Effect of Surfactants on the Binding Properties of a Molecularly Imprinted Polymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Binding Measurements
3. Results
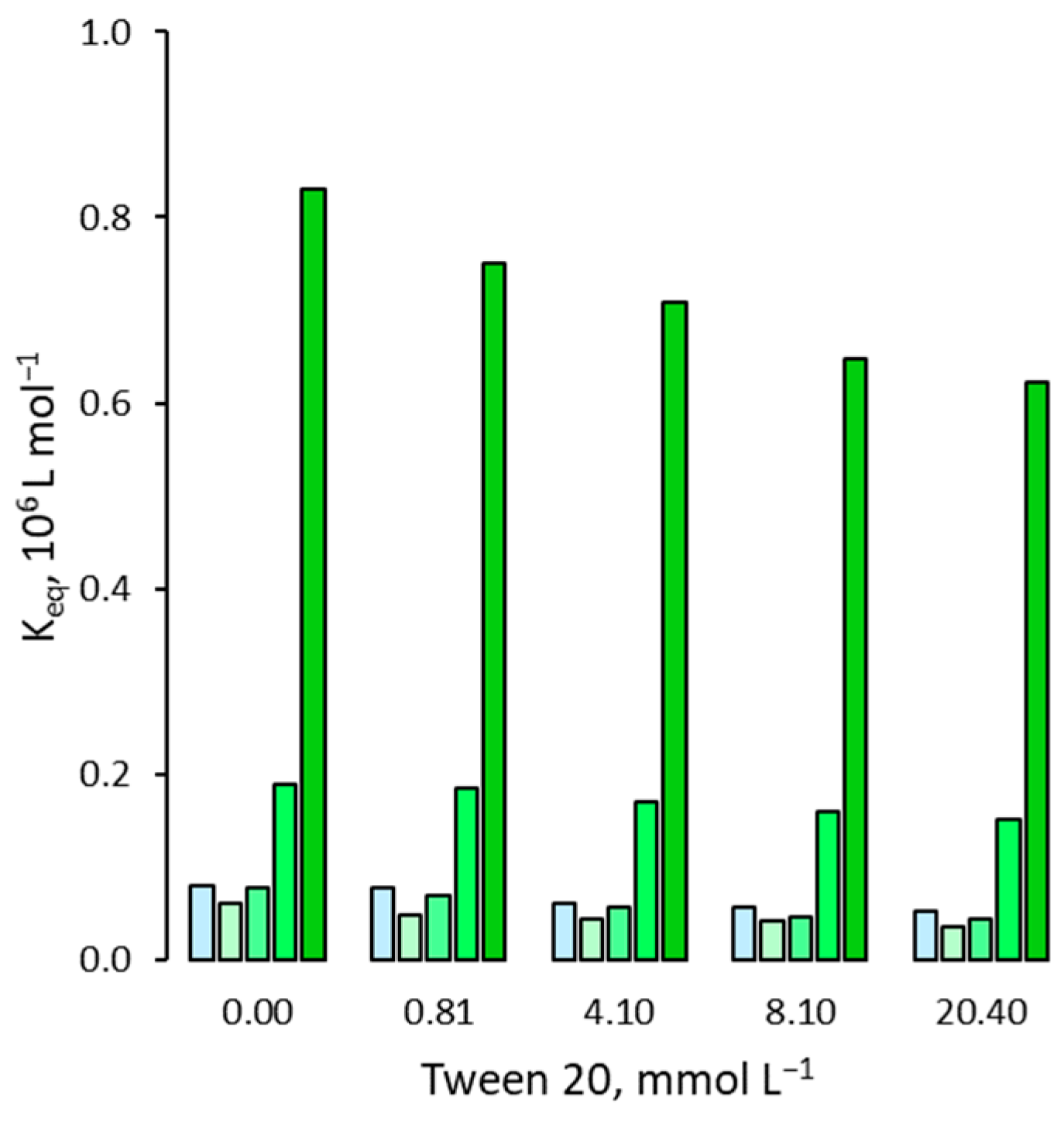
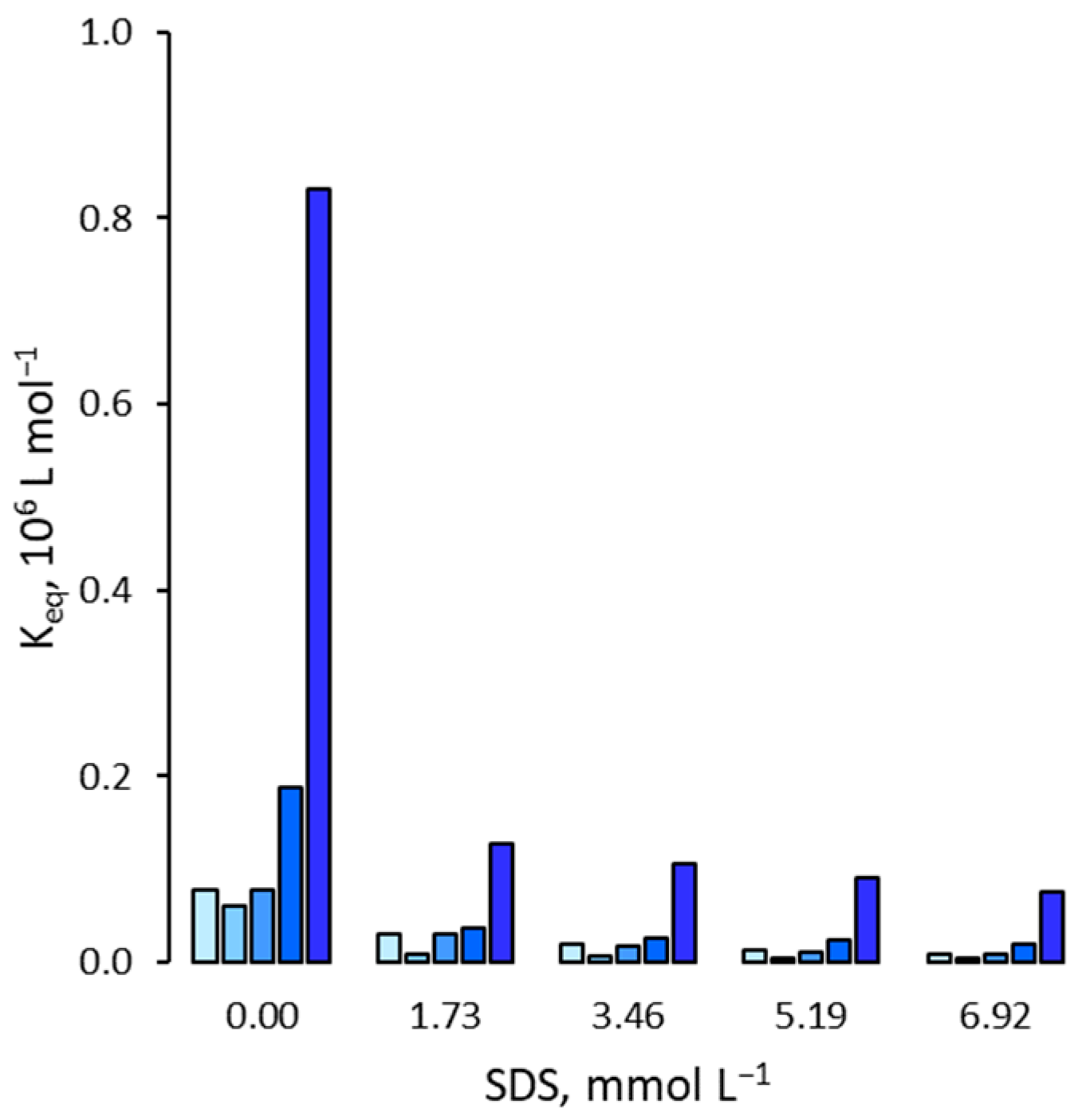
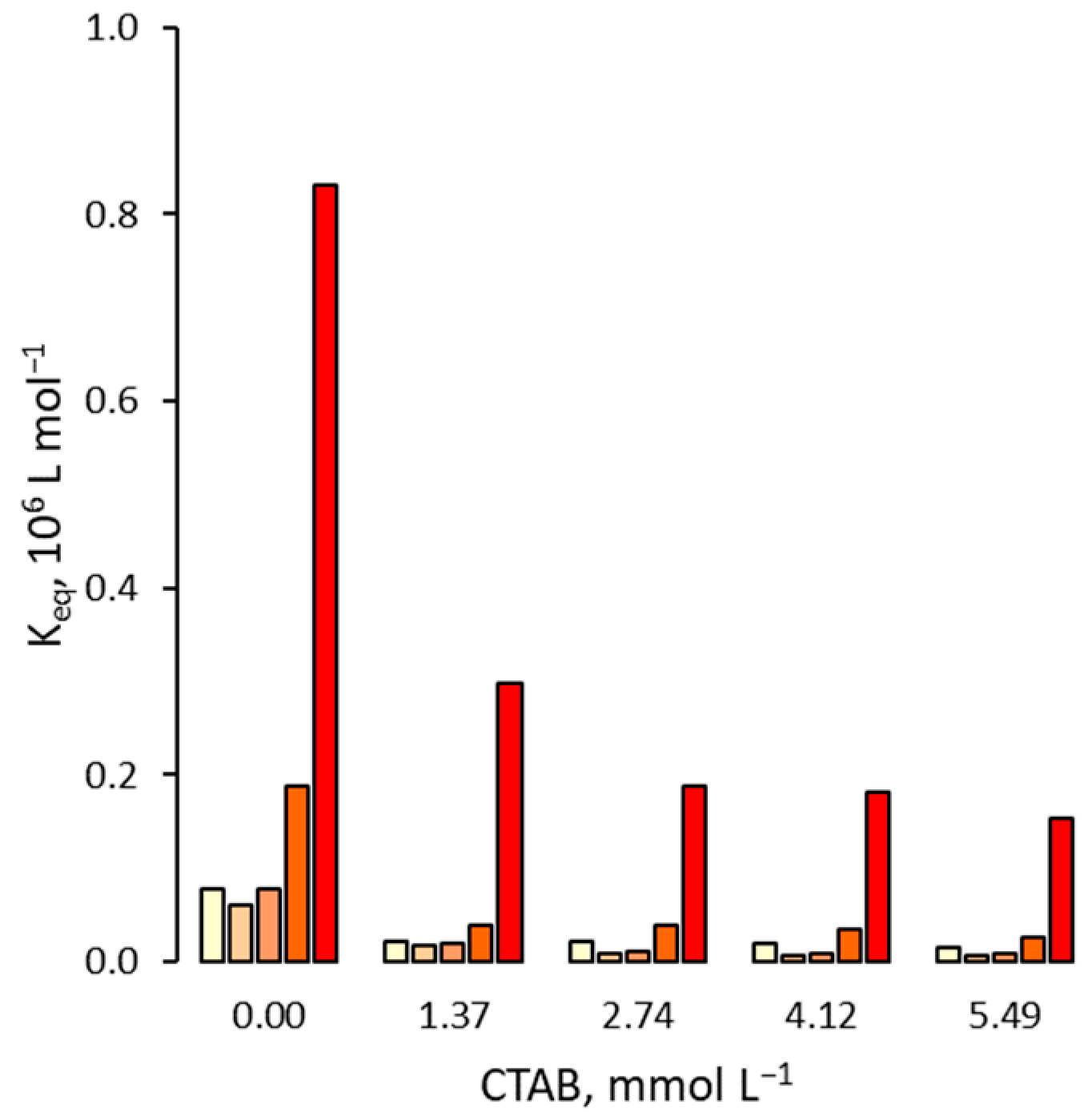
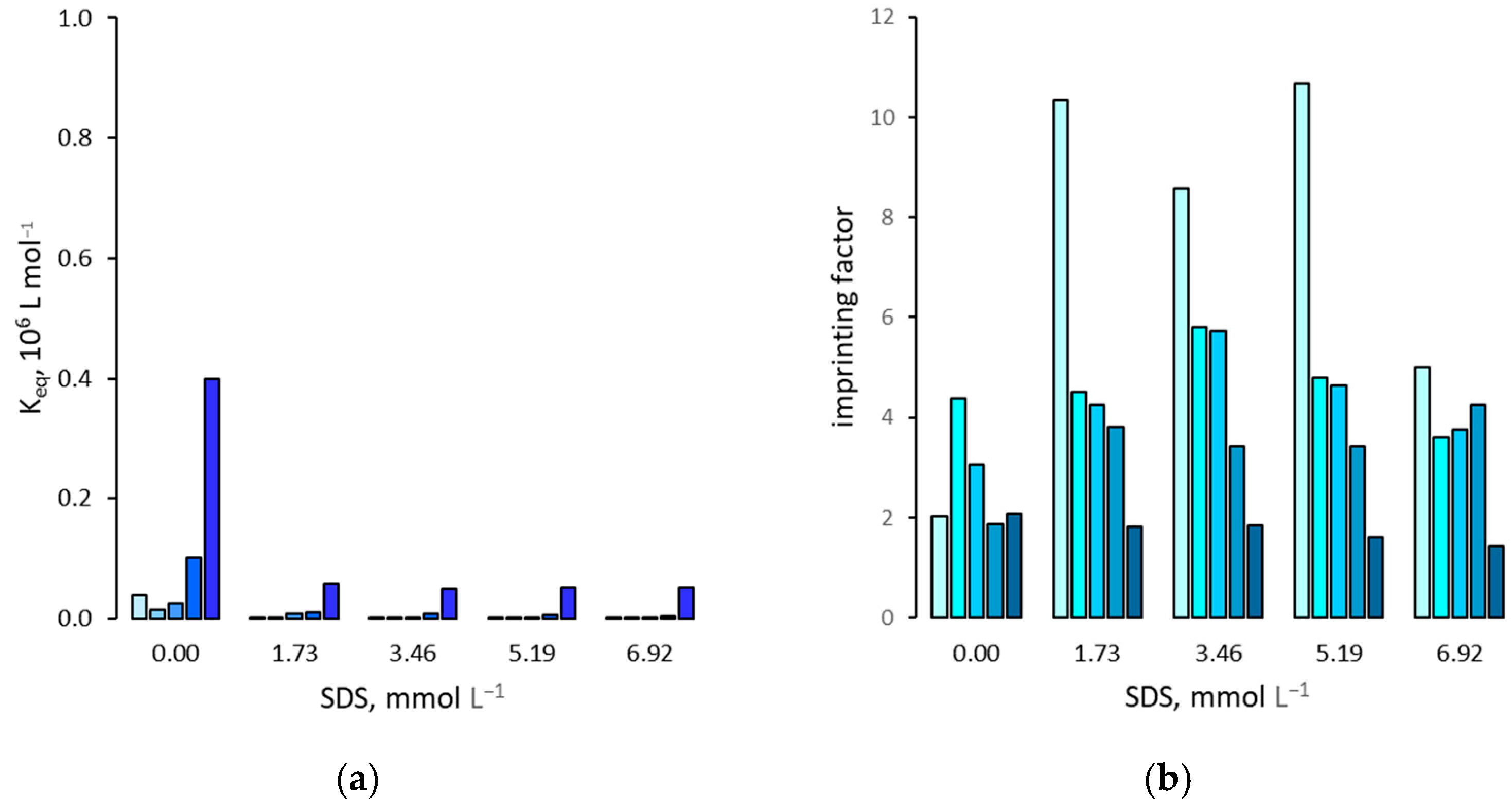
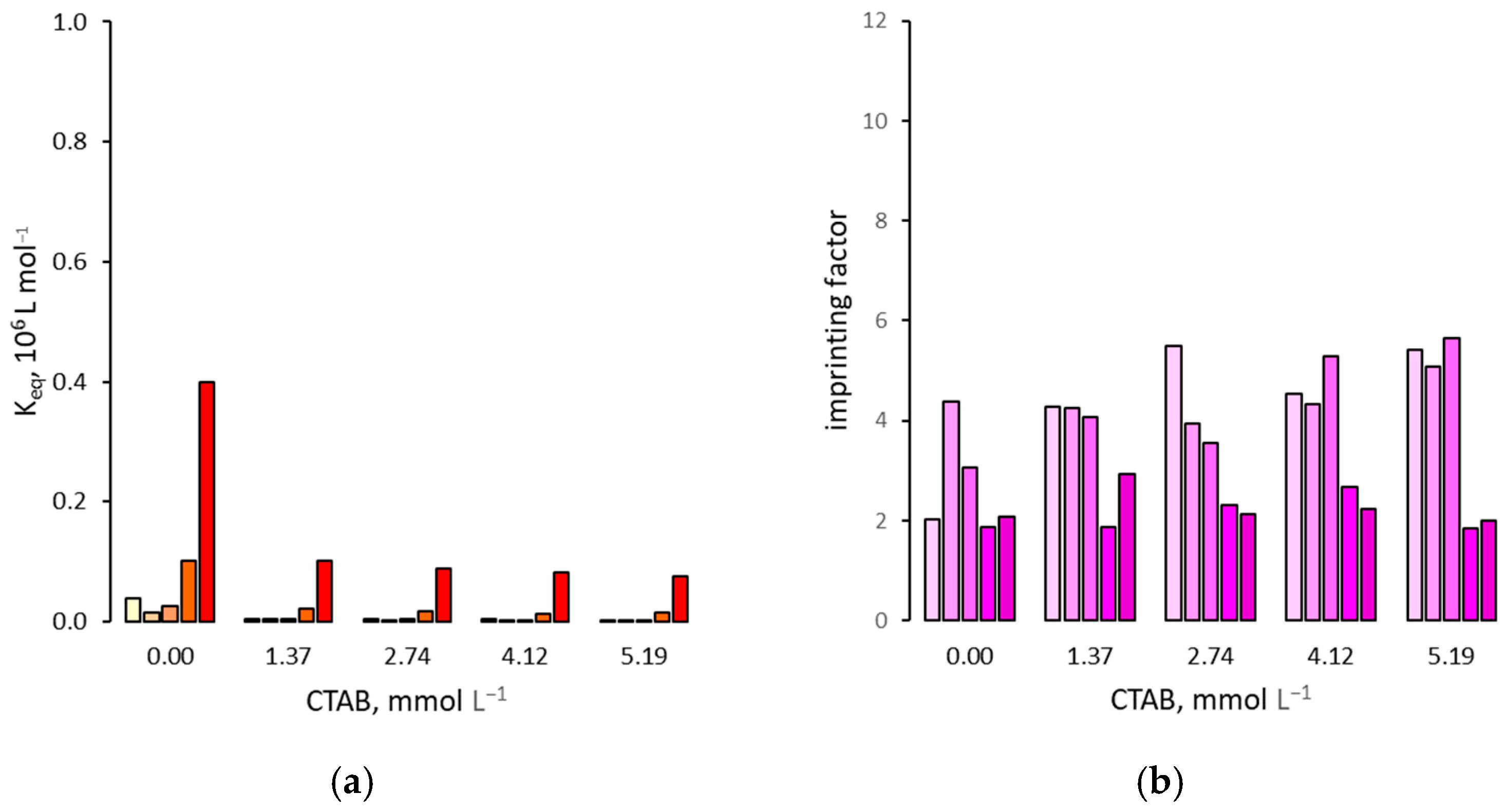
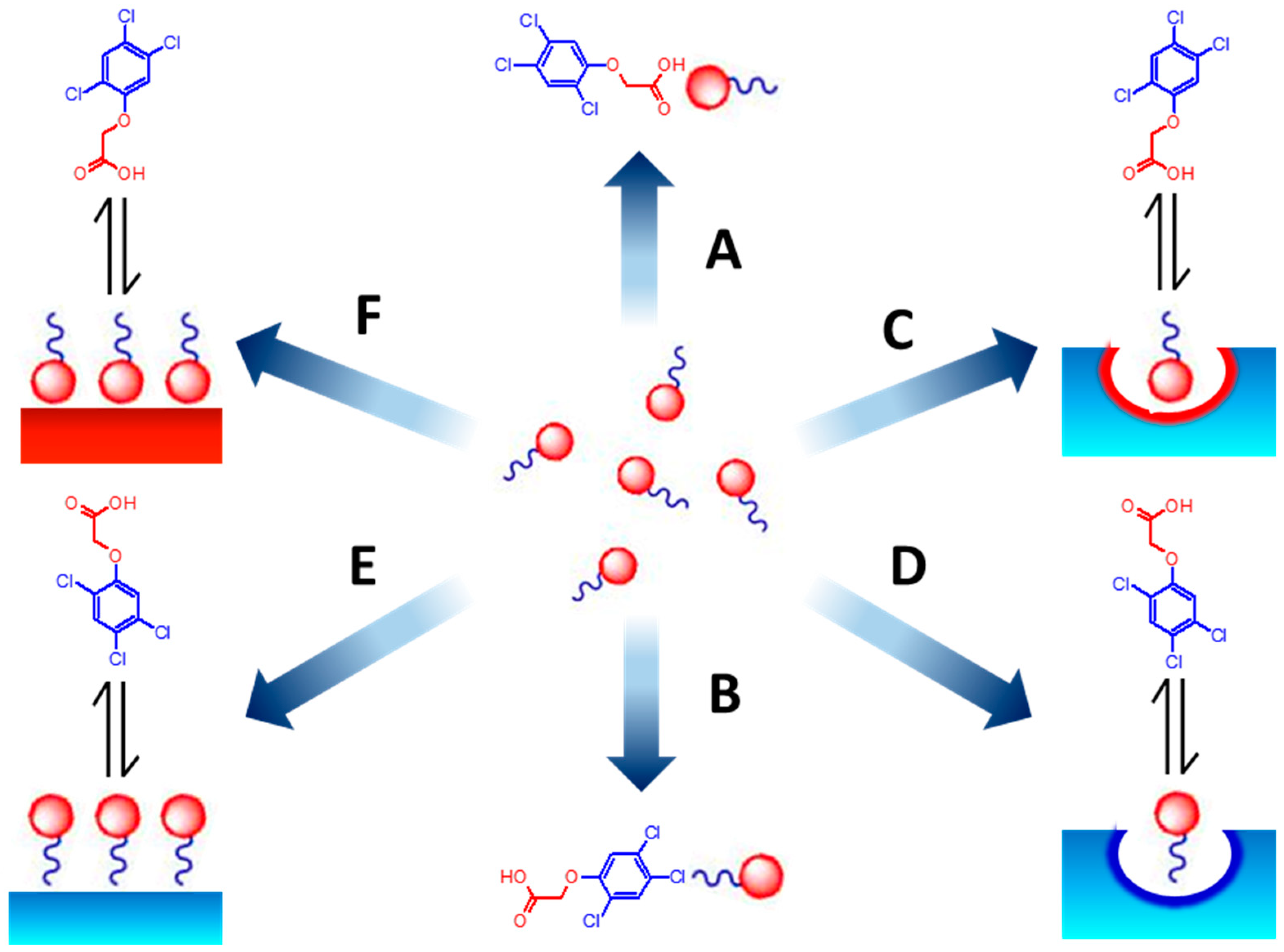
3.1. Effect on the Binding Affinity for the Template
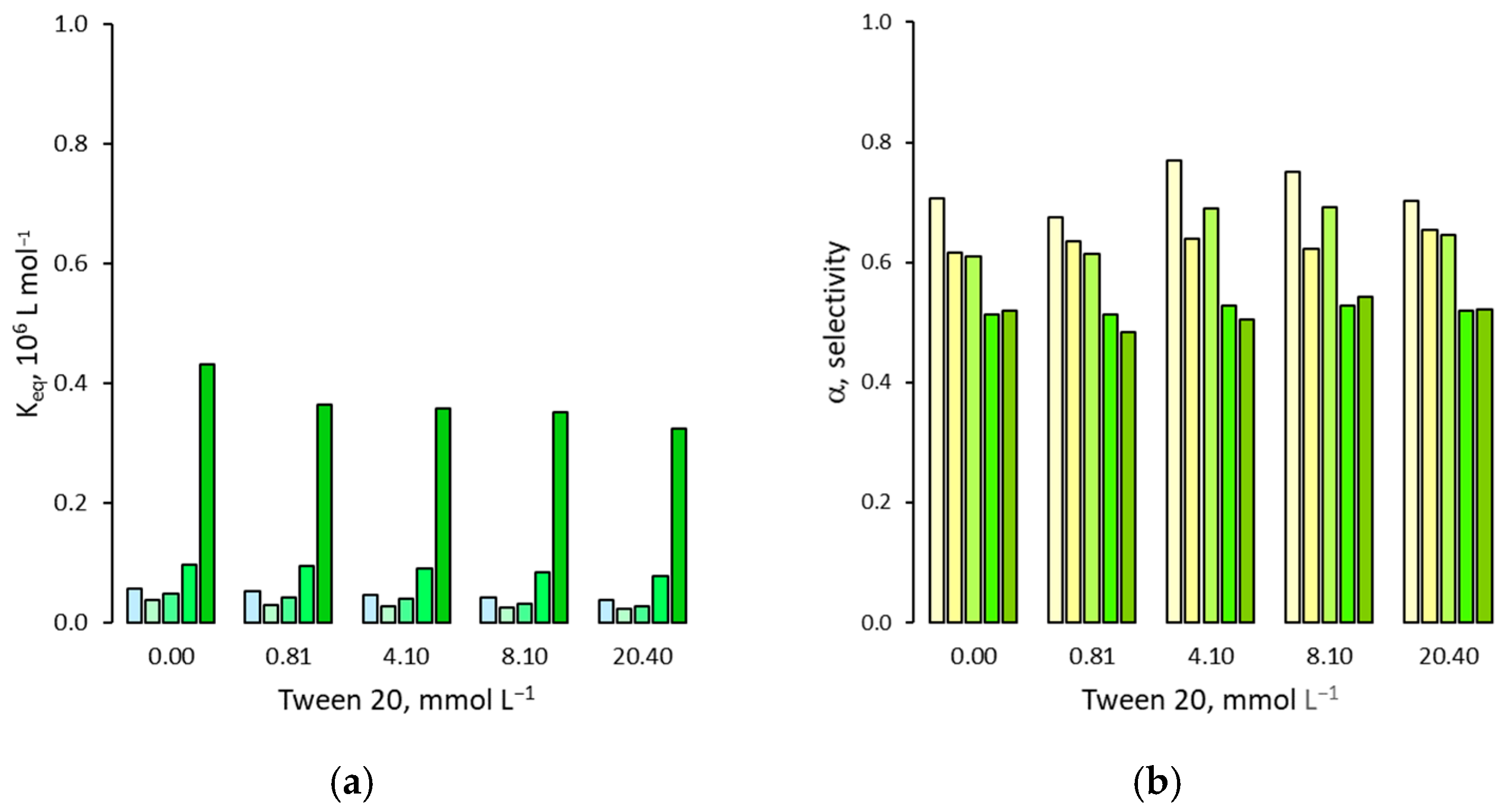
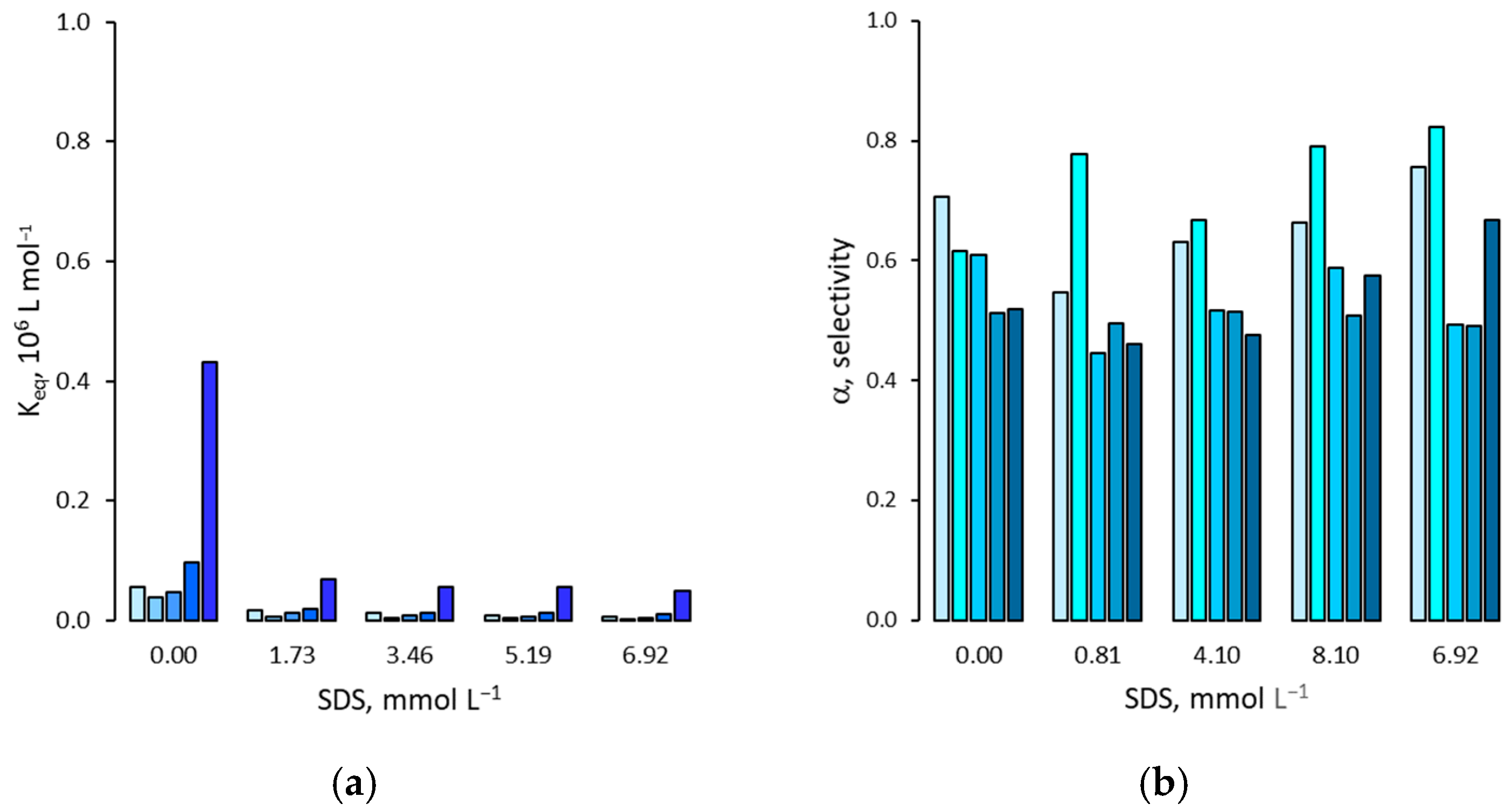
3.2. Effect on Non Specific Binding
3.3. Effect on the Binding Selectivity
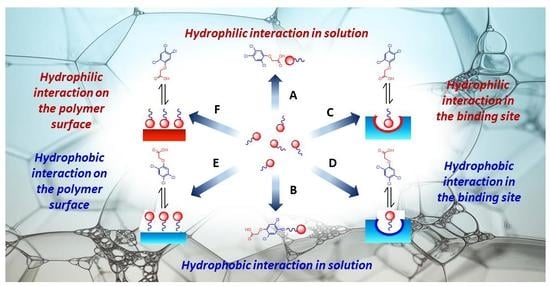
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pichon, V.; Delaunay, N.; Combès, A. Sample preparation using molecularly imprinted polymers. Anal. Chem. 2020, 92, 16–33. [Google Scholar] [CrossRef]
