Hemicellulose and Nano/Microfibrils Improving the Pliability and Hydrophobic Properties of Cellulose Film by Interstitial Filling and Forming Micro/Nanostructure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Methods
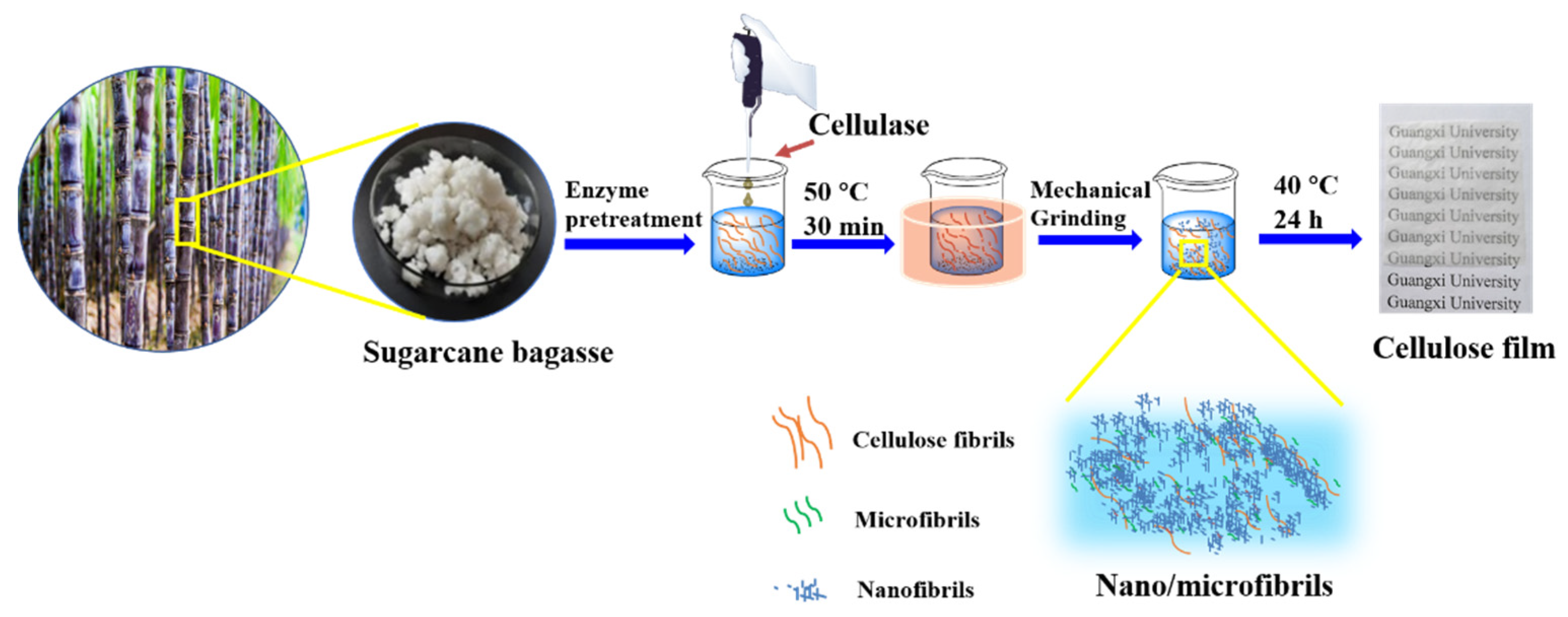
2.2.1. Enzyme Pretreatment
2.2.2. Preparation of Nano/Microfibrils by Mechanical Grinding
2.2.3. Preparation of Nano/Microfibrils Film
2.3. Characterization
2.3.1. Characterization of Morphological Features
2.3.2. XRD Analysis
2.3.3. Particles Size Analysis
2.3.4. IC Analysis
2.3.5. UV–Vis Analysis
2.3.6. Water Resistance Analysis
2.3.7. Mechanical Properties
3. Results
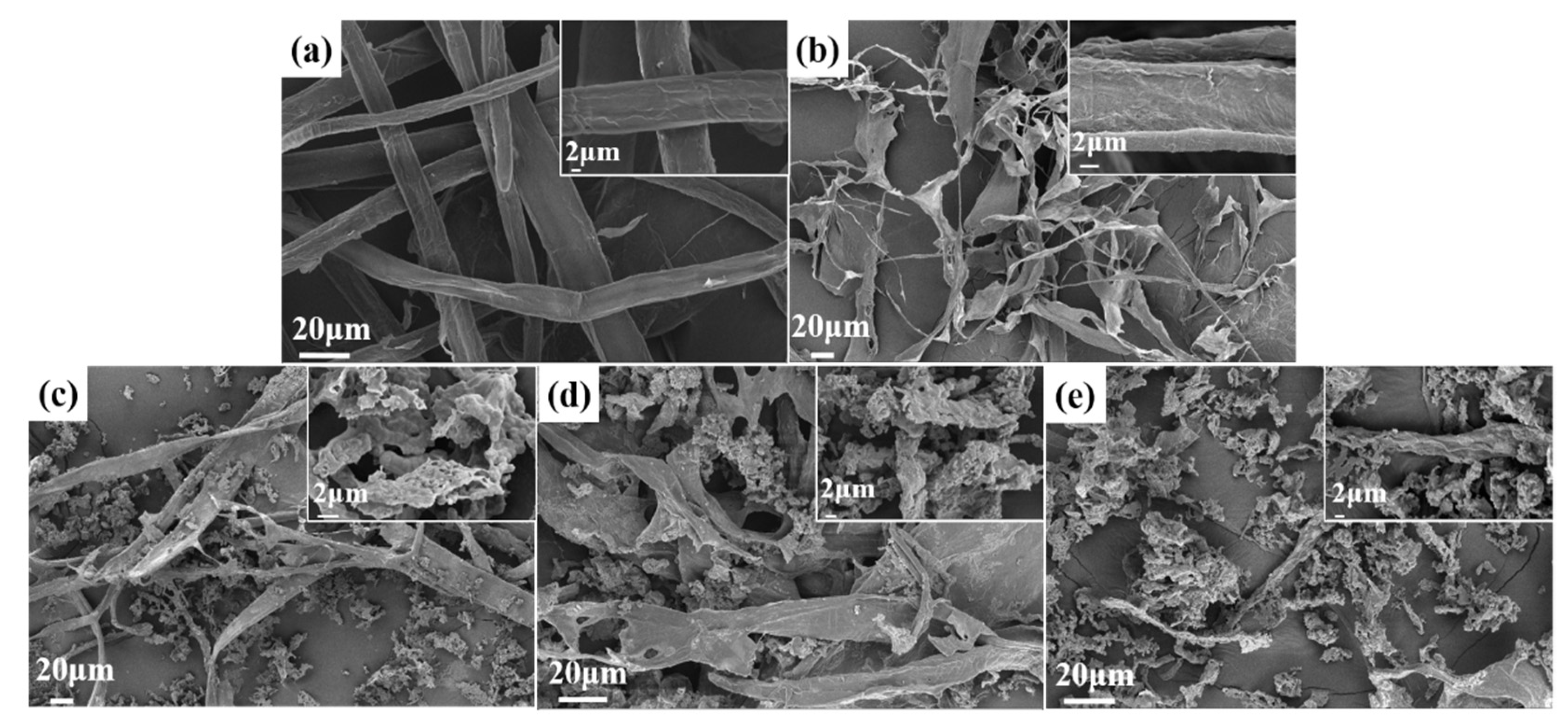
3.1. SEM Analysis of Fibers after Different Stages of Treatments
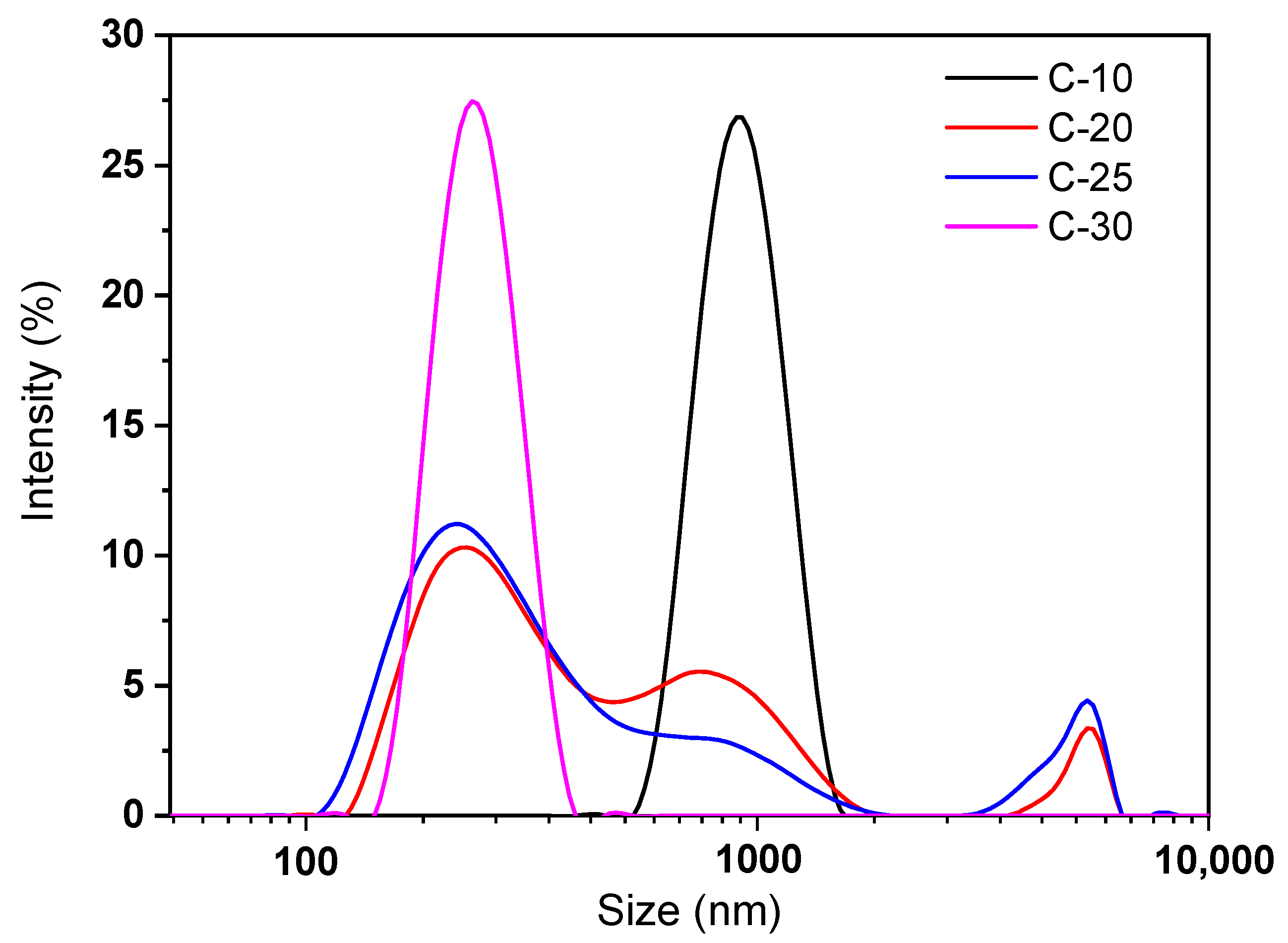
3.2. Particle Size and Components Analysis
3.3. Crystallinity of Fibers after Different Treatment Stages
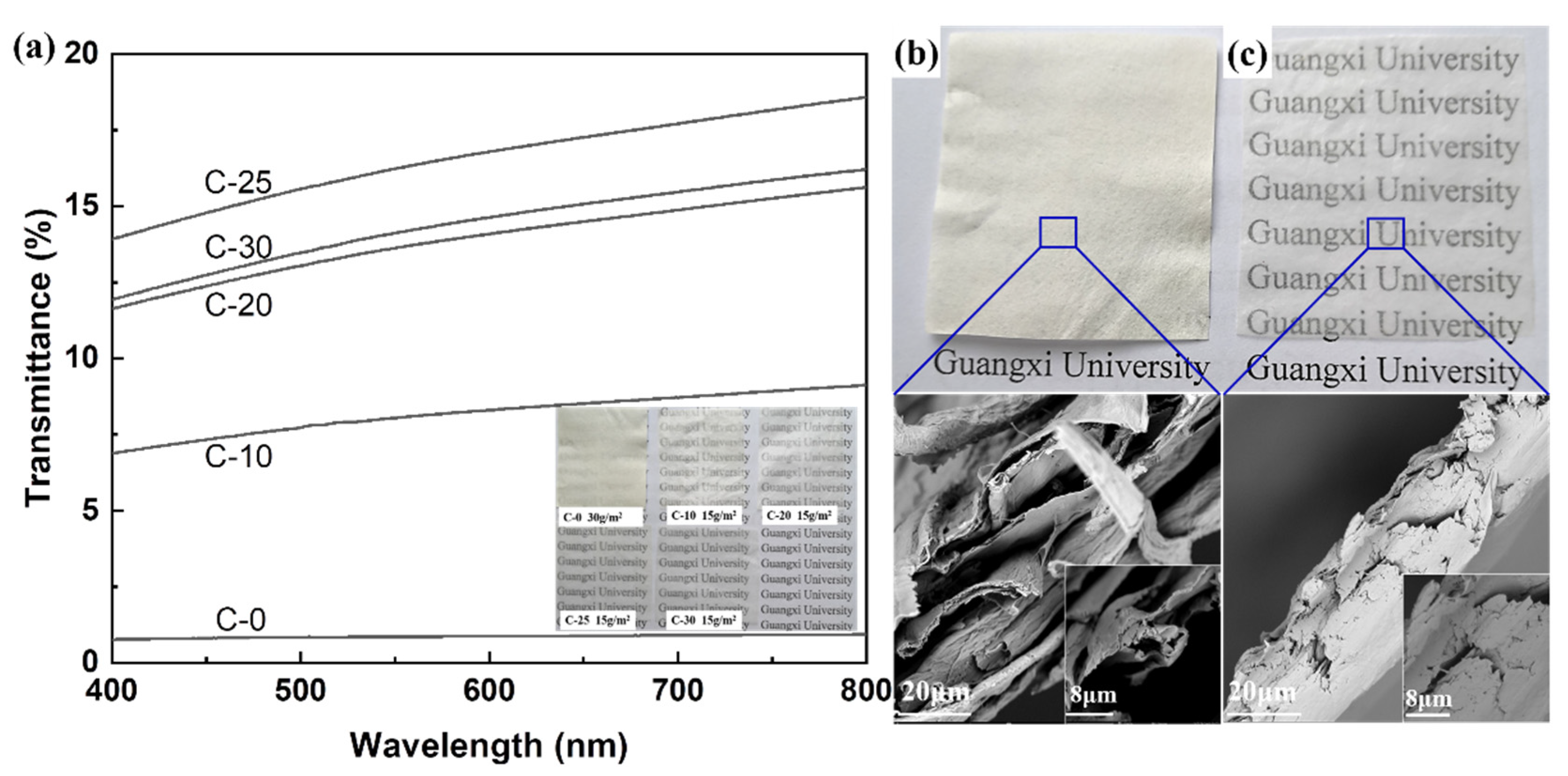
3.4. Optical Transparency of Nano/Microfibrils Films
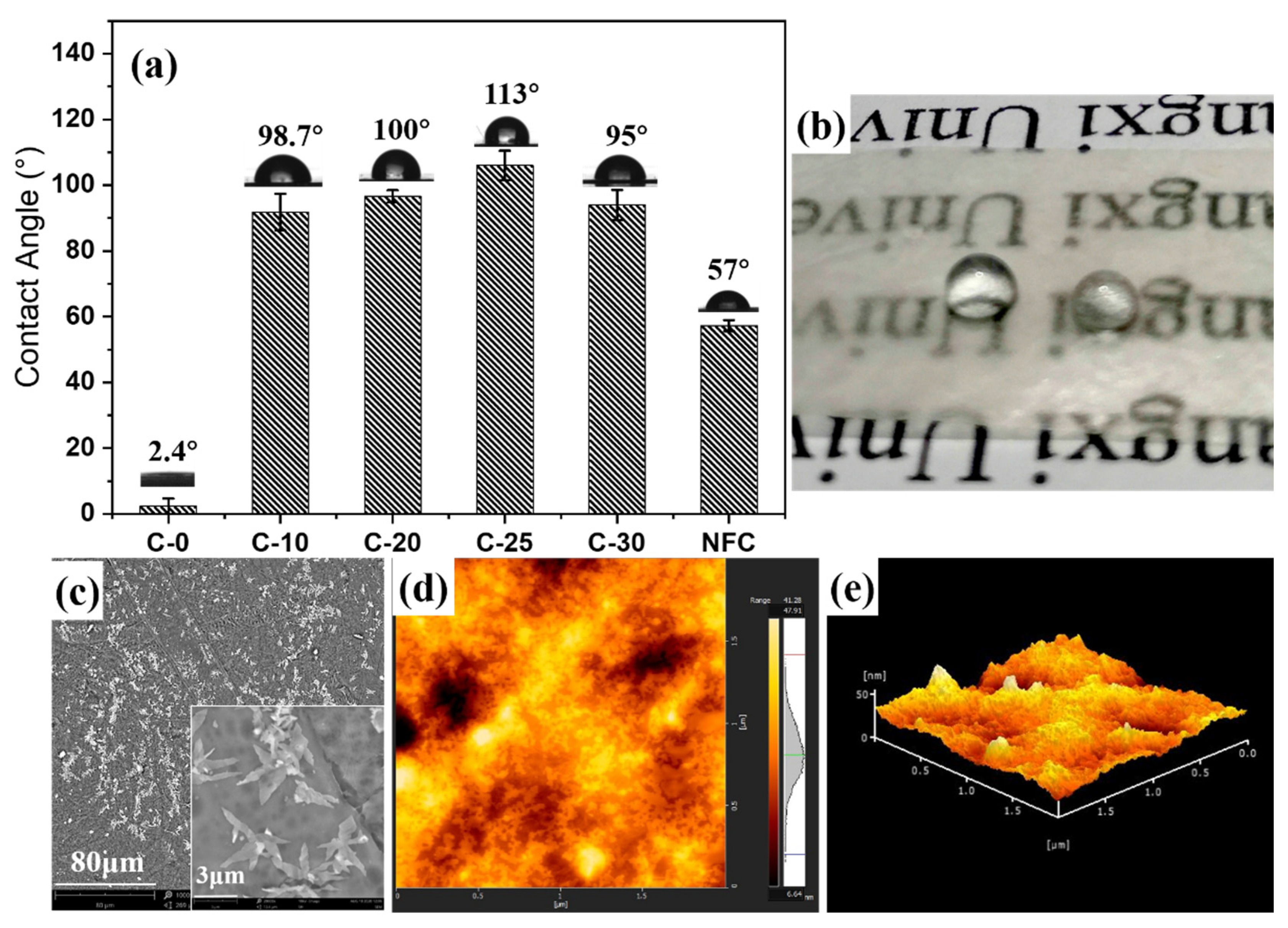
3.5. Hydrophobic Properties and Principle of Nano/Microfibrils Film
3.6. Barrier Performance of Nano/Microfibrils Film
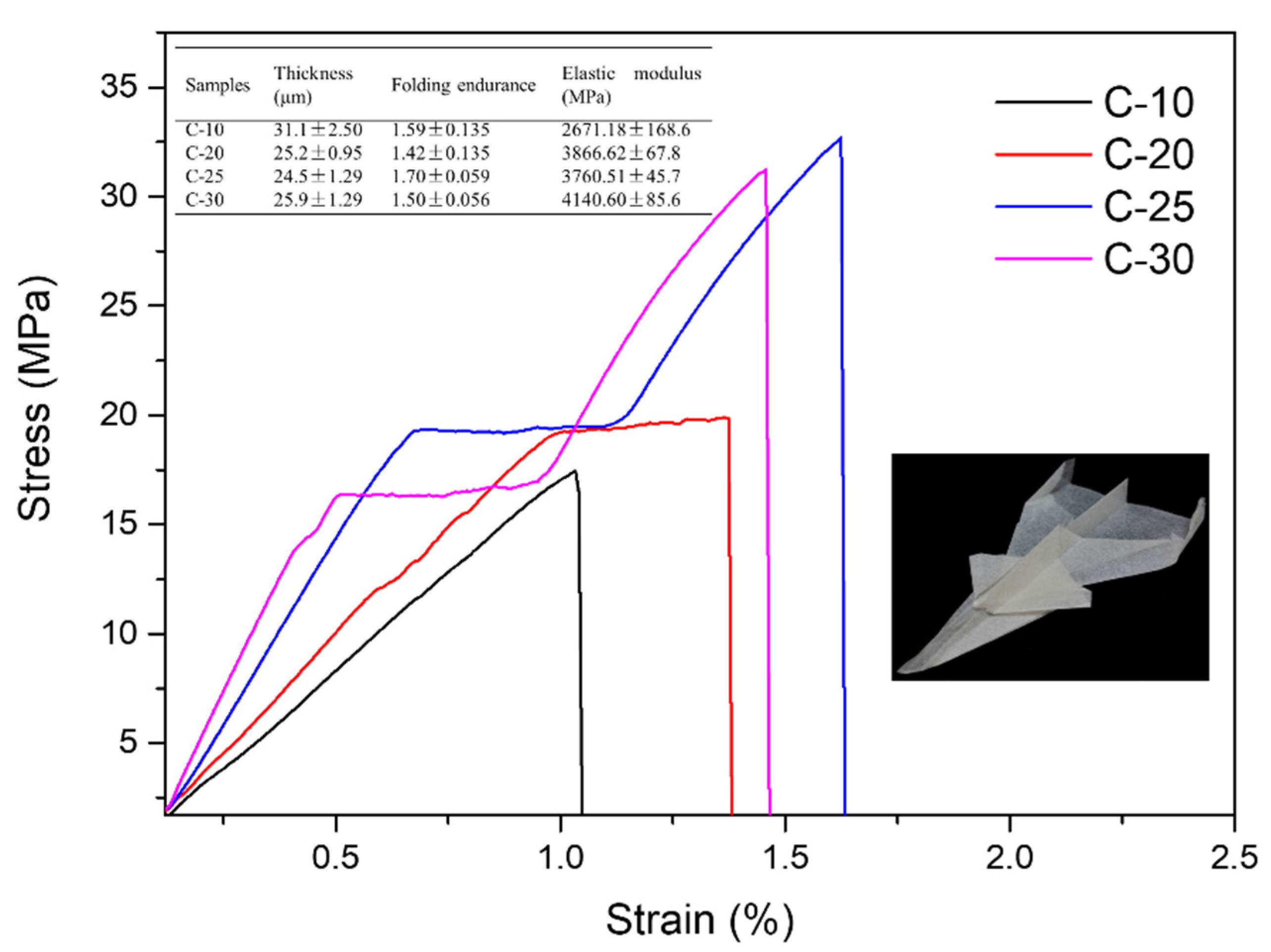
3.7. Mechanical Properties of Films
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohanty, A.K.; Vivekanandhan, S.; Pin, J.M.; Misra, M. Composites from renewable and sustainable resources: Challenges and innovations. Science 2018, 362, 536–542. [Google Scholar] [CrossRef] [Green Version]
