Recent Developments in Hyaluronic Acid-Based Hydrogels for Cartilage Tissue Engineering Applications
Abstract
:1. Introduction
2. Cartilage
2.1. Articular Cartilage
2.1.1. Composition
2.1.2. Structure: Zones and Regions
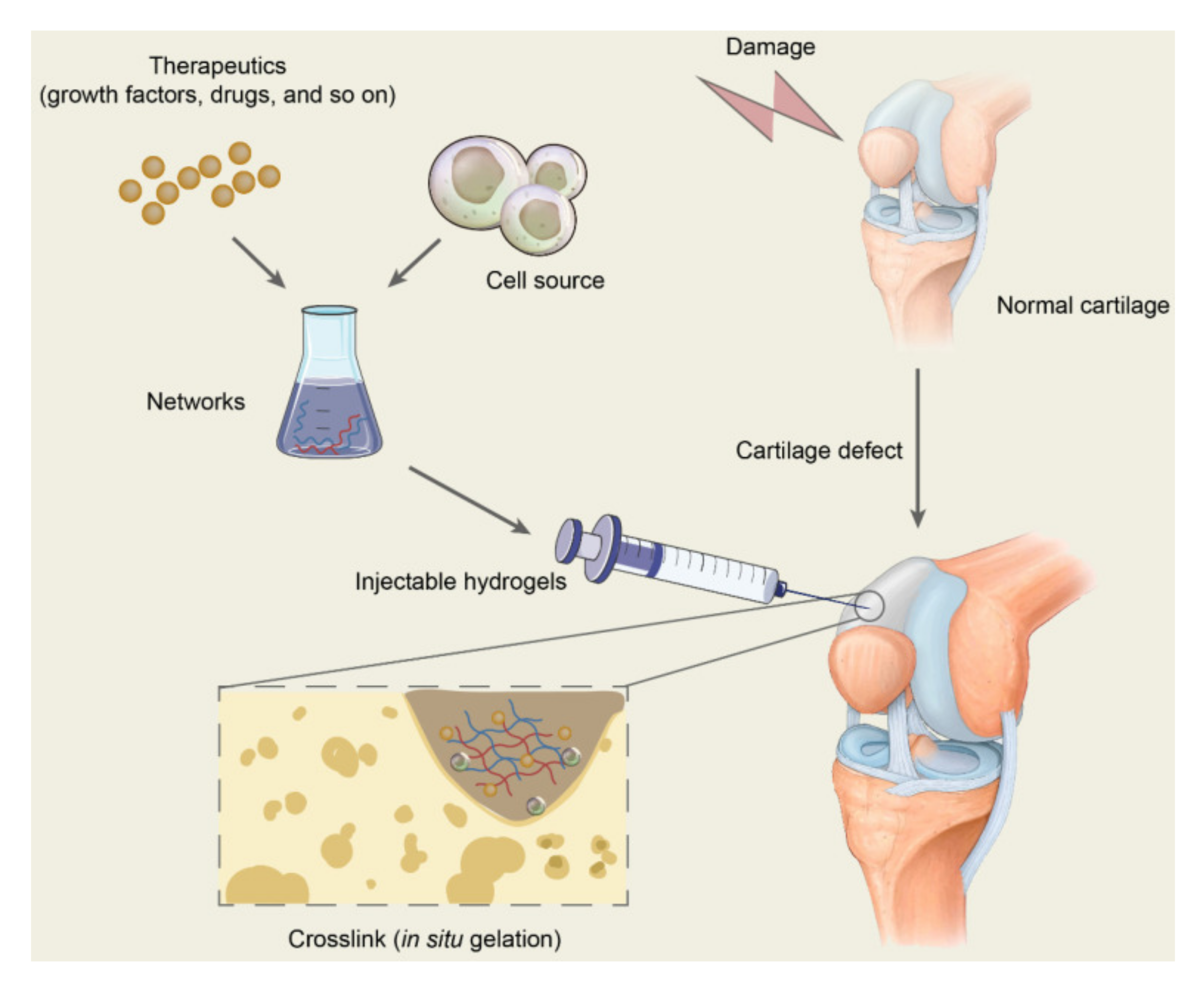
3. Cartilage Tissue Engineering
4. Hydrogels—Preclinical Evaluation
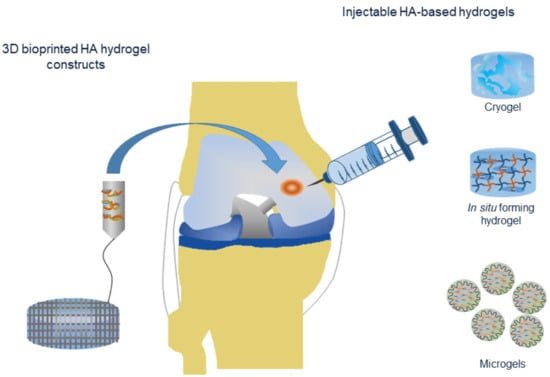
4.1. Injectable Hydrogels
4.1.1. In Situ Forming Hydrogels
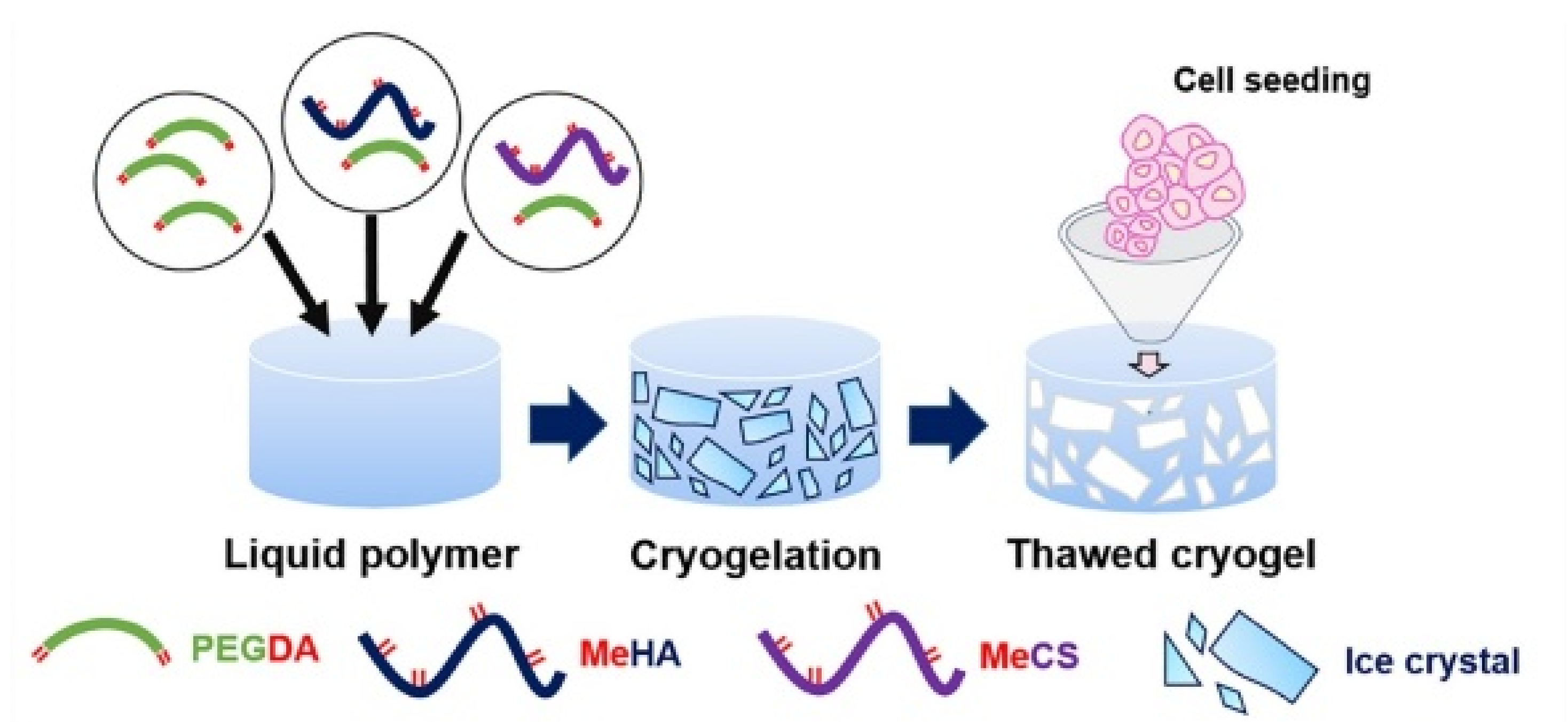
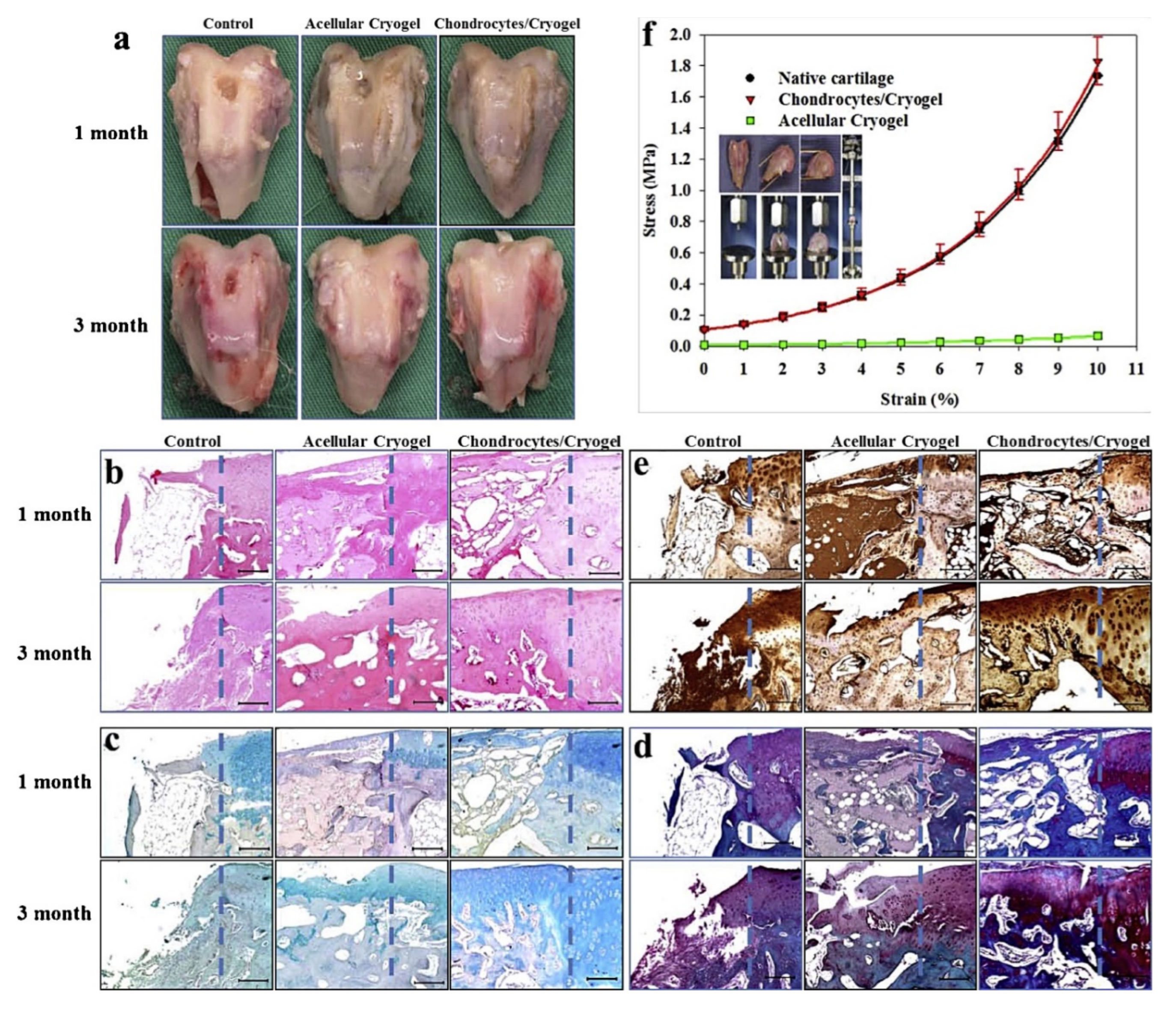
4.1.2. Cryogels
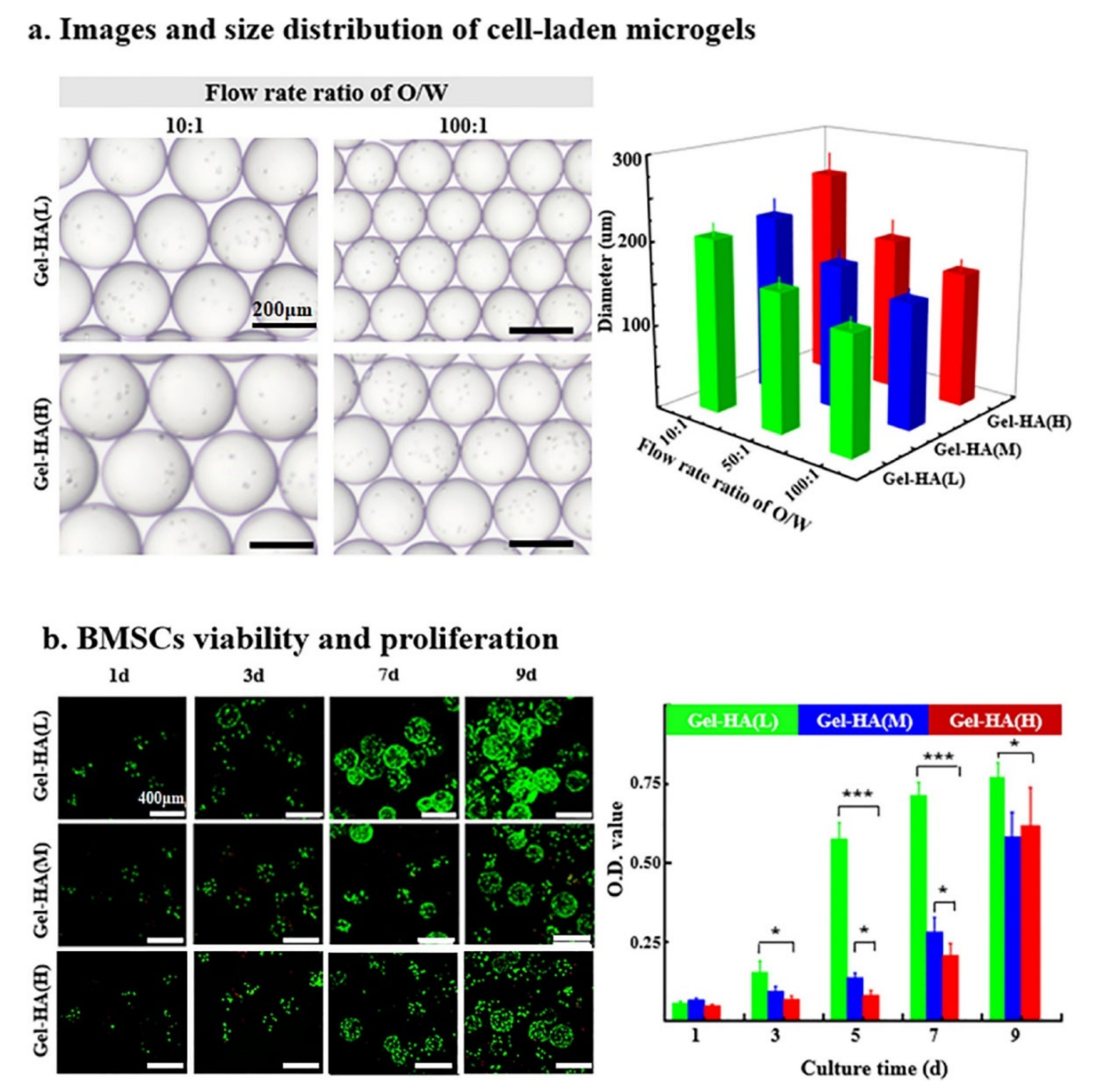
4.1.3. Microgels
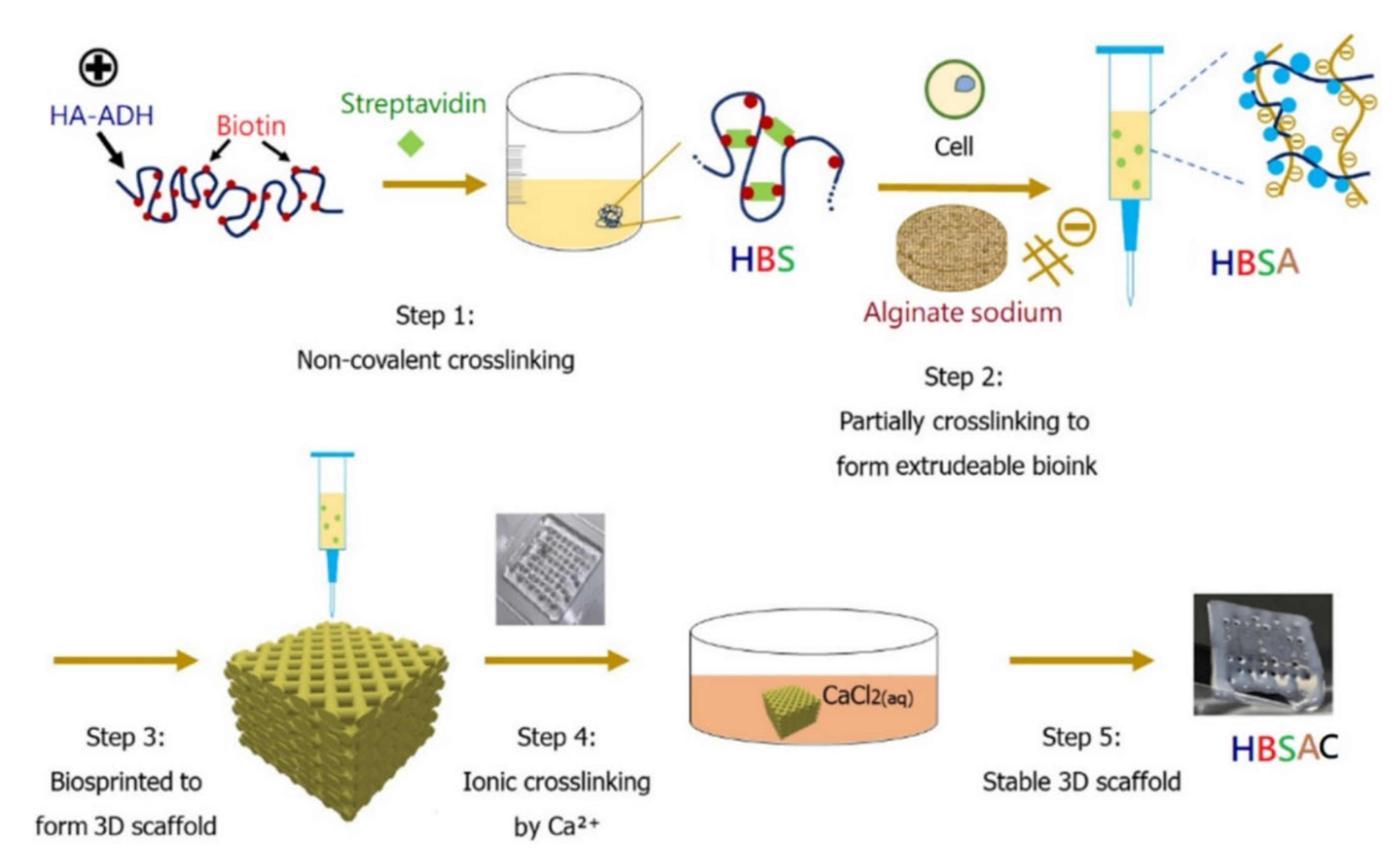
4.2. Three-Dimensional Bioprinted Hydrogel Constructs
5. Clinical Evaluation
6. Conclusions
7. Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Athanasiou, K.; Darling, E.M.; Hu, J.C. Articular cartilage tissue engineering. Synthesis Lectures on Tissue Engineering #3; Morgan & Claypool Publishers: San Rafael, CA, USA, 2010; ISBN 9781598298758. [Google Scholar]
- Tsanaktsidou, E.; Kammona, O.; Labude, N.; Neuss, S.; Krüger, M.; Kock, L.; Kiparissides, C. Biomimetic cell-laden MeHA hydrogels for the regeneration of cartilage tissue. Polymers 2020, 12, 1598. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef] [PubMed]
- Biant, L.C.; McNicholas, M.J. Current surgical options for the treatment of symptomatic articular cartilage lesions of the knee. Orthop. Trauma 2019, 33, 127–132. [Google Scholar] [CrossRef]
- Li, J.; Chen, G.; Xu, X.; Abdou, P.; Jiang, Q.; Shi, D.; Gu, Z. Advances of injectable hydrogel-based scaffolds for cartilage regeneration. Regen. Biomater. 2019, 6, 129–140. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhang, Y.S.; Yue, K.; Khademhosseini, A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017, 57, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Knutsen, G.; Richardson, J.B. A clinical review of cartilage repair techniques. J. Bone Jt. Surg. Br. Vol. 2005, 87, 445–449. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, L.-Q.; Nugraha, B.; Gao, Y.; Leo, H.L. Current hydrogel solutions for repairing and regeneration of complex tissues. Curr. Med. Chem. 2014, 21, 2480–2496. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.W.; Ackerman, G.; Dines, J.S.; Grande, D. Emerging technologies and fourth generation issues in cartilage repair. Sports Med. Arthrosc. 2008, 16, 246–254. [Google Scholar] [CrossRef]
- Tsanaktsidou, E.; Kammona, O.; Kiparissides, C. On the synthesis and characterization of biofunctional hyaluronic acid based injectable hydrogels for the repair of cartilage lesions. Eur. Polym. J. 2019, 114, 47–56. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Healthc. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- MEDIPOST—CARTISTEM®. Available online: https://www.medi-post.com/intro/ (accessed on 11 January 2022).
- Temenoff, J.S.; Mikos, A.G. Review: Tissue engineering for regeneration of articular cartilage. Biomaterials 2000, 21, 431–440. [Google Scholar] [CrossRef]
- Parvizi, J. High Yield Orthopaedics, 1st ed.; Saunders Elsevier: Philadelphia, PA, USA, 2010; pp. 40–41, 80–81. ISBN 978-1-4160-0236-9. [Google Scholar]
- Chang, L.R.; Marston, G.; Martin, A. Anatomy, Cartilage; StatPearls Publishing: Treasure Island, FL, USA, 2021; Bookshelf ID: NBK532964. [Google Scholar] [PubMed]
- Zhang, L.; Hu, J.; Athanasiou, K.A. The role of tissue engineering in articular cartilage repair and regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1–57. [Google Scholar] [CrossRef]
- Bhosale, A.M.; Richardson, J.B. Articular cartilage: Structure, injuries and review of management. Br. Med. Bull. 2008, 87, 77–95. [Google Scholar] [CrossRef]
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- James, C.-B.; Uhl, T.L. A review of articular cartilage pathology and the use of glucosamine sulfate. J. Athl. Train. 2001, 36, 413–419. [Google Scholar] [PubMed]
- Petty, R.E.; Laxer, R.M.; Lindsley, C.B.; Wedderbum, L.R. Textbook of Pediatric Rheumatology, 7th ed.; Elsevier: Philadelphia, PA, USA, 2016; ISBN 978-0-323-24145-8. [Google Scholar]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Ngadimin, K.D.; Stokes, A.; Gentile, P.; Ferreira, A.M. Biomimetic hydrogels designed for cartilage tissue engineering. Biomater. Sci. 2021, 9, 4246. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Burdick, J.A. Engineering cartilage tissue. Adv. Drug Deliv. Rev. 2008, 60, 243–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, W.; Li, M.; Yang, Y.; Wan, Y.; Wang, X.; Bi, N.; Li, C. Advancements and frontiers in the high performance of natural hydrogels for cartilage tissue engineering. Front. Chem. 2020, 8, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Del Bakhhayesh, A.R.; Asadi, N.; Alihemmati, A.; Nasrabadi, H.T.; Montaseri, A.; Davaran, S.; Saghati, S.; Akbarzadeh, A.; Abedelah, A. An overview of advanced biocompatible and biomimetic materials for creation of replacement structures in the musculoskeletal systems: Focusing on cartilage tissue engineering. J. Biol. Eng. 2019, 13, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 307–3329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega, S.L.; Kwon, M.Y.; Burdick, J.A. Recent advances in hydrogels for cartilage tissue engineering. Eur. Cells Mater. 2018, 33, 59–75. [Google Scholar] [CrossRef]
