2.1. Materials and Instrument
Acrylamide (AM) was purchased from Shandong Duofeng Chemical Co., Ltd., Shandong, China. 2-acrylamide-2-methylpropanesulfonic acid (AMPS) was purchased from Jingwen Dongxin Biotechnology Co., Ltd., Beijing, China. Potassium persulfate, sodium hydroxide, sodium carbonate, sodium bicarbonate, sodium chloride, anhydrous calcium chloride, magnesium chloride, fumaric acid, and maleic acid were purchased from Beijing Yili Fine Chemicals Co., Ltd., Beijing, China. Fumaric acid sludge (FAS) was obtained from Karamay Zhengcheng Co., Ltd., Karamay, China. Except for AM, AMPS, and FAS, which were industrial grade, the other reagents were analytical grade.
Roller heating furnace GRL-9, six-speed rotary viscometer ZNN-D6, digital display high-speed mixer GJ-2S, and multi-linked medium-pressure filtrate loss instrument ZNS-2A were manufactured in Qingdao Tongda Special Instrument Co., Ltd., Qingdao, China. Multi-functional high-speed blender SUS-304 was manufactured in Wuyi Hainan Electric Co., Ltd., Haikou, China. Electric thermostatic blast drying oven DHG-9035A was manufactured in Beijing Lushi Technology Co., Ltd., Beijing, China. Infrared spectrometer Nicolet IS5 was manufactured in Nicolet Corporation, Waltham, MA, USA. Full Digital NMR Spectrometer BRUKER-400MHz was manufactured in Bruker (Beijing) Technology Co., LTD., Beijing, China. X-ray Diffractometer XD-3 was manufactured in Beijing Pu-Analysis General Instrument Co., Ltd., Beijing, China. Gas chromatography-mass spectrometry GC-7890A/MS-5975c was manufactured in Agilent Technologies (China) Co., Ltd., Beijing, China. Pyrolysis gas chromatograph-mass spectrometer PY-3030D/QP2010Ultra was manufactured in Shimadzu (China) Co., Ltd., Beijing, China. Liquid chromatograph-mass spectrometer Acquity-TQD was manufactured in Waters Technology (Shanghai) Co., Ltd., Shanghai, China.
2.2. Analysis Method of FAS
2.2.1. FT-IR
The samples were prepared using the KBr method. Firstly, the sample and KBr were ground into a fine powder in an agate mortar with a mass ratio of approximately 1:150. After being fully dried under an infrared lamp, the sample was pressed into a thin sheet using a tablet machine. Then, the thin sheet was carefully mounted on the magnetic sample rack and placed in the sample chamber of the Fourier infrared spectrometer for measurement. The prepared samples were scanned in a wave-number range of 4000 cm−1 to 400 cm−1 with a resolution of 4 cm−1 and a signal-to-noise ratio of 50,000:1. All spectrums were obtained by accumulating 64 scans.
2.2.2. 1H-NMR
The FAS samples were fully dried in the oven and then dissolved with D2O and CD3OD as solvents, respectively. After 30 min of ultrasonic shaking at 40 °C, the samples were placed in a nuclear magnetic resonance hydrogen spectrometer and tested at a resonance frequency of 400 MHz.
2.2.3. XRD
The FAS samples were ground into a fine powder, compacted onto a test block to form a very flat surface, and then placed in the sample chamber of the X-ray diffractometer. The samples were tested under the parameters of a scan range of 2theta = 10–80°, step width = 0.01°, and a light tube power of 35 kV and 30 mA.
2.2.4. XRF
Take 3–5 g FAS and place it in the sample mold. Then, put the sample mold into the tablet machine and press it for 15 s at a pressure of 20 MPa. Determination of the elemental composition and its proportion in FAS was conducted using the X-ray fluorescence spectrometer. The analytical range of elements is Be (4)–U (92).
2.2.5. GC-MS
The FAS samples were pre-treated by the solid phase microextraction (SPME) method. The samples were determined by gas chromatography and mass spectrometry (GC-MS) and retrieved using the NIST mass spectrometry library. GC conditions: initial temperature of 50 °C, hold for 5 min, ramp up to 100 °C at 10 °C/min, then ramp up to 300 °C at 30 °C/min, hold for 4 min; the chromatographic column was DB-5(30 m × 0.25 mm × 0.25 μm). MS conditions: ion source was ESI; ion source temperature was 230 °C, maximum 250 °C; quadrupole temperature was 150 °C, maximum 200 °C; scan mass range was 29–500 AMU, ion source energy of 70 eV, emission current of 34.6 μA; run time of 10 min.