- Vanitjinda, G.; Nimchua, T.; Sukyai, P. Effect of xylanase-assisted pretreatment on the properties of cellulose and regenerated cellulose films from sugarcane bagasse. Int. J. Biol. Macromol. 2019, 122, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.T.; Chen, X.Q. Preparation and characterization of spherical cellulose nanocrystals with high purity by the composite enzymolysis of pulp fibers. Bioresour. Technol. 2019, 291, 121842. [Google Scholar] [CrossRef] [PubMed]
- Molina, N.F.; Brito, Y.F.; Benavides, J.M.P. Recycling of Residual Polymers Reinforced with Natural Fibers as a Sustainable Alternative: A Review. Polymers 2021, 13, 3612. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Fowler, I.J. Thermal, mechanical, and rheological properties of micro-fibrillated cellulose-reinforced starch foams crosslinked with polysiloxane-based cross-linking agents. Int. J. Biol. Macromol. 2022, 205, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Kontturi, E.; Spirk, S. Ultrathin Films of Cellulose: A Materials Perspective. Front. Chem. 2019, 7, 488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botta, L.; Titone, V.; Mistretta, M.C.; La Mantia, F.P.; Modica, A.; Bruno, M.; Sottile, F.; Lopresti, F. PBAT Based Composites Reinforced with Microcrystalline Cellulose Obtained from Softwood Almond Shells. Polymers 2021, 13, 2643. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Romero, M.; Aguado, R.; Moral, A.; Brindley, C.; Ballesteros, M. From traditional paper to nanocomposite films: Analysis of global research into cellulose for food packaging. Food Packag. Shelf Life 2022, 31, 100788. [Google Scholar] [CrossRef]