- Zhao, W.; Jin, X.; Cong, Y.; Liu, Y.; Fu, J. Degradable natural polymer hydrogels for articular cartilage tissue engineering. J. Chem. Technol. Biotechnol. 2013, 88, 327–339. [Google Scholar] [CrossRef]
- Li, H.; Qi, Z.; Zheng, S.; Chang, Y.; Kong, W.; Fu, C.; Yu, Z.; Yang, X.; Pan, S. The Application of Hyaluronic Acid-Based Hydrogels in Bone and Cartilage Tissue Engineering. Adv. Mater. Sci. Eng. 2019, 2019, 3027303. [Google Scholar] [CrossRef] [Green Version]
- Lam, J.; Truong, N.F.; Segura, T. Design of cell–matrix interactions in hyaluronic acid hydrogel scaffolds. Acta Biomater. 2014, 10, 1571–1580. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Ma, Y.; Yao, X.; Zhou, W.; Wang, X.; Li, C.; Lin, J.; He, Q.; Leptihn, S.; Quyang, H. Advanced hydrogels for the repair of cartilage defects and regeneration. Bioact. Mater. 2021, 6, 998–1011. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Luo, Z.; Li, D.; Lu, J.; Wang, Q.; Xiao, Y.; Zhang, X. Development of an injectable thiolated icariin functionalized collagen/hyaluronic hydrogel to promote cartilage formation in vitro and in vivo. J. Mater. Chem. B 2019, 7, 2845–2854. [Google Scholar] [CrossRef] [PubMed]
- Mellati, A.; Akhtari, J. Injectable hydrogels: A review of injectability mechanisms and biomedical applications. Res. Mol. Med. (RMM) 2018, 6, 1–19. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Mariatti, M.; Abdul Hamid, Z.A.; Nurul, A.A.; Teramoto, N. Injectable hydrogel scaffold from natural biomaterials—An overview of recent studies. AIP Conf. Proc. 2020, 2267, 020068. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Q.; Deng, C.; Xu, B.; Zhang, Z.; Yang, Y.; Lu, T. Exquisite design of injectable hydrogels in cartilage repair. Theranostics 2020, 10, 9843–9864. [Google Scholar] [CrossRef]
- Chircov, C.; Grumezescu, A.M.; Bejenaru, L.E. Hyaluronic acid-based scaffolds for tissue engineering. Rom. J. Morphol. Embryol. 2018, 59, 71–76. [Google Scholar]
- Jeznach, O.; Kołbuk, D.; Sajkiewicz, P. Injectable hydrogels and nanocomposite hydrogels for cartilage regeneration. J. Biomed. Mater. Res. A 2018, 106A, 2762–2776. [Google Scholar] [CrossRef] [PubMed]
- Menaa, F.; Menaa, A.; Menaa, B. Hyaluronic acid and derivatives for tissue engineering. J. Biotechnol. Biomater. 2011, S3, 001. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Cao, Y.; Zhao, H.; Zhang, L.; Ni, T.; Liu, Y.; An, Z.; Liu, M.; Pei, R. An injectable BMSC-laden enzyme-catalyzed crosslinking collagen-hyaluronic acid hydrogel for cartilage repair and regeneration. J. Mater. Chem. B 2020, 8, 4237. [Google Scholar] [CrossRef]
- Wang, G.; Cao, X.; Dong, H.; Zeng, L.; Yu, C.; Chen, X. A hyaluronic acid based injectable hydrogel formed via photo-crosslinking reaction and thermal-induced Diels-Alder reaction for cartilage tissue engineering. Polymers 2018, 10, 949. [Google Scholar] [CrossRef] [Green Version]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascual-Garrido, C.; Rodriguez-Fontan, F.; Aisenbrey, E.A.; Payne, K.A.; Chahla, J.; Goodrich, L.R.; Bryant, S.J. Current and novel injectable hydrogels to treat focal chondral lesions: Properties and applicability. J. Orthop. Res. 2018, 36, 64–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eslahi, N.; Abdorahim, M.; Simchi, A.A. Smart polymeric hydrogels for cartilage tissue engineering: A review on the chemistry and biological functions. Biomacromolecules 2016, 17, 3441–3463. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.A.; Nair, L.S. Injectable hydrogels for bone and cartilage repair. Biomed. Mater. 2012, 7, 024105. [Google Scholar] [CrossRef]
- Piantanida, E.; Alonci, G.; Bertucci, A.; De Cola, L. Design of nanocomposite injectable hydrogels for minimally invasive surgery. Acc. Chem. Res. 2019, 52, 2101–2112. [Google Scholar] [CrossRef]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic acid: Molecular mechanisms and therapeutic trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr. Opin. Biotechnol. 2016, 40, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Tsao, C.T.; Kyomoto, M.; Zhang, M. Injectable natural polymer hydrogels for treatment of knee osteoarthritis. Adv. Healthcare Mater. 2021, 2101479. [Google Scholar] [CrossRef]
