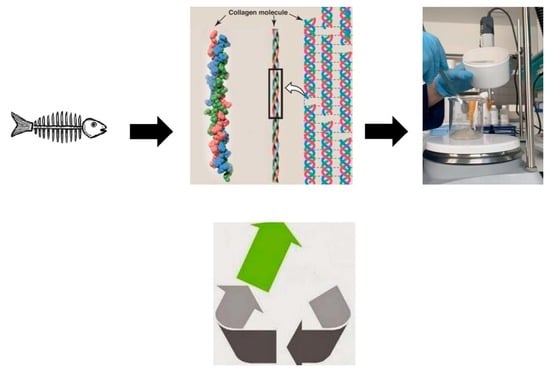
The Use of Natural Collagen Obtained from Fish Waste in Hair Styling and Care
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Tests Confirming the Safety and the Manufacturer’s Declaration for the Collagen Laminate
2.3. Apparatus Tests Carried Out with the Help of TrichoScope Polarizer Dino-Lite (MEDL4HM)
2.4. Apparatus Test Performed Using the SEM(Scanning Electron Microscope) Method
3. Results and Discussion
3.1. Instrument Tests Carried Out with the TrichoScope Polarizer Dino-Lite (MEDL4HM)
3.2. Apparatus Test Performed Using the SEM (Scanning Electron Microscope) Method
4. Conclusions


Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- George, A.; Sanjay, M.R.; Srisuk, R.; Parameswaranpillai, J.; Siengchin, S. A comprehensive review on chemical properties and applications of biopolymers and their composites. Int. J. Biol. Macromol. 2020, 154, 329–338. [Google Scholar] [CrossRef]
- Kalia, S.; Avérous, L. Biopolymers: Biomedical and Environmental Applications; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Jain, S.; Tote, D.; Kolte, G.; Jajoo, S.; Tote, S. Effect of moist dressing, collagen sheet dressing and epidermal growth factor in healing of chronic wounds. Int. Surg. J. 2017, 4, 2594–2599. [Google Scholar] [CrossRef] [Green Version]
- Rahul, V.G.; Nair, P.D. Biomaterials and designs supporting cartilage regeneration. In Biomaterials and Nanotechnology for Tissue Engineering; Sethuraman, S., Krishnan, U.M., Subramanian, A., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 93–103. ISBN 9781498743747. [Google Scholar]
- Kasalkova, N.S.; Slepicka, P.; Kolska, Z.; Svorcik, Z.K.A.V. Wettability and other surface properties of modified polymers. Wetting Wettability 2015, 12, 323–356. [Google Scholar]
- Rey, C.; Combes, C. Physical chemistry of biological apatites. In Biomineralization and Biomaterials: Fundamentals and Applications; Aparicio, C., Ginebra, M.P., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 95–127. ISBN 9781782423560. [Google Scholar]
- Savoji, H.; Godau, B.; Hassani, M.S.; Akbari, M. Skin tissue substitutes and biomaterial risk assessment and testing. Front. Bioeng. Biotechnol. 2018, 6, 86. [Google Scholar] [CrossRef]
- Pahuja, M.P.; Arora, S.; Pawar, P. Ocular drug delivery system: A reference to natural polymers. Expert Opin. Drug Deliv. 2012, 9, 837–861. [Google Scholar] [CrossRef]
- Przybylski, J.E.; Siemaszko-Przybylska, K. Electrical Conductivity of Fish Skin Collagen in the Temperature Range 290–380 K. Polish Patent 190737, July 2002. [Google Scholar]
- Matsushita, S.; Deki, S. Anomalous behavior of electrical conductivity of solubilized collagen solutions with thermal denaturation. J. Appl. Polym. Sci. 2003, 50, 1969–1975. [Google Scholar] [CrossRef]
- Mitkowski, P.; Szaferski, W. Production of emulsion in tank mixer with sieve bottom. ChERD 2016, 109, 618–627. [Google Scholar] [CrossRef]
- The Cosmetics Act of 4.10.2018. Pol. J. Laws 2018, 11, 2227. Available online: https://www.cosmeticscare.eu/en/legal-provisions/ (accessed on 31 December 2021).