- Haney, R.; Kollarigowda, R.H.; Wiegart, L.; Ramakrishnan, S. Surface-Functionalized Cellulose Nanocrystals as Nanofillers for Crosslinking Processes: Implications for Thermosetting Resins. ACS Appl. Nano Mater. 2022, 5, 1891–1901. [Google Scholar] [CrossRef]
- Tekinalp, H.L.; Meng, X.; Lu, Y.; Kunc, V.; Love, L.J.; Peter, W.H.; Ozcan, S. High modulus biocomposites via additive manufacturing: Cellulose nanofibril networks as “microsponges”. Compos. Part B Eng. 2019, 173, 106817. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, W.; Teng, A.; Zhang, K.; Ma, Y.; Duan, S.; Li, S.; Guo, Y. Using cellulose nanofibers to reinforce polysaccharide films: Blending vs layer-by-layer casting. Carbohydr. Polym. 2020, 227, 115264. [Google Scholar] [CrossRef]
- Chowdhury, R.A.; Clarkson, C.; Youngblood, J. Continuous roll-to-roll fabrication of transparent cellulose nanocrystal (CNC) coatings with controlled anisotropy. Cellulose 2018, 25, 1769–1781. [Google Scholar] [CrossRef]
- Li, K.; Jin, S.; Li, J.; Chen, H. Improvement in antibacterial and functional properties of mussel-inspired cellulose nanofibrils/gelatin nanocomposites incorporated with graphene oxide for active packaging. Ind. Crop. Prod. 2019, 132, 197–212. [Google Scholar] [CrossRef]
- Chowdhury, R.A.; Nuruddin, M.; Clarkson, C.; Montes, F.; Howarter, J.; Youngblood, J.P. Cellulose Nanocrystal (CNC) Coatings with Controlled Anisotropy as High-Performance Gas Barrier Films. ACS Appl. Mater. Interfaces 2019, 11, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M.C.; Rosa, M.F.; Mattoso, L.H.C. Nanocellulose in bio-based food packaging applications. Ind. Crop. Prod. 2017, 97, 664–671. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Chen, S.; Zhang, X.; Ma, J.; He, J. Oxidized cellulose-based hemostatic materials. Carbohydr. Polym. 2020, 230, 115585. [Google Scholar] [CrossRef]