- Wan, Q.Q.; Liu, H.; Deng, Z.W.; Bu, J.Q.; Li, T.H.; Yang, Y.J.; Zhong, S. A critical review of molecularly imprinted solid phase extraction technology. J. Polym. Res. 2021, 28, 401. [Google Scholar] [CrossRef]
- Wang, H.; Huang, C.; Ma, S.; Bo, C.; Ou, J.; Gong, B. Recent advances of restricted access molecularly imprinted materials and their applications in food and biological samples analysis. Trends Anal. Chem. 2022, 147, 116526. [Google Scholar] [CrossRef]
- Shahhoseini, F.; Azizi, A.; Bottaro, C.S. A critical evaluation of molecularly imprinted polymer (MIP) coatings in solid phase microextraction devices. Trends Anal. Chem. 2022, 156, 116695. [Google Scholar] [CrossRef]
- Moreno-Bondi, M.C.; Benito-Peña, M.E.; Urraca, J.L.; Orellana, G. Immuno-like assays and biomimetic microchips. In Molecular Imprinting, 1st ed.; Haupt, K., Ed.; Springer: Heidelberg, Germany, 2012; pp. 111–164. [Google Scholar]
- Bedwell, T.S.; Whitcombe, M.J. Analytical applications of MIPs in diagnostic assays: Future perspectives. Anal. Bioanal. Chem. 2016, 408, 1735–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Luo, J.; Li, C.; Ma, M.; Yu, W.; Shen, J.; Wang, Z. Molecularly imprinted polymer as an antibody substitution in pseudo-immunoassays for chemical contaminants in food and environmental samples. J. Agric. Food Chem. 2018, 66, 2561–2571. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Chacon, A.; Ceto, X.; del Valle, M. Molecularly imprinted polymers—Towards electrochemical sensors and electronic tongues. Anal. Bioanal. Chem. 2021, 413, 6117–6140. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, S.; Jagminas, A.; Ramanavicius, A. Advances in molecularly imprinted polymers based affinity sensors. Polymers 2021, 13, 974. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Jia, M.; Zhao, H.; Kang, L.; Shi, L.; Zhou, L.; Kong, W. Molecularly imprinted polymer-based optical sensors for pesticides in foods: Recent advances and future trends. Trends Food Sci. Technol. 2021, 116, 387–404. [Google Scholar] [CrossRef]
- Yang, W.; Ma, Y.; Sun, H.; Huang, C.; Shen, X. Molecularly imprinted polymers based optical fiber sensors: A review. Trends Anal. Chem. 2022, 152, 116608. [Google Scholar] [CrossRef]
- Chen, L.; Xu, S.; Li, J. Recent advances in molecular imprinting technology: Current status, challenges and highlighted applications. Chem. Soc. Rev. 2011, 40, 2922–2942. [Google Scholar] [CrossRef] [PubMed]
- Tov, O.Y.; Luvitch, S.; Bianco-Peled, H. Molecularly imprinted hydrogel displaying reduced non-specific binding and improved protein recognition. J. Sep. Sci. 2010, 33, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, J.; Tian, L.; Wang, Y.; Zhang, B.; Zhang, H.; Zhang, Q. Preparation of anti-nonspecific adsorption polydopamine-based surface protein-imprinted magnetic microspheres with the assistance of 2-methacryloyloxyethylphosphorylcholine and its application for protein recognition. Sens. Act. B 2017, 241, 413–421. [Google Scholar] [CrossRef]
- Refaat, D.; Aggour, M.G.; Farghali, A.A.; Mahajan, R.; Wiklander, J.G.; Nicholls, I.A.; Piletsky, S.A. Strategies for molecular imprinting and the evolution of MIP nanoparticles as plastic