- Levinson, C.; Lee, M.; Applegate, L.A.; Zenobi-Wong, M. An injectable heparin-conjugated hyaluronan scaffold for local delivery of transforming growth factor β1 promotes successful chondrogenesis. Acta Biomater. 2019, 99, 168–180. [Google Scholar] [CrossRef]
- Nguyen, K.T.; West, J.L. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef]
- Jooybar, E.; Abdekhodaie, M.J.; Alvi, M.; Mousavi, A.; Karperien, M.; Dijkstra, P.J. An injectable platelet lysate-hyaluronic acid hydrogel supports cellular activities and induces chondrogenesis of encapsulated mesenchymal stem cells. Acta Biomater. 2019, 83, 233–244. [Google Scholar] [CrossRef] [Green Version]
- Thomas, L.V.; Prabha, R.V.G.; Nair, D. Effect of stiffness of chitosan-hyaluronic acid dialdehyde hydrogels on the viability and growth of encapsulated chondrocytes. Int. J. Biol. Macromol. 2017, 104(Pt. B), 1925–1935. [Google Scholar] [CrossRef]
- Chen, Y.; Sui, J.; Wang, Q.; Yin, Y.; Liu, J.; Wang, Q.; Han, X.; Sun, Y.; Fan, Y.; Zhang, X. Injectable self-crosslinking HA-SH/Col I blend hydrogels for in vitro construction of engineered cartilage. Carbohydr. Polym. 2018, 190, 57–66. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Wang, L.; Zhang, X.; Heng, B.C.; Wang, D.-A.; Ge, Z. Modified hyaluronic acid hydrogels with chemical groups that facilitate adhesion to host tissues enhance cartilage regeneration. Bioact. Mater. 2021, 6, 1689–1698. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Song, J.; Li, X.; Zhang, X.; Zhou, Z.; Chen, D.; Ma, P.X.; Peng, W.; Wang, W.; et al. Cartilage regeneration using arthroscopic flushing fluid-derived mesenchymal stem cells encapsulated in a one-step rapid cross-linked hydrogel. Acta Biomater. 2018, 79, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Koh, R.H.; Kim, S.-H.; Kim, K.M.; Park, G.K.; Hwang, N.S. Injectable anti-inflammatory hyaluronic acid hydrogel for osteoarthritic cartilage repair. Mater. Sci. Eng. C 2020, 115, 111096. [Google Scholar] [CrossRef]
- Lin, H.; Beck, A.M.; Shimomura, K.; Sohn, J.; Fritch, M.R.; Deng, Y.; Kilroy, E.J.; Tang, Y.; Alexander, P.G.; Tuan, R.S. Optimization of photocrosslinked gelatin/hyaluronic acid hybrid scaffold for the repair of cartilage defect. J. Tissue Eng. Regen. Med. 2019, 13, 1418–1429. [Google Scholar] [CrossRef]
- Vaca-González, J.J.; Clara-Trujillo, S.; Guillot-Ferriols, M.; Ródenas-Rochina, J.; Sanchis, M.J.; Ribelles, J.J.G.; Garzón-Alvarado, D.A.; Ferrer, G.G. Effect of electrical stimulation on chondrogenic differentiation of mesenchymal stem cells cultured in hyaluronic acid-Gelatin injectable hydrogels. Bioelectrochemistry 2020, 134, 107536. [Google Scholar] [CrossRef]
- Cavalli, E.; Levinson, C.; Hertl, M.; Broguiere, N.; Brück, O.; Mustjoki, S.; Gerstenberg, A.; Weber, D.; Salzmann, G.; Steiwachs, M.; et al. Characterization of polydactyly chondrocytes and their use in cartilage engineering. Sci. Rep. 2019, 9, 4275. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.Z.; Eswaramoorthy, R.; Lin, T.H.; Chen, C.H.; Fu, Y.C.; Wang, C.K.; Wu, S.C.; Wang, G.J.; Chang, J.K.; Ho, M.-L. Enhancement of chondrogenesis of adipose-derived stem cells in HA-PNIPAAm-CL hydrogel for cartilage regeneration in rabbits. Sci. Rep. 2018, 8, 10526. [Google Scholar] [CrossRef] [Green Version]
- Lynch, B.; Crawford, K.; Baruti, O.; Abdulahad, A.; Webster, M.; Puetzer, J.; Ryu, C.; Bonassar, L.J.; Mendenhall, J. The effect of hypoxia on thermosensitive poly(N-Vinylcaprolactam) hydrogels with tunable mechanical integrity for cartilage tissue engineering. J. Biomed. Mater. Res. Part B 2016, 105, 1863–1873. [Google Scholar] [CrossRef]
- Toh, W.S.; Lim, T.C.; Kurisawa, M.; Spector, M. Modulation of mesenchymal stem cell chondrogenesis in a tunable hyaluronic acid hydrogel microenvironment. Biomaterials 2012, 33, 3835–3845. [Google Scholar] [CrossRef]
- Feng, Q.; Zhu, M.; Wei, K.; Bian, L. Cell-mediated degradation regulates human mesenchymal stem cell chondrogenesis and hypertrophy in MMP-sensitive hyaluronic acid hydrogels. PLoS ONE 2014, 9, e99587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Liu, D.; Wang, Y.; Li, Y.; Li, T.; Zhou, Z.; Yang, Z.; Wang, J.; Zhang, Q. Glycol chitosan/oxidized hyaluronic acid hydrogels functionalized with cartilage extracellular matrix particles and incorporating BMSCs for cartilage repair. Artif. Cells Nanomed. Biotechnol. 2018, 46, S721–S732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, N.; Mohanan, P.V.; Sabareeswaan, A.; Nair, P. Chitosan-hyaluronic acid hydrogel for cartilage repair. Int. J. Biol. Macromol. 2017, 104, 1936–1945. [Google Scholar] [CrossRef]
- Kim, D.Y.; Park, H.; Kim, S.W.; Lee, J.W.; Lee, K.Y. Injectable hydrogels prepared from partially oxidized hyaluronate and glycol chitosan for chondrocyte encapsulation. Carbohydr. Polym. 2017, 157, 1281–1287. [Google Scholar] [CrossRef]