- Rudnicka, L.; Rakowska, A.; Kerzeja, M.; Olszewska, M. Hair shafts in trichoscopy: Clues for diagnosis of hair and scalp diseases. Dermatol. Clin. 2013, 31, 695–708. [Google Scholar] [CrossRef]
- Rudnicka, L.; Olszewska, M.; Rakowska, A.; Slowinska, M. Trichoscopy update. J. Dermatol. Case Rep. 2011, 5, 82–88. [Google Scholar] [PubMed]
- Phan, D.-N.; Khan, M.Q.; Nguyen, N.-T.; Phan, T.-T.; Ullah, A.; Khatri, M.; Kien, N.N.; Kim, I.-S. A review on the fabrication of several carbohydrate polymers into nanofibrous structures using electrospinning for removal of metal ions and dyes. Carbohydr. Polym. 2021, 252, 117175. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Zohra, R.R.; Qader, S.A.; Mumtaz, M. Scanning electron microscopy analysis of hair index on Karachi’s population for social and professional appearance enhancement. Int. J. Cosmet. Sci. 2015, 37, 312–320. [Google Scholar] [CrossRef]
- Harding, C.R. The stratum corneum: Structure and function in health and disease. Dermatol. Ther. 2004, 17, 6–15. [Google Scholar] [CrossRef]
- Del Rosso, J.Q.; Levin, J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J. Clin. Aesthet. Dermatol. 2011, 4, 22–42. [Google Scholar] [PubMed]
- Holmes, D.F.; Lu, Y.; Starborg, T.; Kadler, K.E. Collagen fibril assembly and function. In Current Topics in Developmental Biology; Litscher, E.S., Wassarman, P.M., Eds.; Academic Press Inc.: Cambridge, MA, USA, 2018; Volume 130, pp. 107–142. ISBN 978-0-12-809802-8. [Google Scholar]
- Sheehy, E.J.; Cunniffe, G.M.; O’Brien, F.J. Collagen-based biomaterials for tissue regeneration and repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Barbosa, M.A., Martins, M.C.L., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 127–150. ISBN 978-0-08-100803-4. [Google Scholar]
- Henriksen, K.; Karsdal, M.A. Type I collagen. In Biochemistry of Collagens, Laminins and Elastin: Structure, Function and Biomarkers; Karsdal, M.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–11. ISBN 9780128098998. [Google Scholar]
- Naomi, R.; Bahari, H.; Ridzuan, P.M.; Othman, F. Natural-Based Biomaterial for Skin Wound Healing (Gelatin vs. Collagen): Expert Review. Polymers 2021, 13, 2319. [Google Scholar] [CrossRef]
- Kubyshkin, V. Stabilization of the triple helix in collagen mimicking peptides. Org. Biomol. Chem. 2019, 17, 8031–8047. [Google Scholar] [CrossRef]
- Silvipriya, K.; Kumar, K.; Bhat, A.; Kumar, B.; John, A.; Lakshmanan, P. Collagen: Animal sources and biomedical application. J. Appl. Pharm. Sci. 2015, 5, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Jeng, W.M. Preparation of Biomaterials using Fish Collagen and Seaweed Alginate to Promote In Vivo Cell Growth and Proliferation Activity. Ph.D. Thesis, Universiti Tunku Abdul Rahman, Ipoh, Malaysia, 2019. [Google Scholar]
- Yamada, S.; Yamamoto, K.; Ikeda, T.; Yanagiguchi, K.; Hayashi, Y. Potency of fish collagen as a scaffold for regenerative medicine. BioMed Res. Int. 2014, 2014, 302932. [Google Scholar] [CrossRef]
- Law, J.X.; Liau, L.L.; Bin Saim, A.; Yang, Y.; Idrus, R. Electrospun collagen nanofibers and their applications in skin tissue engineering. Tissue Eng. Regen. Med. 2017, 14, 699–718. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.D.; Jeon, S.Y.; Ji, J.H.; Lee, W.S. Development of a classification system for extrinsic hair damage: Standard grading of electron microscopic findings of damaged hairs. Am. J. Dermatopathol. 2010, 32, 432–438. [Google Scholar] [CrossRef]
- Activoil Kerox Pro Release; Innovacos Corp: Mount Arlington, NJ, USA, 2018–2020.
- Igielska-Kalwat, J.; Kilian-Pięta, E. The Use of Dalmatian Tansy and Auxiliary Substances as Natural Substitutes in the Treatment of Dermatosis. SSRG 2021, 8, 1–9. [Google Scholar]
- Bouwstra, J.A.; Dubbelaar, F.E.R.; Gooris, G.S.; Weerheim, A.M.; Ponec, M. The Role of Ceramide Composition in the Lipid Organisation of the Skin Barrier. Biochim. Biophys. Acta 1999, 1419, 127–136. [Google Scholar] [CrossRef] [Green Version]









| Trade Name | INCI |
|---|---|
| Phase I | |
| Ceramide G and A | Grapeseed/avocado Oil Aminopropanediol Esters |
| Raspberry | Raspberry Seed Oil/Tocopherol Succinate Aminopropanediol Esters |
| Phase II | |
| Collagen | Collagen |
| Hyaluronic acid | Sodium Hyaluronate |
| Parfum | Srublet Bukiet |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Igielska-Kalwat, J.; Kilian-Pięta, E.; Połoczańska-Godek, S. The Use of Natural Collagen Obtained from Fish Waste in Hair Styling and Care. Polymers 2022, 14, 749. https://doi.org/10.3390/polym14040749
Igielska-Kalwat J, Kilian-Pięta E, Połoczańska-Godek S. The Use of Natural Collagen Obtained from Fish Waste in Hair Styling and Care. Polymers. 2022; 14(4):749. https://doi.org/10.3390/polym14040749
Chicago/Turabian StyleIgielska-Kalwat, Joanna, Ewa Kilian-Pięta, and Sława Połoczańska-Godek. 2022. "The Use of Natural Collagen Obtained from Fish Waste in Hair Styling and Care" Polymers 14, no. 4: 749. https://doi.org/10.3390/polym14040749