2.2.6. Py-GC-MS
The FAS samples were placed in a pyrolysis reactor for pyrolysis, and the pyrolysis temperature increased from 50 °C to 550 °C at a rate of 10 °C/min. Volatile components pyrolysis from FAS samples were trapped in the cold trap at the port of the chromatographic column. Then, it enters GC-MS for separation and detection. The detection conditions are the same as those of
Section 2.2.5.
2.2.7. LC-MS
The high-performance liquid chromatography (HPLC) system was used to separate the samples, and a mass spectrometry (MS) system was used for detection. LC conditions: the chromatographic column was Shimadzu XR-ODS 2.0 mmI.D. × 75 mmL, 2.2 μm; mobile phase: A is an aqueous solution; B is methanol; flow rate: 0.3 mL/min; column temperature: constant temperature 30 °C; elution mode: binary gradient elution; gradient elution procedure is shown in
Table 1.
MS conditions: ion source: ESI; atomized gas: nitrogen at a flow rate of 3.0 L/min; DL temperature: 250 ℃; heating module temperature: 400 ℃; dry gas: nitrogen at a flow rate of 10.0 L/min.
Preparation of standard solution: the standard solution is diluted in a gradient with water. Phthalate and malic acid are prepared as standard working solutions with concentrations of 0.15, 0.65, 1.30, 2.60, and 6.40 μg/mL; fumaric acid and sodium citrate are prepared as standard working solutions with concentrations of 0.60, 1.10, 2.20, 5.50, and 11.00 μg/mL; Sulfourea is prepared as a standard working solution with concentrations of 0.10, 0.50, 1.00, 2.00, and 5.00 μg/mL.
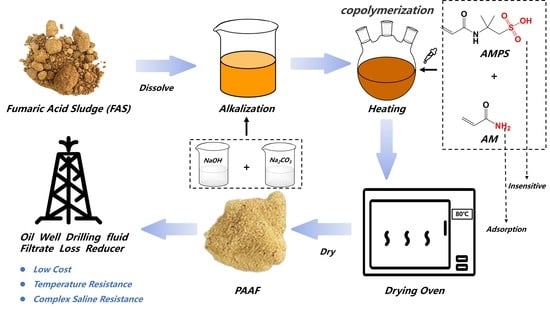
2.3. Preparation of PAAF Based on FAS
FAS pretreatment: FAS pretreatment: Block FAS was dried in an oven at 80 °C for 24 h and then crushed into a fine powder with a high-speed blender until ready to use.
Preparation method of the PAAF: Dissolve a certain mass of sodium hydroxide and sodium carbonate in deionized water, and then stir while adding a certain mass of FAS fine powder to obtain solution A; solution B was obtained by dissolving a certain mass of AMPS and AM in deionized water. Solution C was obtained by dissolving a certain mass of initiator K
2S
2O
8 and cross-linking agent [
25,
26,
27,
28,
29] Na
2SiO
3 in deionized water. The detailed composition of the solutions is shown in
Table 2.
Transfer solution A and solution B to a three-mouth flask, mix well, and adjust pH to alkalinity. After 30 min of nitrogen injection into the three-mouth flask, the solution temperature was raised to 55 °C, and solution C was added drop by drop. After 5 h of reaction, the mixed solution turned into a brown viscous liquid, which was poured out and put into an oven at 80 °C to obtain the PAAF after drying.
2.4. PAAF Performance Evaluation Method
The performance evaluation of the PAAF is reflected by two parts of data: rheological parameters and Filtration loss FL
API. The test methods are based on the test methods used by these researchers [
30]. The specific test steps are as follows:
Rheological parameters: Pour the drilling fluid mud sample into the sample cup of the rotational viscometer; make the liquid level reach the scale line in the sample cup of the rotational viscometer; put the sample cup on the bottom frame of the viscometer; move the bottom frame so that the sample liquid level coincides with the scale line on the outer cylinder; measure and record the temperature of the drilling fluid sample. Adjust the rotational speed of the outer cylinder of the rotational viscometer, and after the dial reading value is stabilized, read and record the dial reading value under different rotational speeds. Calculate the apparent viscosity, plastic viscosity, and dynamic shear force based on the following equation:
where:
R600 = the reading of the viscometer at 600 r/min (dia);
R300 = the reading of the viscometer at 300 r/min (dia);
AV = apparent viscosity (mPa·s);
PV = plastic viscosity (mPa·s);
YP = yield point (Pa).
Filtration loss FLAPI: Pour the drilling fluid mud sample into the filtration loss meter cup; make the liquid level reach the scale line in the filtration loss meter cup, and put on the filtrate paper, and install the filtration loss meter; put the dry measuring cylinder underneath to receive the filtrate; close the pressure relief valve, and adjust the pressure regulator to make the pressure reach 690 ± 35 kPa (100 ± 5 psi) in 30 s or less; start timing while pressurizing; after reaching 30 min, measure the collected filtrate volume, which is the room temperature filtration loss FLAPI.