- Pahoff, S.; Meinert, C.; Bas, O.; Nguyen, L.; Klein, T.J.; Hutmacher, D.W. Effect of gelatin source and photoinitiator type on chondrocyte redifferentiation in gelatin methacryloyl-based tissue-engineered cartilage constructs. J. Mater. Chem. B 2019, 7, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.Y.; Vega, S.L.; Gramlich, W.M.; Kim, M.; Mauck, R.L.; Burdick, J.A. Dose and timing of N-Cadherin mimetic peptides regulate MSC chondrogenesis within hydrogels. Adv. Healthc. Mater. 2018, 7, 1701199. [Google Scholar] [CrossRef]
- Shirzaei Sani, E.; Portillo-Lara, R.; Spencer, A.; Yu, W.; Geilich, B.M.; Noshadi, I.; Webster, T.J.; Annabi, N. Engineering adhesive and antimicrobial hyaluronic acid/elastin-like polypeptide hybrid hydrogels for tissue engineering applications. ACS Biomater. Sci. Eng. 2018, 4, 2528–2540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, E.C.; Barragan, M.; Libeer, T.B.; Kieweg, S.L.; Converse, G.L.; Hopkins, R.A.; Berkland, C.J.; Detamore, M.S. Chondroinduction from naturally derived cartilage matrix: A comparison between devitalized and decellularized cartilage encapsulated in hydrogel pastes. Tissue Eng. Part A 2016, 22, 665–679. [Google Scholar] [CrossRef] [Green Version]
- Fisher, M.B.; Belkin, N.S.; Milby, A.H.; Henning, E.A.; Söegaard, N.; Kim, M.; Pfeifer, C.; Saxena, V.; Dodge, G.R.; Burdick, J.A.; et al. Effects of mesenchymal stem and growth factor delivery on cartilage repair in a mini-pig model. Cartilage 2016, 7, 174–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Feng, Q.; Sun, Y.; Li, G.; Bian, L. Effect of cartilaginous matrix components on the chondrogenesis and hypertrophy of mesenchymal stem cells in hyaluronic acid hydrogels. J. Biomed. Mater. Res. Part B 2017, 105, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Feng, Q.; Bian, L. Differential effect of hypoxia on human mesenchymal stem cell chondrogenesis and hypertrophy in hyaluronic acid hydrogels. Acta Biomater. 2014, 10, 1333–1340. [Google Scholar] [CrossRef]
- Snyder, T.N.; Madhavan, K.; Intrator, M.; Dregalla, R.C.; Park, D. A fibrin/hyaluronic acid hydrogel for the delivery of mesenchymal stem cells and potential for articular cartilage repair. J. Biol. Eng. 2014, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Levett, P.A.; Melchels, F.P.W.; Schrobback, K.; Hutmacher, D.W.; Malda, J.; Klein, T.J. A biomimetic extracellular matrix for cartilage tissue engineering centered on photocurable gelatin, hyaluronic acid and chondroitin sulfate. Acta Biomater. 2014, 10, 214–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, L.; Zhai, D.Y.; Mauck, R.L.; Burdick, J.A. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng. Part A 2011, 17, 1137–1145. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Liu, Q.; Kong, W.; Wang, J.; He, L.; Guo, L.; Lin, H.; Fan, H.; Fan, Y.; Zhang, X. Activated hyaluronic acid/collagen composite hydrogel with tunable physical properties and improved biological properties. Int. J. Biol. Macromol. 2020, 164, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Xu, Y.; Chen, M.; Lu, Y.; Liang, J.; Sun, Y.; Fan, Y.; Zhang, X. Effects of the bonding intensity between hyaluronan and gelatin on chondrogenic maintenance. J. Mater. Chem. B 2020, 8, 9062–9074. [Google Scholar] [CrossRef]
- Chen, F.; Ni, Y.; Liu, B.; Zhou, T.; Yu, C.; Su, Y.; Zhu, X.; Yu, X.; Zhou, Y. Self-crosslinking and injectable hyaluronic acid/RGD-functionalized pectin hydrogel for cartilage tissue engineering. Carbohydr. Polym. 2017, 166, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Shariatzadeh, F.J.; Solouk, A.; Khoulenjani, S.B.; Bonakdar, S.; Mirzadeh, H. Injectable and reversible preformed cryogels based on chemically crosslinked gelatin methacrylate (GelMA) and physically crosslinked hyaluronic acid (HA) for soft tissue engineering. Colloids Surf. B Biointerfaces 2021, 203, 111725. [Google Scholar] [CrossRef]
- He, T.; Li, B.; Colombani, T.; Joshi-Navare, K.; Mehta, S.; Kisiday, J.; Bencherif, S.A.; Bajpayee, A.G. Hyaluronic acid-based shape-memory cryogel scaffolds for focal cartilage defect repair. Tissue Eng. Part A 2021, 27, 748–760. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Chen, C.-H.; Hsiao, C.-Y.; Chen, J.-P. Incorporation of chitosan in biomimetic gelatin/chondroitin-6-sulfate/hyaluronan cryogel for cartilage tissue engineering. Carbohydr. Polym. 2015, 117, 722–730. [Google Scholar] [CrossRef]
- Han, M.-E.; Kim, S.-H.; Kim, H.D.; Yim, H.-G.; Bencherif, S.A.; Kim, T.-I.; Hwang, N.S. Extracellular matrix-based cryogels for cartilage tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1410–1419. [Google Scholar] [CrossRef]
- Fan, C.; Ling, Y.; Deng, W.; Xue, J.; Sun, P.; Wang, D.-A. A novel cell encapsulatable cryogel (CECG) with macro-porous structures and high permeability: A three-dimensional cell culture scaffold for enhanced cell adhesion and proliferation. Biomed. Mater. 2019, 14, 055006. [Google Scholar] [CrossRef]