- Hao, P.; Zhao, Z.; Tian, J.; Li, H.; Sang, Y.; Yu, G.; Cai, H.; Liu, H.; Wong, C.P.; Umar, A. Hierarchical porous carbon aerogel derived from bagasse for high performance supercapacitor electrode. Nanoscale 2014, 6, 12120–12129. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Athira, K.B.; Rubiah, M.H.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zhao, Y.; Kuga, S.; Wu, M.; Huang, Y. A versatile method for producing functionalized cellulose nanofibers and their application. Nanoscale 2016, 8, 3753–3759. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Yang, Q.; Saito, T.; Berglund, L.A.; Isogai, A. Cellulose nanofibrils improve the properties of all-cellulose composites by the nano-reinforcement mechanism and nanofibril-induced crystallization. Nanoscale 2015, 7, 17957–17963. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindstrom, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. Engl. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Pinem, M.P.; Wardhono, E.Y.; Nadaud, F.; Clausse, D.; Saleh, K.; Guenin, E. Nanofluid to Nanocomposite Film: Chitosan and Cellulose-Based Edible Packaging. Nanomaterials 2020, 10, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Liu, W.; Huang, W.; Ding, Y.; Song, L.; Zheng, S.; Wang, Z. The toughening of polymeric glasses using cellulose without sacrificing transparency. Ind. Crop. Prod. 2019, 142, 111842. [Google Scholar] [CrossRef]
