Polyvinyl Alcohol/Polyaniline/Carboxylated Graphene Oxide Nanocomposites for Coating Protection of Cast Iron in Simulated Seawater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
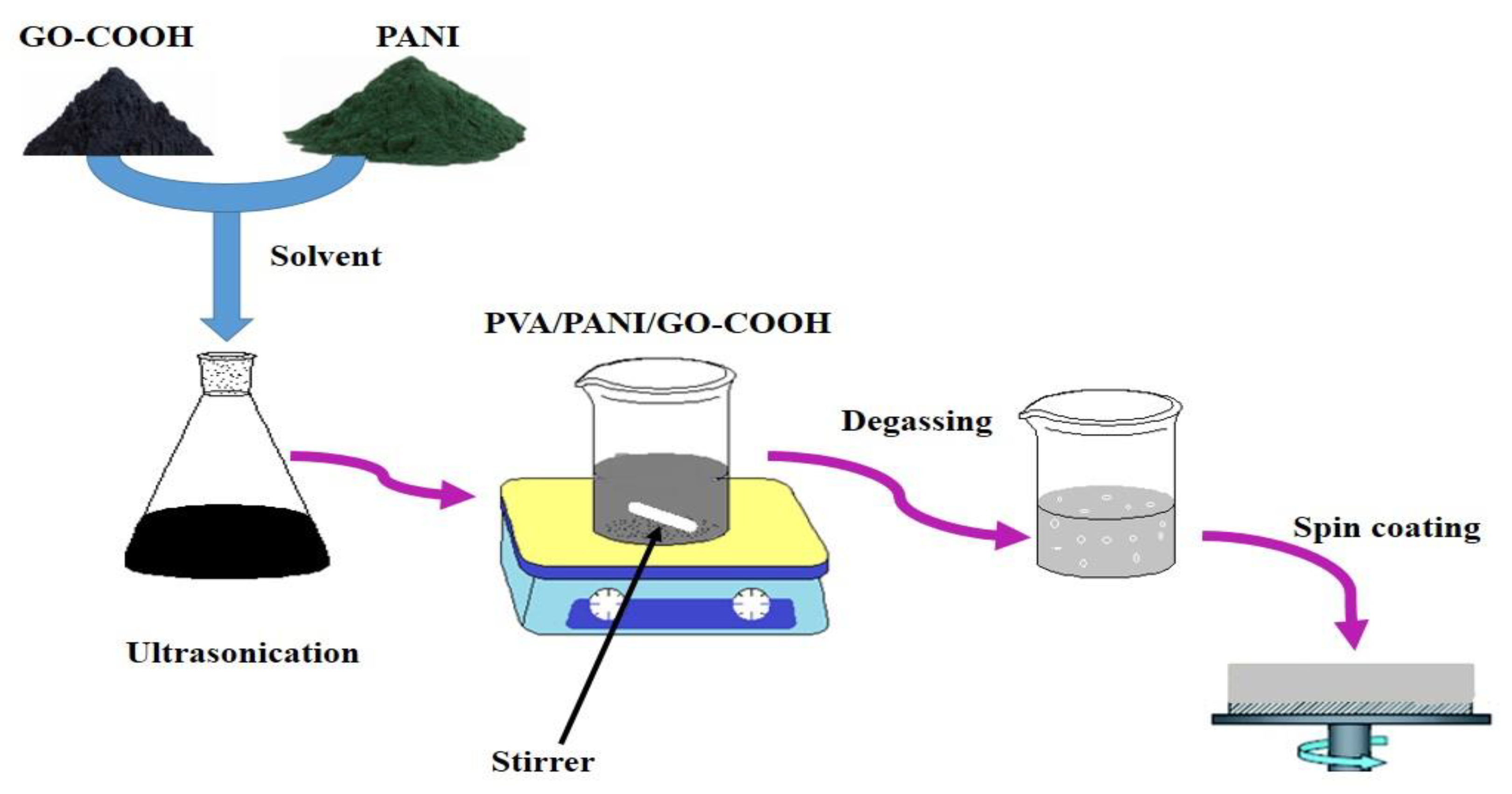
2.2. Preparation of Composite Coatings
2.2.1. Preparation of Carboxylated Graphene Oxide (GO-COOH)
2.2.2. Preparation of PANI
2.2.3. Preparation of PVA Solution
2.2.4. Preparation of PVA/PANI Blended GO-COOH Nanocomposite Coating
2.3. Characterization
3. Results and Discussion
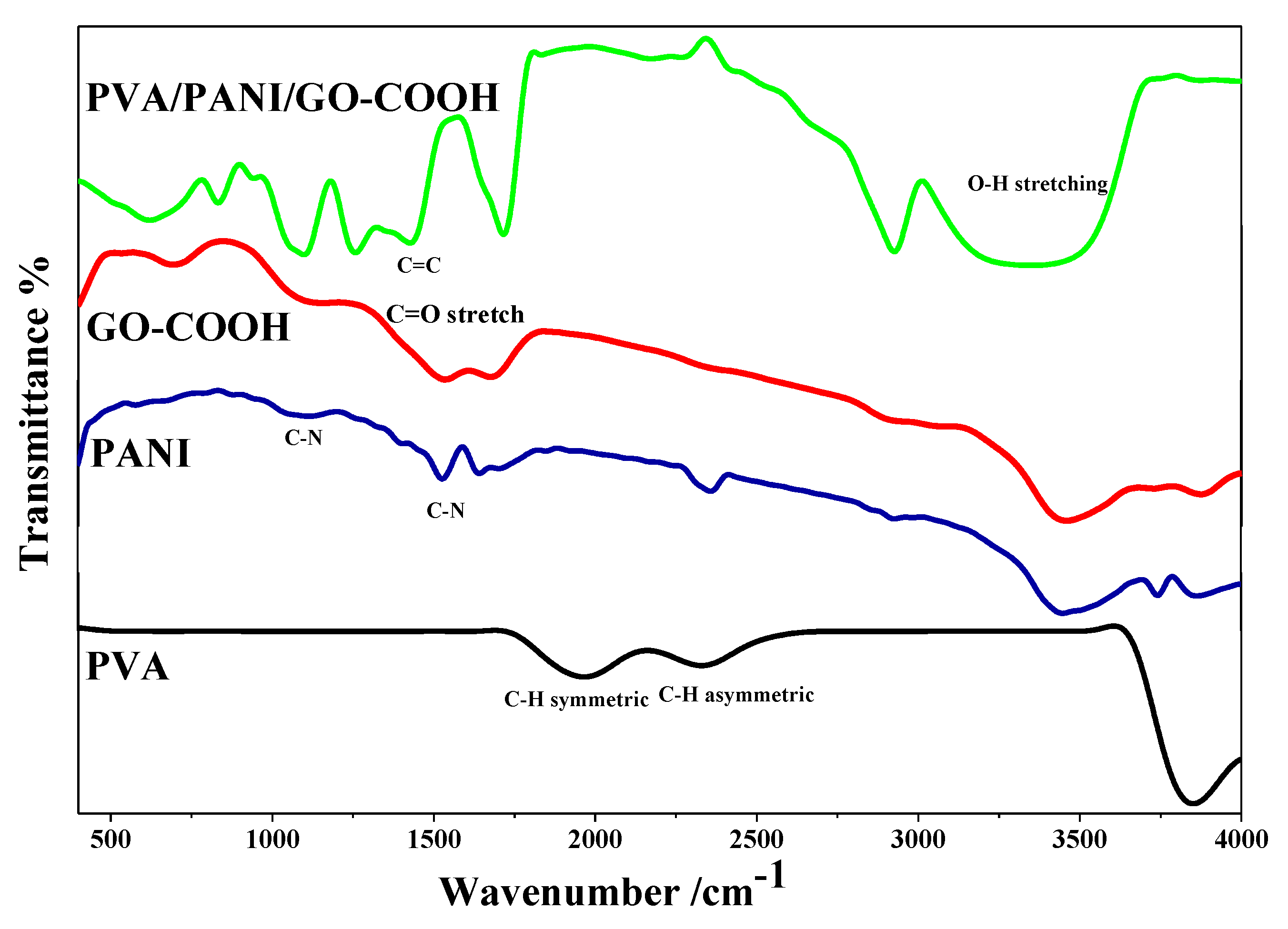
3.1. Characterization of Nanocomposite Coating Using FTIR Spectroscopy
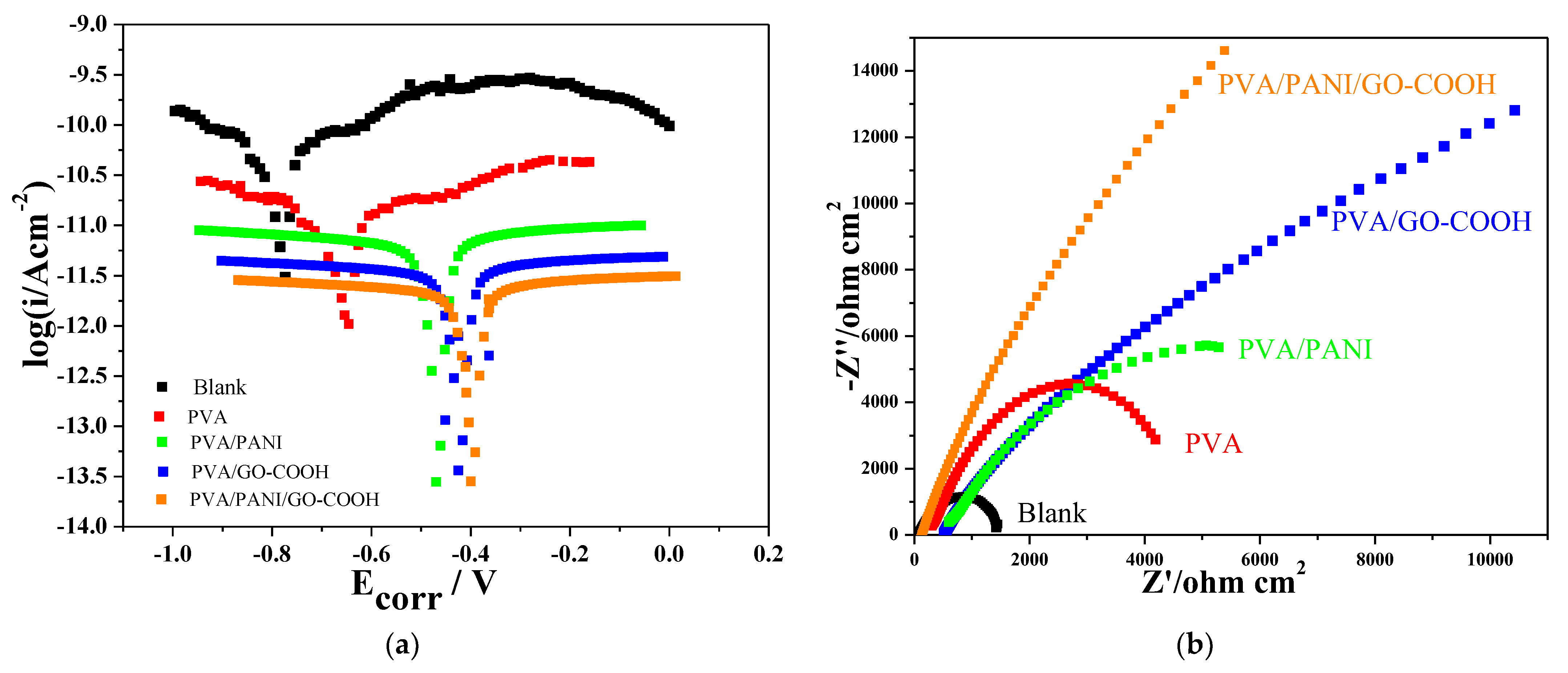
3.2. Corrosion Tests
3.2.1. Corrosion Protection Performance of Prepared Coatings
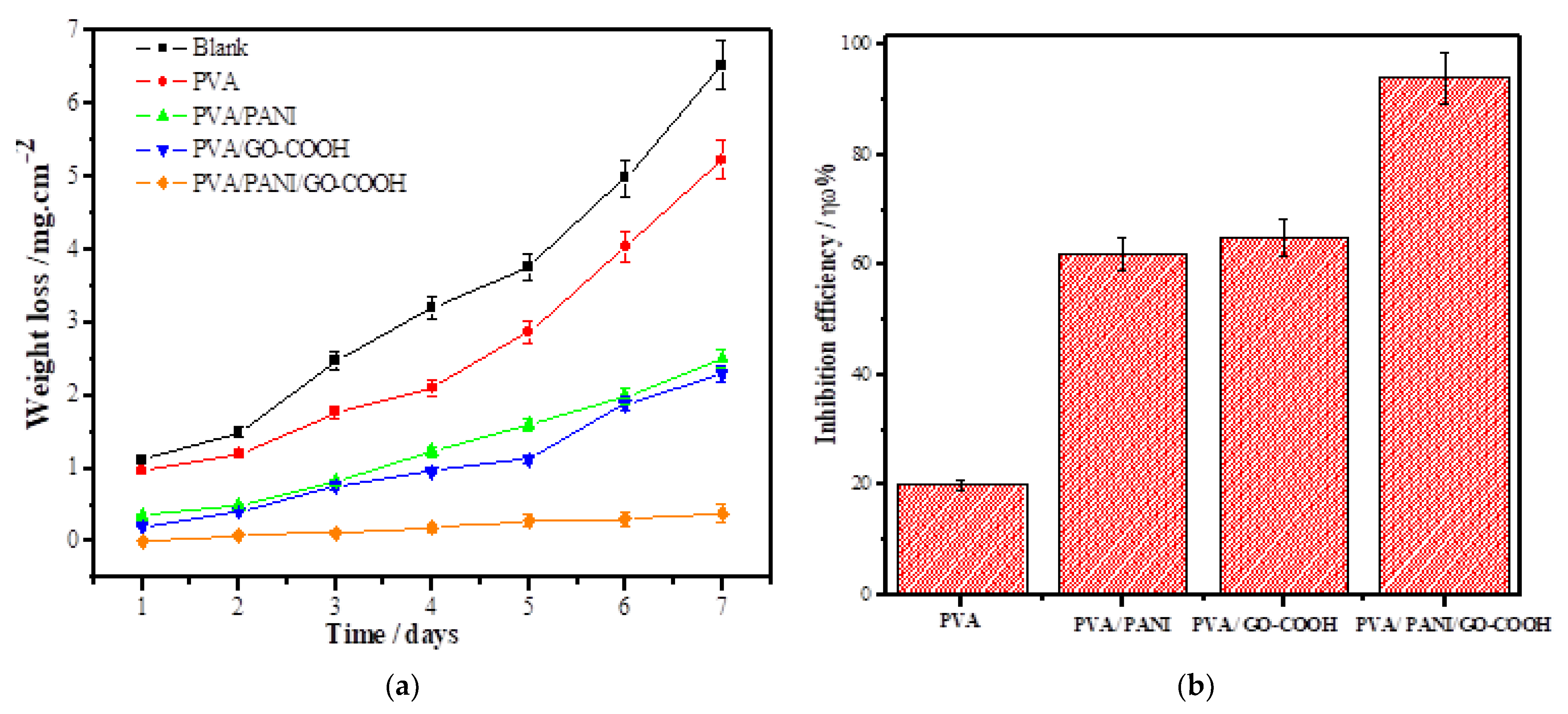
3.2.2. Weight Loss Studies
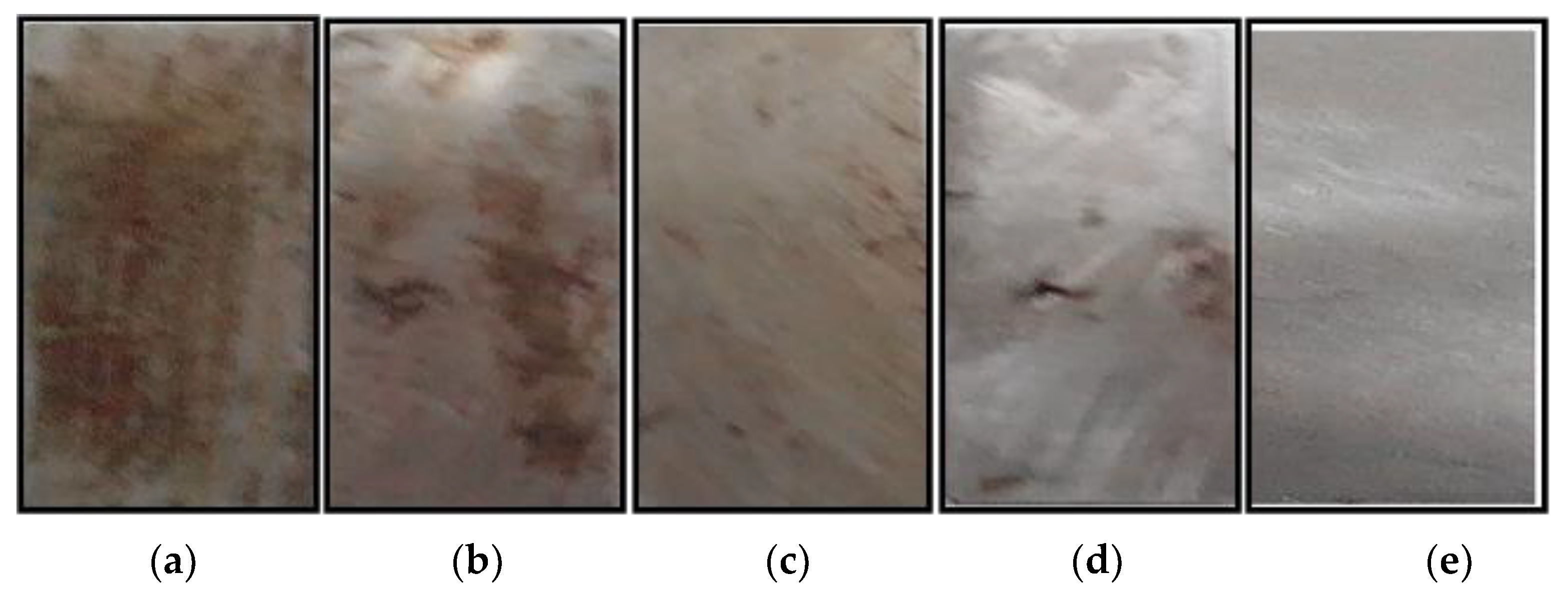
3.2.3. Morphological Characterization
3.2.4. Electrochemical Characterization
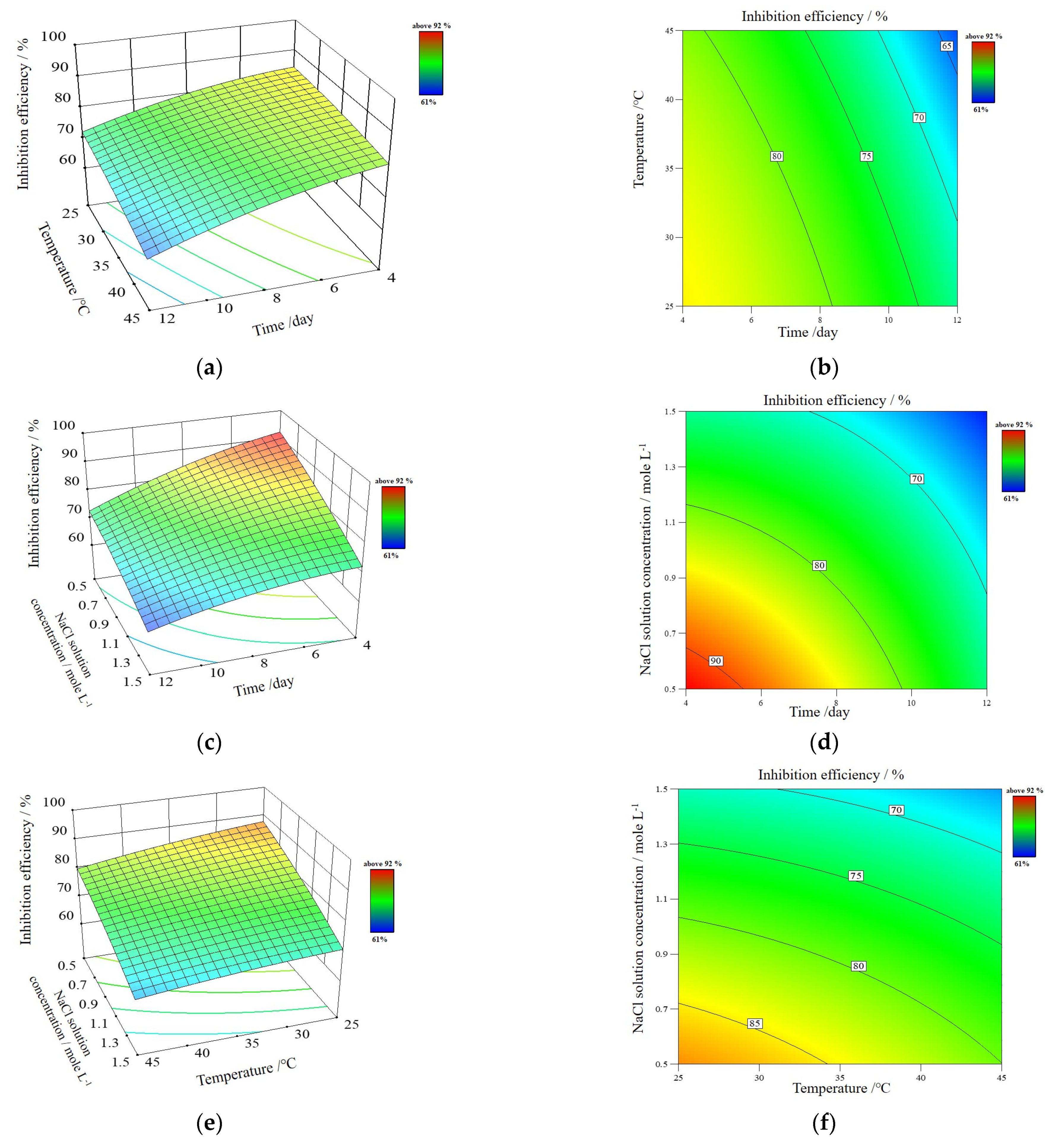
3.2.5. Optimization of Corrosion Conditions for PVA/PANI/GO-COOH Coating