- Chen, C.-H.; Kuo, C.-Y.; Wang, Y.-J.; Chen, J.-P. Dual function of glucosamine in gelatin/hyaluronic acid cryogel to modulate scaffold mechanical properties and to maintain chondrogenic phenotype for cartilage tissue engineering. Int. J. Mol. Sci. 2016, 17, 1957. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Gao, H.; Wen, H.; Huang, H.; Li, Q.; Liang, M.; Liu, Y.; Dong, H.; Cao, X. Engineering the cellular mechanical microenvironment to regulate stem cell chondrogenesis: Insights from a microgel model. Acta Biomater. 2020, 113, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Akkiraju, H.; Srinivasan, P.P.; Xu, X.; Jia, X.; Safran, C.B.K.; Nohe, A. CK2.1, a bone morphogenetic protein receptor type Ia mimetic peptide, repairs cartilage in mice with destabilized medial meniscus. Stem Cell Res. Ther. 2017, 8, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Jha, A.K.; Duncan, R.L.; Jia, X. Heparin-decorated, hyaluronic acid-based hydrogel particles for the controlled release of bone morphogenetic protein 2. Acta Biomater. 2011, 7, 3050–3059. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yu, F.; Shi, J.; Shen, S.; Teng, H.; Yang, J.; Wang, X.; Jiang, Q. In situ repair of bone and cartilage defects using 3D scanning and 3D printing. Sci. Rep. 2017, 7, 9416. [Google Scholar] [CrossRef] [Green Version]
- Dhawan, A.; Kennedy, P.M.; Rizk, E.B.; Ozbolat, I.T. Three-dimensional Bioprinting for bone and cartilage restoration in orthopaedic surgery. J. Am. Acad. Orthop. Surg. 2019, 27, e215–e226. [Google Scholar] [CrossRef]
- Antich, C.; de Vicente, J.; Jiménez, G.; Chocarro, C.; Carrillo, E.; Montañez, E.; Gálvez-Martín, P.; Marchal, J.A. Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater. 2020, 106, 114–123. [Google Scholar] [CrossRef]
- Hauptstein, J.; Böck, T.; Bartolf-Kopp, M.; Forster, L.; Stahlhut, P.; Nadernezhad, A.; Blahetek, G.; Zernecke-Madsen, A.; Detsch, R.; Jüngst, T.; et al. Hyaluronic acid-based bioink composition enabling 3D bioprinting and improving quality of deposited cartilaginous extracellular matrix. Adv. Healthc. Mater. 2020, 9, 2000737. [Google Scholar] [CrossRef] [PubMed]
- Schwab, A.; Hélary, C.; Richards, R.G.; Alini, M.; Eglin, D.; D’Este, M. Tissue mimetic hyaluronan bioink containing collagen fibers with controlled orientation modulating cell migration and alignment. Mater. Today Bio 2020, 7, 100058. [Google Scholar] [CrossRef]
- Abdulghani, S.; Morouço, P.G. Biofabrication for osteochondral tissue regeneration: Bioink printability requirements. J. Mater. Sci. Mater. Med. 2019, 30, 20. [Google Scholar] [CrossRef]
- Costantini, M.; Idaszek, J.; Szöke, K.; Jaroszewicz, J.; Dentini, M.; Barbetta, A.; Brinchmann, J.E.; Święszkowski, W. 3D bioprinting of BM-MSCs-loaded ECM biomimetic hydrogels for in vitro neocartilage formation. Biofabrication 2016, 8, 035002. [Google Scholar] [CrossRef] [PubMed]
- Mouser, V.H.M.; Levato, R.; Mensinga, A.; Dhert, W.J.A.; Gawlitta, D.; Malda, J. Bio-ink development for three-dimensional bioprinting of hetero-cellular cartilage constructs. Connect. Tissue Res. 2020, 61, 137–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-based 3D bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl. Mater. Today 2020, 18, 100479. [Google Scholar] [CrossRef] [PubMed]
- Nedunchezian, S.; Banerjee, P.; Lee, C.-Y.; Lee, S.-S.; Lin, C.-W.; Wu, C.-W.; Wu, S.-C.; Chang, J.-K.; Wang, C.-K. Generating adipose stem cell-laden hyaluronic acid-based scaffolds using 3D bioprinting via the double crosslinked strategy for chondrogenesis. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 124, 112072. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.C.; Critchley, S.E.; Rencsok, E.M.; Kelly, D.J. A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage. Biofabrication 2016, 8, 045002. [Google Scholar] [CrossRef]
- Stichler, S.; Böck, T.; Paxton, N.; Bertlein, S.; Levato, R.; Schill, V.; Smolan, W.; Malda, J.; Teßmar, J.; Blunk, T.; et al. Double printing of hyaluronic acid/poly(glycidol) hybrid hydrogels with poly(ε-caprolactone) for MSC chondrogenesis. Biofabrication 2017, 9, 044108. [Google Scholar] [CrossRef]
- Ma, K.; Zhao, T.; Yang, L.; Wang, P.; Jin, J.; Teng, H.; Xia, D.; Zhu, L.; Li, L.; Jiang, Q.; et al. Application of robotic-assisted in situ 3D printing in cartilage regeneration with HAMA hydrogel: An in vivo study. J. Adv. Res. 2020, 23, 123–132. [Google Scholar] [CrossRef]
- Park, Y.-B.; Ha, C.-W.; Lee, C.-H.; Yoon, Y.C.; Park, Y.-G. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: Results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl. Med. 2017, 6, 613–621. [Google Scholar] [CrossRef]
- NCT01733186. 2012. Available online: https://clinicaltrials.gov/ct2/show/NCT01733186 (accessed on 17 December 2021).