- Shu, L.; Zhang, X.F.; Wang, Z.G.; Yao, J.F. Structure reorganization of cellulose hydrogel by green solvent exchange for potential plastic replacement. Carbohydr. Polym. 2022, 275, 118695. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Qi, G.; Kenny, J.M.; Puglia, D.; Ma, P. Effect of Cellulose Nanocrystals and Lignin Nanoparticles on Mechanical, Antioxidant and Water Vapour Barrier Properties of Glutaraldehyde Crosslinked PVA Films. Polymers 2020, 12, 1364. [Google Scholar] [CrossRef] [PubMed]
- Rol, F.; Belgacem, M.N.; Gandini, A.; Bras, J. Recent advances in surface-modified cellulose nanofibrils. Prog. Polym. Sci. 2019, 88, 241–264. [Google Scholar] [CrossRef]
- Sehaqui, H.; Zimmermann, T.; Tingaut, P. Hydrophobic cellulose nanopaper through a mild esterification procedure. Cellulose 2013, 21, 367–382. [Google Scholar] [CrossRef] [Green Version]
- Dhali, K.; Daver, F.; Cass, P.; Adhikari, B. Surface modification of the cellulose nanocrystals through vinyl silane grafting. Int. J. Biol. Macromol. 2022, 200, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Vergara, L.A.; Ovalle-Serrano, S.A.; Blanco-Tirado, C.; Combariza, M.Y. Influence of post-oxidation reactions on the physicochemical properties of TEMPO-oxidized cellulose nanofibers before and after amidation. Cellulose 2019, 27, 1273–1288. [Google Scholar] [CrossRef]
- Farhat, W.; Venditti, R.A.; Hubbe, M.; Taha, M.; Becquart, F.; Ayoub, A. A Review of Water-Resistant Hemicellulose-Based Materials: Processing and Applications. ChemSusChem 2017, 10, 305–323. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, D.; Ma, Y.; Yang, W. Multiple levels hydrophobic modification of polymeric substrates by UV-grafting polymerization with TFEMA as monomer. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1059–1067. [Google Scholar] [CrossRef]