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hou, X.; Gao, L.; Cui, Z.; Yin, J. Corrosion and protection of metal in the seawater desalination. IOP Conf. Ser. Earth Environ. Sci. 2018, 108, 022037. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Zhao, W.; Miao, L. Designed a novel EP + GO/ZRC + GO coating with bilayered structure for enhancing corrosion resistance of steel substrate. J. Hazard. Mater. 2021, 403, 123670. [Google Scholar] [CrossRef] [PubMed]
- Aghzzaf, A.A.; Rhouta, B.; Rocca, E.; Khalil, A.; Steinmetz, J. Corrosion inhibition of zinc by calcium exchanged beidellite clay mineral: A new smart corrosion inhibitor. Corros. Sci. 2014, 80, 46–52. [Google Scholar] [CrossRef]
- Chen, S.; Li, B.; Xiao, R.; Luo, H.; Yu, S.; He, J.; Liao, X. Design an epoxy coating with TiO2/GO/PANI nanocomposites for enhancing corrosion resistance of Q235 carbon steel. Materials 2021, 14, 2629. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, Y.; Zhou, Y.; Zhao, D.; Wang, D.; Yun, L.; Zhang, D.; Zhang, L. Improved photocathodic protection performance of g-C3N4/rGO/ZnS for 304 stainless steel. J. Phys. Chem. Solids 2021, 148, 109672. [Google Scholar] [CrossRef]
- Xu, D.; Liu, Y.; Liu, Y.; Chen, F.; Zhang, C.; Liu, B. A review on recent progress in the development of photoelectrodes for photocathodic protection: Design, properties, and prospects. Mater. Des. 2021, 197, 109235. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, Y.; Liu, X.; Shi, C.; Wang, Y.; Meng, G.; Zeng, X.; Yang, Y. A study on corrosion protection of different polyaniline coatings for mild steel. Prog. Org. Coat. 2017, 111, 240–247. [Google Scholar] [CrossRef]
- Gao, F.; Mu, J.; Bi, Z.; Wang, S.; Li, Z. Recent advances of polyaniline composites in anticorrosive coatings: A review. Prog. Org. Coat. 2021, 151, 106071. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Vakili, H.; Amini, R. The effects of addition of poly (vinyl) alcohol (PVA) as a green corrosion inhibitor to the phosphate conversion coating on the anti-corrosion and adhesion properties of the epoxy coating on the steel substrate. Appl. Surf. Sci. 2015, 327, 174–181. [Google Scholar] [CrossRef]