- Lim, H.-C.; Park, Y.-B.; Ha, C.-W.; Cole, B.J.; Lee, B.-K.; Jeong, H.-J.; Kim, M.-K.; Bin, S.-I.; Choi, C.-H.; Choi, C.H.; et al. Allogeneic umbilical cord blood–derived mesenchymal stem cell implantation versus microfracture for large, full-thickness cartilage defects in older patients. A multicenter randomized clinical trial and extended 5-Year clinical follow-up. Orthop. J. Sports Med. 2021, 9, 2325967120973052. [Google Scholar] [CrossRef]
- Petrella, R.J.; Emans, P.J.; Alleyne, J.; Dellaert, F.; Gill, D.P.; Maroney, M. Safety and performance of Hydros and Hydros-TA for knee osteoarthritis: A prospective, multicenter, randomized, double-blind feasibility trial. BMC Musculoskelet. Disord. 2015, 16, 57. [Google Scholar] [CrossRef] [Green Version]
- Strand, V.; Baraf, H.S.B.; Lavin, P.T.; Lim, S.; Hosokawa, H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthr. Cartil. 2012, 20, 350–356. [Google Scholar] [CrossRef] [Green Version]
- Strand, V.; Baraf, H.S.B.; Lavin, P.T.; Lim, S.; Hosokawa, H. Effectiveness and safety of a multicenter extension and retreatment trial of Gel-200 in patients with knee osteoarthritis. Cartilage 2012, 3, 297–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takamura, J.; Seo, T.; Strand, V. A pooled analysis of two multicenter, randomized controlled trials of a single intra-articular injection of Gel-200 for treatment of osteoarthritis of the knee, clinical medicine insights. Arthritis Musculoskelet. Disord. 2018, 11, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takamura, J.; Seo, T.; Strand, V. A single intra-articular injection of Gel-200 for treatment of symptomatic osteoarthritis of the knee is more effective than phosphate buffered saline at 6 months: A subgroup analysis of a multicenter, randomized controlled trial. Cartilage 2019, 10, 417–422. [Google Scholar] [CrossRef]
- Sun, S.-F.; Hsu, C.-W.; Lin, H.-S.; Liou, I.-H.; Chen, Y.-H.; Hung, C.-L. Comparison of single intra-articular injection of novel hyaluronan (HYA-JOINT Plus) with Synvisc-One for knee osteoarthritis: A randomized, controlled, double-blind trial of efficacy and safety. J. Bone Jt. Surg. 2017, 99, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Housman, L.; Arden, N.; Schnitzer, T.J.; Birbara, C.; Conrozier, T.; Skrepnik, N.; Wei, N.; Bockow, B.; Waddell, D.; Tahir, H.; et al. Intra-articular hylastan versus steroid for knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Hangody, L.; Szody, R.; Lukasik, P.; Zgadzaj, W.; Lénárt, E.; Dokoupilova, E.; Bichovsk, D.; Berta, A.; Vasarhelyi, G.; Ficzere, A.; et al. Intraarticular injection of a cross-linked sodium hyaluronate combined with triamcinolone hexacetonide (Cingal) to provide symptomatic relief of osteoarthritis of the knee: A randomized, double-blind, placebo-controlled multicenter clinical trial. Cartilage 2018, 9, 276–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petterson, S.C.; Plancher, K.D. Single intra-articular injection of lightly cross-linked hyaluronic acid reduces knee pain in symptomatic knee osteoarthritis: A multicenter, double-blind, randomized, placebo-controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 1992–2002. [Google Scholar] [CrossRef]
- Arden, N.K.; Åkermark, C.; Andersson, M.; Todman, M.G.; Altman, R.D. A randomized saline-controlled trial of NASHA hyaluronic acid for knee osteoarthritis. Curr. Med. Res. Opin. 2014, 30, 279–286. [Google Scholar] [CrossRef]
- Leighton, R.; Åkermark, C.; Therrien, R.; Richardson, J.B.; Andersson, M.; Todman, M.G.; Arden, N.K. on behalf of the DUROLANE Study Group. NASHA hyaluronic acid vs methylprednisolone for knee osteoarthritis: A prospective, multi-centre, randomized, non-inferiority trial. Osteoarthr. Cartil. 2014, 22, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhang, K.; Zhang, X.; Zhu, Z.; Yan, S.; Sun, T.; Guo, A.; Jones, J.; Steen, R.G.; Shan, B.; et al. Comparison of two hyaluronic acid formulations for safety and efficacy (CHASE) study in knee osteoarthritis: A multicenter, randomized, double-blind, 26-week non-inferiority trial comparing Durolane to Artz. Arthritis Res. Ther. 2015, 17, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, C.-W.; Park, Y.-B.; Choi, C.-H.; Kyung, H.-S.; Lee, J.-H.; Yoo, J.D.; Yoo, J.-H.; Choi, C.-H.; Kim, C.-W.; Kim, H.-C.; et al. Efficacy and safety of single injection of cross-linked sodium hyaluronate vs. three injections of high molecular weight sodium hyaluronate for osteoarthritis of the knee: A double-blind, randomized, multi-center, non-inferiority study. BMC Musculoskelet. Disord. 2017, 18, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Kim, Y.N.; Lee, Y.J.; Sim, S.E.; Ko, Y.R.; Shim, J.W.; Lee, K.S.; Joo, M.; Park, H.J. Pilot study to evaluate the efficacy of polynucleotide sodium compared to sodium hyaluronate and crosslinked sodium hyaluronate in patients with knee osteoarthritis. J. Clin. Med. 2021, 10, 1138. [Google Scholar] [CrossRef] [PubMed]
- Strand, V.; Lim, S.; Takamura, J. Evidence for safety of retreatment with a single intra-articular injection of Gel-200 for treatment of osteoarthritis of the knee from the double-blind pivotal and open-label retreatment clinical trials. BMC Musculoskelet. Disord. 2016, 17, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NCT03561779. 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT03561779 (accessed on 17 December 2021).




| Material | Water Solubility | Electrostatic Charge | Functional Group | Cross-Linking Type | Advantages | Disadvantages |
|---|---|---|---|---|---|---|
| Polysaccharides | ||||||
| Hyaluronic Acid | Soluble | Negative | -COOH, -OH, -CH3CO | Ionic, chemical |
|
|
| Chondroitin sulfate | Soluble | Negative | -COOH, -OH | Ionic, chemical |
|
|
| Chitosan | Insoluble; soluble in acetic acid (pH < 4) | Positive at pH < 5.8 | -NH2, -OH | Ionic, chemical |
|
|
| Alginate | Soluble | Negative | -COOH, -OH | Ionic, chemical |
|
|
| Agarose | Soluble in hot water | Neutral | -OH | Temperature-dependent |
|
|
| Proteins | ||||||
| Collagen | Soluble | Neutral | -COOH, -NH2, -OH | Physical, ionic, chemical |
|
|
| Gelatin | Soluble | Neutral | -COOH, -NH2, -OH | Ionic, chemical |
|
|
| Silk fibroin | Soluble | Neutral | -COOH, -NH2 | Sol–gel transition |
|
|
| Fibrin | Soluble | Neutral | Assembly of polypeptides into fibrin via thrombin-mediated cleavage of fibrinogen |
|
| |
| Material | HA a MW b (KDa)/ Functional Groups | DM (%) c/ Functionalization | Cross-Linking Reaction/Cross-Linker/Gelation Onset (s) | Bioactive Agent/Stimulation/Extra | Cell Type/Cell Number per mL | Outcome |
|---|---|---|---|---|---|---|
| Redox/enzymatic reaction | ||||||
| HTG d [57] | -/COOH | 13.38/- | Enzymatic/tyrosinase/108–132 | EGCG e/- | Porcine chondrocytes/2 × 107 |
|
| HA-GEL h [59] | 350/COOH | -/- | Redox/HRP i and H2O2/- | -/electrical | Porcine MSCs j/1 × 106 |
|