- Henriksson, M.; Henriksson, G.; Berglund, L.A.; Lindström, T. An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur. Polym. J. 2007, 43, 3434–3441. [Google Scholar] [CrossRef]
- Hult, E.-L.; Iotti, M.; Lenes, M. Efficient approach to high barrier packaging using microfibrillar cellulose and shellac. Cellulose 2010, 17, 575–586. [Google Scholar] [CrossRef]
- Tenhunen, T.-M.; Peresin, M.S.; Penttilä, P.A.; Pere, J.; Serimaa, R.; Tammelin, T. Significance of xylan on the stability and water interactions of cellulosic nanofibrils. React. Funct. Polym. 2014, 85, 157–166. [Google Scholar] [CrossRef]
- Claro, F.C.; Matos, M.; Jordao, C.; Avelino, F.; Lomonaco, D.; Magalhaes, W.L.E. Enhanced microfibrillated cellulose-based film by controlling the hemicellulose content and MFC rheology. Carbohydr. Polym. 2019, 218, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Besbes, I.; Alila, S.; Boufi, S. Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibres: Effect of the carboxyl content. Carbohydr. Polym. 2011, 84, 975–983. [Google Scholar] [CrossRef]
- Meng, Q.; Fu, S.; Lucia, L.A. The role of heteropolysaccharides in developing oxidized cellulose nanofibrils. Carbohydr. Polym. 2016, 144, 187–195. [Google Scholar] [CrossRef]
- Nogi, M.; Iwamoto, S.; Nakagaito, A.N.; Yano, H. Optically Transparent Nanofiber Paper. Adv. Mater. 2009, 21, 1595–1598. [Google Scholar] [CrossRef]