- Dwivedi, A.; Bharti, P.; Shukla, S. Surface assimilation and corrosion inhibition characteristic of water soluble polyvinyl alcohol on mild steel surface in 0.5M HCl solution. J. Turk. Chem. Soc. 2021, 8, 217–228. [Google Scholar] [CrossRef]
- Peng, L.; Wang, D.; Qi, J. Study on anti-corrosion of PVA-treated wheat straw and its application in reinforcement of a silty soil. Constr. Build. Mater. 2021, 291, 123305. [Google Scholar] [CrossRef]
- Jafari, Y.; Ghoreishi, S.M.; Shabani-Nooshabadi, M. Polyaniline/graphene nanocomposite coatings on copper: Electropolymerization, characterization, and evaluation of corrosion protection performance. Synth. Met. 2016, 217, 220–230. [Google Scholar] [CrossRef]
- Hikku, G.S.; Jeyasubramanian, K.; Venugopal, A.; Ghosh, R. Corrosion resistance behaviour of graphene/polyvinyl alcohol nanocomposite coating for aluminium-2219 alloy. J. Alloy. Compd. 2017, 716, 259–269. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, S.; Hong, R. Graphene oxide/polyaniline nanocomposites used in anticorrosive coatings for environmental protection. Coatings 2020, 10, 1215. [Google Scholar] [CrossRef]
- Li, J.; Shao, L.; Zhou, X.; Wang, Y. Fabrication of high strength PVA/rGO composite fibers by gel spinning. RSC Adv. 2014, 4, 43612–43618. [Google Scholar] [CrossRef]
- Gouda, M.H.; Ali, S.M.; Othman, S.S.; Al-Aziz, S.A.A.; Abu-Serie, M.M.; Elsokary, N.A.; Elessawy, N.A. Novel scaffold based graphene oxide doped electrospun iota carrageenan/polyvinyl alcohol for wound healing and pathogen reduction: In-vitro and in-vivo study. Sci. Rep. 2021, 11, 20456. [Google Scholar] [CrossRef] [PubMed]
- Srimathi, M.; Rajalakshmi, R.; Subhashini, S. Polyvinyl alcohol–sulphanilic acid water soluble composite as corrosion inhibitor for mild steel in hydrochloric acid medium. Arab. J. Chem. 2014, 7, 647–656. [Google Scholar] [CrossRef] [Green Version]
