-Rodríguez, V. Studying the Influence of Surface Topography on Bacterial Adhesion using Spatially Organized Microtopographic Surface Patterns. Langmuir 2014, 30, 4633–4641. [Google Scholar] [CrossRef]
- Tebbs, S.E.; Sawyer, A.; Elliott, T.S.J. Influence of Surface Morphology on in Vitro Bacterial Adherence to Central Venous Catheters. BJA Br. J. Anaesth. 1994, 72, 587–591. [Google Scholar] [CrossRef]
- Gallardo-Moreno, A.M.; Luque-Agudo, V.; González-Martín, M.L.; Hierro-Oliva, M. Micro-structured and self-assembled patterns in PLA-cast films as a function of CTAB content, magnesium and substratum hydrophobicity. Appl. Surf. Sci. 2022, 597, 153676. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Kaelble, D.H. Dispersion-polar surface tension properties of organic solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]
- Fernández-Calderón, M.C.; Cifuentes, S.C.; Pacha-Olivenza, M.A.; Gallardo-Moreno, A.M.; Saldaña, L.; González-Carrasco, J.L.; Blanco, M.T.; Vilaboa, N.; González-Martín, M.L.; Pérez-Giraldo, C. Antibacterial effect of novel biodegradable and bioresorbable PLDA/Mg composites. Biomed. Mater. 2017, 12, 015025. [Google Scholar] [CrossRef] [Green Version]
- Gomes, I.; Simões, M.; Simões, L. The effects of sodium hypochlorite against selected drinking water-isolated bacteria in planktonic and sessile states. Sci. Total Environ. 2016, 565, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, R.; Azeredo, J.; Teixeira, P.; Fonseca, A.P. The Role of Hydrophobicity in Bacterial Adhesion; Bioline: Cardiff, UK, 2001; pp. 11–22. [Google Scholar]
- Abram, A.; Zore, A.; Lipovž, U.; Košak, A.; Gavras, M.; Boltežar, Ž.; Bohinc, K. Bacterial Adhesion on Prosthetic and Orthotic Material Surfaces. Coatings 2021, 11, 1469. [Google Scholar] [CrossRef]
- Hogt, A.H.; Dankert, J.; Feijen, J. Adhesion of Staphylococcus epidermidis and Staphylococcus saprophyticus to a Hydrophobic Biomaterial. Microbiology 1985, 131, 2485–2491. [Google Scholar] [CrossRef] [Green Version]
- Sankar, G.G.; Murthy, P.S.; Das, A.; Sathya, S.; Nankar, R.; Venugopalan, V.P.; Doble, M. Polydimethyl siloxane based nanocomposites with antibiofilm properties for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1075–1082. [Google Scholar] [CrossRef]
- Azeredo, J.; Pacheco, A.; Lopes, I.; Oliveira, R.; Vieira, M.J. Monitoring cell detachment by surfactants in a parallel plate flow chamber. Water Sci. Technol. 2003, 47, 77–82. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- Cullmann, W.; Schlunegger, H. Influence of Exogenous Factors and Antibiotics on the Cytoplasm Ic Membrane Proteins of Staphylococcus Aureus. Microbiol. Chemother. 1992, 38, 211–217. [Google Scholar] [CrossRef]
- Briandet, R.; Meylheuc, T.; Maher, C.; Noe¨, M.N.; Bellon-Fontaine, N. Listeria Monocytogenes Scott A: Cell Surface Charge, Hydrophobicity, and Electron Donor and Acceptor Characteristics under Different Environmental Growth Conditions. Appl. Environ. Microbiol. 1999, 65, 5328–5333. [Google Scholar] [CrossRef] [Green Version]
- Oliver, J.D. The viable but nonculturable state in bacteria. J. Microbiol. 2005, 43, 93–100. [Google Scholar]
- EL Othmany, R.; Zahir, H.; Zanane, C.; El Louali, M.; Latrache, H. Influence of Consistency and Composition of Growth Medium on Surface Physicochemical Properties of Streptomyces. J. Pure Appl. Microbiol. 2021, 15, 1705–1715. [Google Scholar] [CrossRef]
- Sathya, S.; Murthy, P.S.; Devi, V.G.; Das, A.; Anandkumar, B.; Sathyaseelan, V.; Doble, M.; Venugopalan, V. Antibacterial and cytotoxic assessment of poly (methyl methacrylate) based hybrid nanocomposites. Mater. Sci. Eng. C 2019, 100, 886–896. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, B.; Liu, Y.; Suo, X.; Li, H. Influence of surface topography on bacterial adhesion: A review (Review). Biointerphases 2018, 13, 060801. [Google Scholar] [CrossRef] [Green Version]