- Bosmans, T.J.; Stepan, A.M.; Toriz, G.; Renneckar, S.; Karabulut, E.; Wagberg, L.; Gatenholm, P. Assembly of debranched xylan from solution and on nanocellulosic surfaces. Biomacromolecules 2014, 15, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Liang, C.; Yao, S.; Liu, Y.; Zhao, H.; Qin, C. Kinetics and Thermodynamics of Hemicellulose Adsorption onto Nanofibril Cellulose Surfaces by QCM-D. ACS Omega 2021, 6, 30618–30626. [Google Scholar] [CrossRef] [PubMed]
- Linder, Å.; Bergman, R.; Bodin, A.; Gatenholm, P. Mechanism of Assembly of Xylan onto Cellulose Surfaces. Langmuir 2003, 19, 5072–5077. [Google Scholar] [CrossRef]
- Yook, S.; Park, H.; Park, H.; Lee, S.-Y.; Kwon, J.; Youn, H.J. Barrier coatings with various types of cellulose nanofibrils and their barrier properties. Cellulose 2020, 27, 4509–4523. [Google Scholar] [CrossRef]
- An, M.F.; Zhang, Q.L.; Ye, K.; Lin, Y.F.; Wang, D.L.; Chen, W.; Yin, P.C.; Meng, L.P.; Li, L.B. Structural evolution of cellulose triacetate film during stretching deformation: An in-situ synchrotron radiation wide-angle X-Ray scattering study. Polymer 2019, 182, 121815. [Google Scholar] [CrossRef]
- Sharma, A.; Thakre, S.; Kumaraswamy, G. Microstructural differences between Viscose and Lyocell revealed by in-situ studies of wet and dry fibers. Cellulose 2020, 27, 1195–1206. [Google Scholar] [CrossRef]
- Kim, J.W.; Park, S.; Harper, D.P.; Rials, T.G. Structure and Thermomechanical Properties of Stretched Cellulose Films. J. Appl. Polym. Sci. 2013, 128, 181–187. [Google Scholar] [CrossRef]



| C-0 | C-10 | C-20 | C-25 | C-30 | |
|---|---|---|---|---|---|