- Abbes, I.; Srasra, E. Electrical and dielectric properties of polyaniline and polyaniline/montmorillonite nanocomposite prepared by solid reaction using spectroscopy impedance. J. Nanomater. 2015, 2015, 516902. [Google Scholar]
- Schriver, M.; Regan, W.; Gannett, W.J.; Zaniewski, A.M.; Crommie, M.F.; Zettl, A. Graphene as a long-term metal oxidation barrier: Worse than nothing. ACS Nano 2013, 7, 5763–5768. [Google Scholar] [CrossRef]
- El Essawy, N.A.; Konsowa, A.H.; Elnouby, M.; Farag, H.F. A novel one-step synthesis for carbon-based nanomaterials from polyethylene terephthalate (PET) bottles waste. J. Air Waste Manag. Assoc. 2017, 67, 358–370. [Google Scholar] [CrossRef] [Green Version]
- El Essawy, N.A.; Ali, S.M.; Farag, H.A.; Konsowa, A.H.; Elnouby, M.; Hamad, H.A. Green synthesis of graphene from recycled PET bottle wastes for use in the adsorption of dyes in aqueous solution. Ecotoxicol. Environ. Saf. 2017, 145, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowski, R.; Górski, Ł.; Malinowska, E. Carboxylated graphene as a sensing material for electrochemical uranyl ion detection. Sens. Actuators B Chem. 2017, 238, 540–547. [Google Scholar] [CrossRef]