| HA-TA k [52] | 70/COOH | 24 | Oxidative coupling reaction (redox)/HRP i and H2O2/10–500 | Platelet lysate | MSCs j/107 |
|
| HA a [60] | 1010–1800/COOH | /transglutaminase substrate peptides | Enzymatic/thrombin, factor XIII, transglutaminase-modified heparin/60–120 | TGF-β m/- | Human infant chondrocytes/5, 10 or 15 × 106, |
|
| HA a [50] | 1010–1800/COOH | /transglutaminase substrate peptides, heparin | Enzymatic/thrombin, transglutaminase factor XIII/900 | TGF-β m/- | Human chondroprogenitor cells (fetal origin)/15 × 106 |
|
| HA-MA-PNIPAAm-CL n [61] | 2000/OH | 30/- | Redox/-/- | -/- | Rabbit adipose-derived stem cells/1 × 106 |
|
| PVCL-g-HA o (methacrylate HA) [62] | 58 and 1100/OH | -/- | Redox/VA-057 p initiator/- | -/- | Bovine chondrocytes/3.65 × 106 |
|
| HA-Tyr k [63] | 90/COOH | 6/- | Oxidative coupling reaction (Redox)/HRP i and H2O2/60 | Caprine MSCs j/107 |
| |
| Michael-type addition reaction | ||||||
| MeHA q [2] | 66–99/OH | 46.5 ± 5.5/- 46.5 ± 5.5/CS r-binding peptide 46.5 ± 5.5/- | Michael-type addition/MMP7 s-degradable peptide/457 ± 68.1 | -/- | MSCs j/1 × 106 chondrocytes/1.25 × 106 |
|
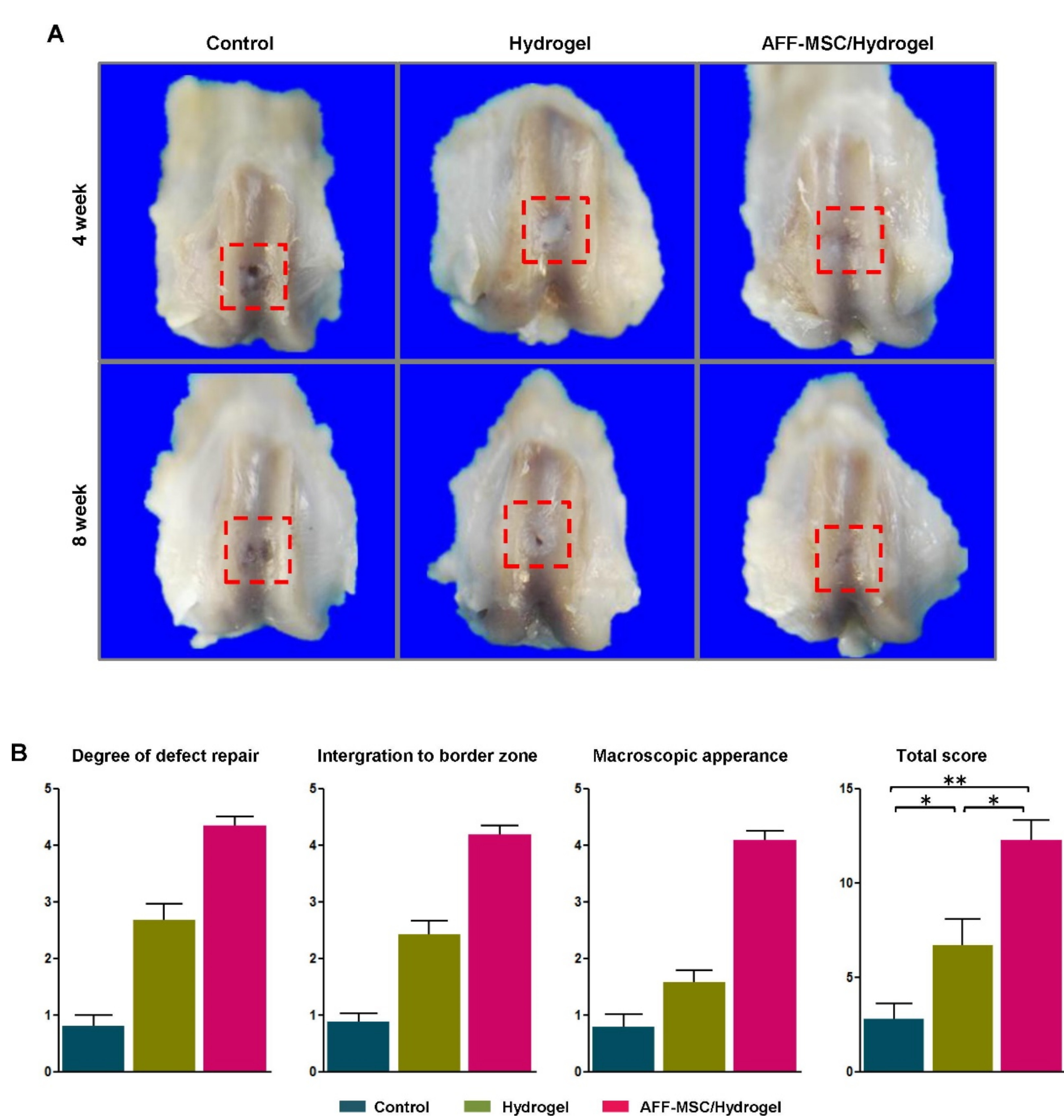
| Hyper-branched PEGDA t-thiolated HA [56] | -/COOH | -/- | Michael-type addition/-/120 | -/- | Human AFF-MSCs u/5 × 106 |
|
| MA-HA v [64] | 59/COOH | 30/- | Michael-type addition/MMP w-cleavable peptides/- | -/- | Human MSCs j/20 × 106 |
|
| Schiff base reaction | ||||||
| Glycol chitosan-oxidized HA a [65] | 100/OH | 33.4/- | Schiff base reaction/-/- | Cartilage ECM f particles/- | BMSCs x/2 × 107 |
|
| Collagen-HAD z [66] | 1500–1800/ | -/- | Schiff base reaction/-/- | -/- | Rabbit chondrocytes/5 × 104 |
|
| CH-HAD aa [53] | -/OH | 50/- | Schiff base raction//25–60 | -/- | Rabbit chondrocytes/5 × 106 |
|
| OHA/GC ab [67] | 1000/OH | ~6.8–33.8/- | Schiff base raction//97–120 | -/- | ATDC5 chondrogenic cell line/106 |
|
| Photocross-linking | ||||||
| AHAMA ac [55] | 100–200/OH | 24/- | Photopolymerization/Irgacure 2959/- | -/- | BMSCs x/5 × 106 |
|
| mGL/mHA ad [58] | 66–99/OH | Photocross-linking/LAP ae | Human BMSCs x/ 20 × 106 |
| ||
| GelMA af/HAMA ag [68] | 860/OH | Photocross-linking/LAP ae and visible light (405 nm), Irgacure 2959 and UV ah light (365 nm) | /MEW-mPCL ai reinforcement | Human articular chondrocytes/107 |
| |
| MeHA ag [69] | 75/OH | 37/± HAV, ADAM-cleavable domain | Photocross-linking/Irgacure 2959 | MSCs j/20 × 106 |
| |
| MeHA ag/ELP aj [70] | 1600/OH | Photocross-linking/ | ZnO ak (antimicrobial) | Human MSCs j, NIH-3T3/2 × 106, 5 × 106 |
| |
| MeHA ag [71] | 1000/OH | 1.2/ | Photocross-linking/Irgacure 2959 | TGFβ3 m/DCC al or DVC am microparticles | Rat BMSC x/20 × 106 |
|
| MeHA ag [72] | 74/OH | Photocross-linking/Irgacure 2959 | TGFβ3 m | Allogeneic MSCs j/60 × 106 |
| |
| MeHA ag, MeHA ag+ColI an, MeHA ag+MeCS ao [73] | 74/OH | 30 | Photocross-linking/Irgacure 2959 | Human MSCs j/20 × 106 |
| |
| MeHA ag [74] | 74/OH | 29 | Photocross-linking/Irgacure 2959 | Human MSCs j/20 × 106 |
| |
| Fibrinogen/HA-MA ag [75] | 1500–1800/OH | 95 ± 13/- | Ionic and chemical interactions, Photocross-linking/Irgacure 2959/ | TGFβ m/- | BMSCs x/104/well |
|
| GelMA af and HA-MA ag [76] | 860/OH | Photocross-linking/Irgacure 2959/900 | Human chondrocytes/107 |
| ||
| MeHA ag [77] | 74/OH | 27 | Photocross-linking/Irgacure 2959 | MSCs j and/or chondrocytes/20 × 106 |
| |
| Self-cross-linking and other reactions | ||||||
| ColI an/HA-sNHS ap [78] | 61/COOH | 32, 50, 83/ | Self-cross-linking/no initiators and no cross-linkers/93–130 | Rabbit chondrocytes/5 × 106 |
| |
| HA-SH aq/GelSH ar, HA-SH aq/GelMA af, HA-SH aq/Gel as [79] | 340/COOH | 35.3/ | Strong disulfide bonding between HA-SH aq and GelSH ar/7.19, Michael addition between HA-SH aq and GelMA af/7.31, Physical interaction/7.27 | -/- | Rabbit chondrocytes/3 × 106 |
|
| Thiolated HA a—collagen [32] | 100, 300, 1000/COOH | -/- | Formation of disulfide bonds/thiolated icariin/1800 | -/- | Chondrocytes/5 × 106 |
|
| Thiolated HA—collagen I [54] | 300/COOH | -/- | Self-cross-linking/10 | -/- | Rabbit chondrocytes/5 × 106 |
|
| HA a-ADH at/PAD au, HA a-ADH at/PAD-RGD av [80] | 740/COOH | 41.5/- | Hydrazone reaction/PAD-RGD av/112–399 | -/- | Chondrocytes/6 × 106 |
|
| Objective | Trial/Phase | Number/Age/BMI a (kg/m2)/K-L b Grade/WOMAC c (Pain)/Sex of Participants | Treatment | Administration Route/Dose/Clinical Evaluation | Results |
|---|---|---|---|---|---|
| To assess Hydros d and Hydros d-TA e regarding their safety and initial performance in comparison with Synvisc-One f in patients with knee OA g [106] | Prospective, multicenter, randomized, double-blind feasibility trial/II | 98/60 years (average)/29.0 (average)/II and III/50–90 mm (using VAS 0–100 mm)/male and female | Hydros d Hydros d-TA e Synvisc-One | i.a. h injection/6 mL of Hydros d or Hydros d-TA e, or Synvisc-One f, single dose/2, 6, 13 and 26 weeks p.i. i |
|
| To investigate the safety and efficiency of Gel-One® j in treating patients with symptomatic knee OA g [107] To examine the continued safety and efficacy of Gel-One® j(extension of the aforementioned clinical trial) [108] | Double-blind, multicenter, RCT k/-Multicenter extension and retreatment trial | Gel-One® j: 247, PBS l: 128/40–80 years old/28.3/I, II and III/≥40 mm (using VAS m 0–100 mm)/male and femaleContinued observation/≥ 64, second injection/≥ 196/40–80 years old/28.8/I, II and III/≥40 mm (using VAS m 0–100 mm)/male and female | Gel-One® j PBS l (control) Gel-One® j PBS l (control) | i.a. h injection/3 mL (30 mg HA n/3 mL), 3 mL PBS l, single dose/1 wk, 3, 6, 9 and 13 wks p.i. iSecond injection: i.a. h injection/3 mL (30 mg cross-linked HA n/3 mL), 3 mL PBS l, single dose/13 wks p.i. i |
|
| Integrated analysis of two RCTs k aiming to investigate the safety and efficiency of a single i.a. h injection of Gel-One® j in treating knee OA g [109] | Multicenter, double-blind RCT k/- Multicenter, double-blind RCT k/- | SI-6606/01: -/60 years old (average)/~28.8 (average)/I-III/≥40 mm (using VAS m 0–100 mm)/male and female Gel/1133: -/60 years old (average)/~28.8 (average)/I-III/≥40 mm (using VAS m 0–100 mm)/male and female Pooled ITT o population: Gel-One® j: 649, PBS l: 5345 | Gel-One® j PBS l (control) Gel-One® j PBS l (control) | i.a. h injection/single dose/3, 6, 9 and 13 wks p.i. ii.a. h injection/single dose/3, 6, 12, 18 and 26 wks p.i. i |
|
| To demonstrate the benefit of a single i.a. injection of Gel-One® j as treatment of knee OA g in a population similar to those of viscosupplementation-reported trials [110] | Subgroup analysis of a multicenter RCT k | Subgroup: 311 (Gel-One® j:152, PBS:159)/40–80 years old/II and III/40–80 mm (using VAS m 0–100 mm)/male and female | Gel-One® j PBS l (control) | i.a. h injection/single dose/3, 6, 12, 18 and 26 wks p.i. i |
|