| L(n), mm | 0.34 ± 0.08 | 0.29 ± 0.02 | 0.28 ± 0.07 | 0.27 ± 0.02 | 0.27 ± 0.03 |
| L(w), mm | 1.20 ± 0.07 | 0.86 ± 0.04 | 0.80 ± 0.03 | 0.80 ± 0.05 | 0.78 ± 0.08 |
| Fines, % | 42.08 ± 1.46 | 46.16 ± 1.21 | 47.94 ± 0.98 | 50.96 ± 1.82 | 53.52 ± 1.63 |
| Sample | Neutral Sugars and Acidic Oligomers (%) | ||
|---|---|---|---|
| Glucose | Xylose | Arabinose | |
| C-0 | 74.95 ± 4.43 | 21.21 ± 0.90 | 1.16 ± 0.0006 |
| C-E | 75.48 ± 5.48 | 20.97 ± 0.56 | 1.14 ± 0.0006 |
| C-10 | 72.64 ± 5.32 | 19.52 ± 1.52 | 1.05 ± 0.0006 |
| C-20 | 72.07 ± 8.89 | 17.46 ± 1.45 | 1.01 ± 0.0011 |
| C-25 | 73.02 ± 7.65 | 17.16 ± 0.96 | 0.86 ± 0.0018 |
| C-30 | 73.39 ± 4.09 | 18.66 ± 0.98 | 0.96 ± 0.0004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yao, M.; Liang, C.; Zhao, H.; Liu, Y.; Zong, Y. Hemicellulose and Nano/Microfibrils Improving the Pliability and Hydrophobic Properties of Cellulose Film by Interstitial Filling and Forming Micro/Nanostructure. Polymers 2022, 14, 1297. https://doi.org/10.3390/polym14071297
Li Y, Yao M, Liang C, Zhao H, Liu Y, Zong Y. Hemicellulose and Nano/Microfibrils Improving the Pliability and Hydrophobic Properties of Cellulose Film by Interstitial Filling and Forming Micro/Nanostructure. Polymers. 2022; 14(7):1297. https://doi.org/10.3390/polym14071297
Chicago/Turabian StyleLi, Yan, Mingzhu Yao, Chen Liang, Hui Zhao, Yang Liu, and Yifeng Zong. 2022. "Hemicellulose and Nano/Microfibrils Improving the Pliability and Hydrophobic Properties of Cellulose Film by Interstitial Filling and Forming Micro/Nanostructure" Polymers 14, no. 7: 1297. https://doi.org/10.3390/polym14071297