- Meinshausen, A.-K.; Herbster, M.; Zwahr, C.; Soldera, M.; Müller, A.; Halle, T.; Lasagni, A.F.; Bertrand, J. Aspect ratio of nano/microstructures determines Staphylococcus aureus adhesion on PET and titanium surfaces. J. Appl. Microbiol. 2021, 131, 1498–1514. [Google Scholar] [CrossRef]
- Wojciechowski, K.; Kaczorowski, M.; Mierzejewska, J.; Parzuchowski, P. Antimicrobial dispersions and films from positively charged styrene and acrylic copolymers. Colloids Surf. B Biointerfaces 2018, 172, 532–540. [Google Scholar] [CrossRef]
- Ribeiro, R.T.; Galvão, C.N.; Betancourt, Y.P.; Mathiazzi, B.I.; Carmona-Ribeiro, A.M. Microbicidal Dispersions and Coatings from Hybrid Nanoparticles of Poly (Methyl Methacrylate), Poly (Diallyl Dimethyl Ammonium) Chloride, Lipids, and Surfactants. Int. J. Mol. Sci. 2019, 20, 6150. [Google Scholar] [CrossRef] [Green Version]
- Naves, A.F.; Palombo, R.R.; Carrasco, L.D.; Carmona-Ribeiro, A.M. Antimicrobial Particles from Emulsion Polymerization of Methyl Methacrylate in the Presence of Quaternary Ammonium Surfactants. Langmuir 2013, 29, 9677–9684. [Google Scholar] [CrossRef] [PubMed]
- Eoknin, H.; Esteinberg, D.; Eshemesh, M. Magnesium ions mitigate biofilm formation of Bacillus species via downregulation of matrix genes expression. Front. Microbiol. 2015, 6, 907. [Google Scholar] [CrossRef]
- Song, B.; Leff, L.G. Influence of magnesium ions on biofilm formation by Pseudomonas fluorescens. Microbiol. Res. 2006, 161, 355–361. [Google Scholar] [CrossRef]
- Reffuveille, F.; Josse, J.; Velard, F.; Lamret, F.; Varin-Simon, J.; Dubus, M.; Haney, E.F.; Hancock, R.; Mongaret, C.; Gangloff, S.C. Bone Environment Influences Irreversible Adhesion of a Methicillin-Susceptible Staphylococcus aureus Strain. Front. Microbiol. 2018, 9, 2865. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Li, Y.; Wang, J.; Wang, C.; Liu, D.; Wang, G.; Liu, S. Mechanically Robust, Self-Healing, Polymer Blends and Polymer/Small Molecule Blend Materials with High Antibacterial Activity. ACS Appl. Mater. Interfaces 2020, 12, 26966–26972. [Google Scholar] [CrossRef]



| Sample | γd (mJ/m2) | γnd (mJ/m2) | γs (mJ/m2) | ΔGSWS (mJ/m2) | Sample | γd (mJ/m2) | γnd (mJ/m2) | γs (mJ/m2) | ΔGSWS (mJ/m2) |
|---|---|---|---|---|---|---|---|---|---|
| 0/00 | 26 ± 1 | 0 ± 0 | 26 ± 1 | −94 ± 3 | 3/00 | 30 ± 7 | 1 ± 1 | 31 ± 8 | −84 ± 17 |
| 0/01 | 28 ± 3 | 3 ± 2 | 31 ± 4 | −70 ± 9 | 3/01 | 28 ± 3 | 4 ± 3 | 32 ± 6 | −68 ± 14 |
| 0/03 | 20 ± 3 | 3 ± 2 | 23 ± 5 | −80 ± 13 | 3/03 | 23 ± 2 | 6 ± 3 | 29 ± 5 | −66 ± 12 |
| 0/10 | 31 ± 1 | 0 ± 1 | 32 ± 1 | −84 ± 6 | 3/10 | 26 ± 1 | 4 ± 2 | 30 ± 2 | −70 ± 7 |
| 0/11 | 27 ± 2 | 6 ± 2 | 33 ± 4 | −63 ± 7 | 3/11 | 16 ± 3 | 3 ± 3 | 19 ± 5 | −83 ± 13 |
| 0/13 | 22 ± 6 | 7 ± 3 | 28 ± 9 | −65 ± 15 | 3/13 | 26 ± 1 | 18 ± 4 | 45 ± 5 | −36 ± 8 |
| 0/50 | 23 ± 2 | 9 ± 6 | 32 ± 8 | −58 ± 16 | 3/50 | 29 ± 1 | 13 ± 1 | 42 ± 1 | −43 ± 2 |
| 0/51 | 25 ± 3 | 10 ± 5 | 36 ± 8 | −53 ± 14 | 3/51 | 21 ± 3 | 10 ± 4 | 31 ± 7 | −58 ± 12 |
| 0/53 | 25 ± 1 | 8 ± 3 | 33 ± 4 | −59 ± 10 | 3/53 | 25 ± 2 | 33 ± 5 | 58 ± 6 | −17 ± 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Grajera, M.; Gallardo-Moreno, A.M.; Luque-Agudo, V.; González-Martín, M.L.; Hierro-Oliva, M. Bacterial Response to the Surface Aging of PLA Matrices Loaded with Active Compounds. Polymers 2022, 14, 4976. https://doi.org/10.3390/polym14224976
Fernández-Grajera M, Gallardo-Moreno AM, Luque-Agudo V, González-Martín ML, Hierro-Oliva M. Bacterial Response to the Surface Aging of PLA Matrices Loaded with Active Compounds. Polymers. 2022; 14(22):4976. https://doi.org/10.3390/polym14224976
Chicago/Turabian StyleFernández-Grajera, María, Amparo María Gallardo-Moreno, Verónica Luque-Agudo, María Luisa González-Martín, and Margarita Hierro-Oliva. 2022. "Bacterial Response to the Surface Aging of PLA Matrices Loaded with Active Compounds" Polymers 14, no. 22: 4976. https://doi.org/10.3390/polym14224976