antibodies—Synthesis and applications. Int. J. Mol. Sci. 2019, 20, 6304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chianella, I.; Guerreiro, A.; Moczko, E.; Caygill, J.S.; Piletska, E.V.; Perez De Vargas Sansalvador, I.M.; Whitcombe, M.J.; Piletsky, S.A. Direct replacement of antibodies with molecularly imprinted polymer nanoparticles in ELISA. Development of a novel assay for vancomycin. Anal. Chem. 2013, 85, 8462–8468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, Y.; Smolinska-Kempisty, K.; Pereira, E.; Piletska, E.; Piletsky, S.A. Development of competitive ‘pseudo’-ELISA assay for measurement of cocaine and its metabolites using molecularly imprinted polymer nanoparticles. Anal. Methods 2017, 9, 4592–4598. [Google Scholar] [CrossRef]
- Spano, G.; Cavalera, S.; Di Nardo, F.; Giovannoli, C.; Anfossi, L.; Baggiani, C. Development of a biomimetic enzyme-linked immunosorbent assay based on a molecularly imprinted polymer for the detection of cortisol in human saliva. Anal. Methods 2019, 11, 2320–2326. [Google Scholar] [CrossRef]
- Khan, A. Phase science of surfactants. Curr. Opin. Coll. Interf. Sci. 1996, 1, 614–623. [Google Scholar] [CrossRef]
- Pramauro, E.; Pelizetti, E. Surfactants in Analytical Chemistry. In Comprehensive Analytical Chemistry, 1st ed.; Weber, S.G., Ed.; Elsevier: Amsterdam, The Netherlands, 1994; Volume 31, pp. 1–91. [Google Scholar]
- Spano, G.; Giovannoli, C.; Di Nardo, F.; Anfossi, L.; Baggiani, C. Full vs. partial competitive binding behavior in molecularly imprinted polymers. The case for a chlorinated phenoxyacids-binding polymer. RSC Adv. 2016, 6, 78317–78321. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Testa, V.; Anfossi, L.; Cavalera, S.; Chiarello, M.; Di Nardo, F.; Serra, T.; Baggiani, C. Effect of Surfactants on the Binding Properties of a Molecularly Imprinted Polymer. Polymers 2022, 14, 5210. https://doi.org/10.3390/polym14235210
Testa V, Anfossi L, Cavalera S, Chiarello M, Di Nardo F, Serra T, Baggiani C. Effect of Surfactants on the Binding Properties of a Molecularly Imprinted Polymer. Polymers. 2022; 14(23):5210. https://doi.org/10.3390/polym14235210
Chicago/Turabian StyleTesta, Valentina, Laura Anfossi, Simone Cavalera, Matteo Chiarello, Fabio Di Nardo, Thea Serra, and Claudio Baggiani. 2022. "Effect of Surfactants on the Binding Properties of a Molecularly Imprinted Polymer" Polymers 14, no. 23: 5210. https://doi.org/10.3390/polym14235210