| To compare the safety and efficiency of HYA-JOINT Plus p with Synvisc-One f in subjects with kneeOA g [111] | Prospective, double-blind RCT k/- | HYA-JOINT Plus p: 62, Synvisc-One f: 59/40–85 years old/~25 (average)/II, III//≥30 mm (using VAS m 0–100 mm)/male and female | HYA-JOINT Plus p Synvisc-One f | i.a. h injection/3 mL of HYA-JOINT Plus p (20 mg/mL), 6 mL of Synvisc-One f (8 mg/mL), single dose/1, 3 and 6 months p.i. i |
|
| To examine the efficacy of hylastan SGL-80 q regarding pain reduction in patients with knee OA g, in comparison with corticosteroid injection [112] | Multicenter, double-blind, randomized, parallel group, trial/- | Hylasatan SGL-80 q (single dose): 130, hylasatan SGL-80 q (double dose): 129, methylprednisolone acetate: 132/>40 years old/-/I–III/1.5–3.5 (using Likert scale)/male and female | hylastan SGL-80 qmethylprednisolone acetate | i.a. h injection/4 mL of hylastan SGL-80 q on day 0, or 2 × 4 mL of hylastan SGL-80 q on day 0 and week 2, or 40 mg of methylprednisolone acetate on Day 0/4, 8, 12, 16, 20 and 26 weeks |
|
| To evaluate the efficacy and safety of Cingal® r in comparison with Monovisc® s for the treatment of knee OA g [113] | Prospective, randomized, multicenter, double-blind, placebo-controlled trial/- | Cingal® r:149, Monovisc® s:150, saline:69/40–75 years old/40–90/I, II or III/40–90 mm (using VAS m 0–100)/male and female | Cingal® r Monovisc® s Saline | i.a. c injection/4 mL of Cingal® r (88 mg cross-linked HA and 18 mg TH), 4 mL of Monovisc® s (88 mg cross-linked HA), 4 mL of saline, single dose/1, 3, 6, 12, 18 and 26 wks p.i. i |
|
| To prove the safety and efficacy of Monovisc® s in relieving joint pain inidiopathic knee OA g patients [114] | Multicenter, double-blind, randomized, placebo-controlled trial/- | Monovisc® s: 184, saline: 185/35–75 years old/20–40 kg/m2/II or III/200–400 mm (VAS m pain score 0–500 mm)/male and female | Monovisc® s Saline | i.a. h injection/4 mL of Monovisc® s, 4 mL of saline (0.9%), single dose/2, 4, 8, 12, 20 and 26 wks p.i. i |
|
| To assess thesafety and efficiency of Durolane® t in unilateral knee OA g patients [115] | Randomized, double-blind, saline-controlled trial/- | Durolane® t: 108, saline: 110/> 50 years old/20.1–41/Likert version of WOMAC c pain score: 7–17/male and female | Durolane® t Saline | i.a. c injection/3 mL of Durolane® t (20 mg/mL) or 3 mL saline, single dose/2, 4 and 6 wks p.i. i |
|
| To compare Durolane® t with MPA u for the treatment of unilateralknee OA g [116] | Prospective, multicenter, randomized, active-controlled, double-blind, noninferiority trial (blinded phase)Open label extension phase | Durolane® t: 221, MPA u: 221/35–80 years old/≤40/II, III/7–17/male and female | Durolane® t MPA u | Blinded phase: i.a. h injection/3 mL of Durolane® t (20 mg/mL) or 1 mL of MPA u (40 mg/mL), single dose/2, 4, 6, 12, 18 and 26 wks p.i. iOLE v: i.a. h injection/3 mL of Durolane® t (20 mg/mL), single dose/28, 39 and 52 wks post initial i.a. h |
|
| To compare safety and effectiveness of Durolane® t and Artz w in treating knee OA g [117] | Multicenter, randomized, double-blind, noninferiority trial/- | Durolane ® t:175, Artz w:174/40–80 years old/-/II or III/7–17 (Likert pain score range 0–20)/male and female | Durolane® t Artz w | i.a. h injection/1 × 3 mL of Durolane® t (and 4 sham s.c. x injections on weeks 1, 2, 3 and 4); or 5 × 2.5 mL of Artz w on weeks 0, 1, 2, 3 and 4/0, 6, 10, 14, 18 and 26 wks |
|
| To evaluate the safety and efficiency of XLHA y in comparison with HMWHA zin treating symptomatic knee OA g [118] | Double-blind, randomized, multicenter, noninferiority trial | XLHA y (single dose): 141, HMWHA z (three doses): 146/>40 years old/<32/I-III/≥40 mm (using VAS m 0–100 mm)/male and female | XLHA y HMWHA z | i.a. h injection/XLHA y group: 2 × 2 mL of PBS l (9 mg/mL) and 3 mL of XLHA y (20 mg/mL), HMWHA z group: 3 × 2 mL of HMWHA z (10 mg/mL)/1 wk, 2, 3, 4, 9, 12 and 15 wks p.i. i |
|
| To compare Conjuran® ab with Synovian® acand Hyruan Plus® ad regarding their analgesic efficiency in patients with knee OA [119] | Pilot study | Synovian® ac: 5, Hyruan Plus® ad: 5, Conjuran® ab: 5/≥40 years old/-/I- III/≥40 mm (using VAS 0–100 mm)/male and female | Synovian® acHyruan Plus® adConjuran® ab | i.a. h injection/3 i.a. h injections at 1 week interval (all three groups), 3 mL of Synovian® ac (20 mg/mL) and 2 × 3 mL of saline, 3 × 2 mL of Hyruan Plus® ad (10 mg/mL), 3 × 2 mL of Conjuran® ab (20 mg/mL)/4 wks after the last injection |
|
| To examine the safety and efficiency of YYD302 ae for knee OA g [121] | Randomized, double-blind, active-controlled, multicenter trial/III | 190/≥40 years old/≤32/I- III/≥40 mm (using VAS m 0–100 mm)/male and female | YYD302 aeSynovian® ac | i.a. h injection/2 mL of YYD302 ae, 3 mL of Synovian® ac, single dose/2, 4 and 12 wks after the i.a. h injection | |
| To examine the safety and efficiency of Cartistem® af with respect to the regeneration of articular cartilage [103] | Open-label, single-arm, single-center trial/I/II | 7/51–77 years old/-/III (ICRS ag grade of defect: 4)/40–60 mm (using VAS m 0–100 mm)/male and female | Cartistem® af | Transplantation, closure of wound and application of a splint/0.5 mL of Cartistem® af per cm2 of defect (0.5 × 107 cells per ml), low-dose: 2.3–2.5 mL of Cartistem® af, high dose: 3.3–4.0 mL of Cartistem® af/24 weeks (short term), 7 years (long term) |
|
| To investigate the ability of Cartistem® af to reliably restore cartilage in patients with large cartilage lesions and to examine the long-term maintenance of the potential clinical improvements [105] | Randomized controlled trial/III | Cartistem® af: 57, microfracture: 57/55.9 years old (average)/~ 26 (average)/II, III (ICRS ag grade 4)/-/male or female | Cartistem® afmicrofracture | Surgical implantation, closure of wound and application of a splint/-/48 weeks, 36, 48 and 60 months |
|
| To evaluate the safety and efficiency of Cartistem® af, in treating articular cartilage lesions in the knee due to trauma, ageing, or degenerative diseases [104] | Open label trial/I/IIa | 12/>18 years old/≤35/ICRS ag grade 3 or 4/20–60 mm (using VAS m 0–100 mm)/male and female | Cartistem® af | Surgical implantation/0.5 mL of the medicinal product per cm2 of cartilage lesion/12 months |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsanaktsidou, E.; Kammona, O.; Kiparissides, C. Recent Developments in Hyaluronic Acid-Based Hydrogels for Cartilage Tissue Engineering Applications. Polymers 2022, 14, 839. https://doi.org/10.3390/polym14040839
Tsanaktsidou E, Kammona O, Kiparissides C. Recent Developments in Hyaluronic Acid-Based Hydrogels for Cartilage Tissue Engineering Applications. Polymers. 2022; 14(4):839. https://doi.org/10.3390/polym14040839
Chicago/Turabian StyleTsanaktsidou, Evgenia, Olga Kammona, and Costas Kiparissides. 2022. "Recent Developments in Hyaluronic Acid-Based Hydrogels for Cartilage Tissue Engineering Applications" Polymers 14, no. 4: 839. https://doi.org/10.3390/polym14040839