- Alaoui, K.; El Kacimi, Y.; Galai, M.; Touir, R.; Dahmani, K.; Harfi, A.; Ebn Touhami, M. Anti-corrosive properties of polyvinyl-alcohol for carbon steel in hydrochloric acid media: Electrochemical and thermodynamic investigation. J. Mater. Environ. Sci. 2016, 7, 2389–2403. [Google Scholar]
- Dong, Y.; Zhou, Y.; Ding, Y.; Chu, X.; Wang, C. Sensitive detection of Pb(II) at gold nanoparticle/polyaniline/graphene modified electrode using differential pulse anodic stripping voltammetry. Anal. Methods 2014, 6, 9367–9374. [Google Scholar] [CrossRef]
- Chhabra, V.A.; Deep, A.; Kaur, R.; Kumar, R. functionalization of graphene using carboxylation process. Int. J. Sci. Emerg. Technol. Latest Trends 2012, 4, 13–19. [Google Scholar]
- Bandeira, R.M.; Drunen, J.; Ferreira, F.A.; Rodrigues-Filho, U.P.; Tremiliosi-Filho, G. Chemically synthesized polyaniline/polyvinyl chloride blended coatings for the corrosion protection of AA7075 aluminum alloy. Corros. Sci. 2018, 139, 35–46. [Google Scholar] [CrossRef]
- Mahato, N.; Cho, M.H. Graphene integrated polyaniline nanostructured composite coating for protecting steels from corrosion: Synthesis, characterization, and protection mechanism of the coating material in acidic environment. Constr. Build. Mater. 2016, 115, 618–633. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, W.; Liu, S.; Cen, Q.; Xue, Q. Comparative tribological and corrosion resistance properties of epoxy composite coatings reinforced with functionalized fullerene C60 and graphene. Surf. Coat. Technol. 2016, 286, 354–364. [Google Scholar] [CrossRef]
- Ammar, A.U.; Shahid, M.; Ahmed, M.K.; Khan, M.; Khalid, A.; Khan, Z.A. Electrochemical study of polymer and ceramic-based nanocomposite coatings for corrosion protection of cast iron pipeline. Materials 2018, 11, 332. [Google Scholar] [CrossRef] [Green Version]
- Box, G.; Behnken, D.W. Some new three level designs for the study of quantitative variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
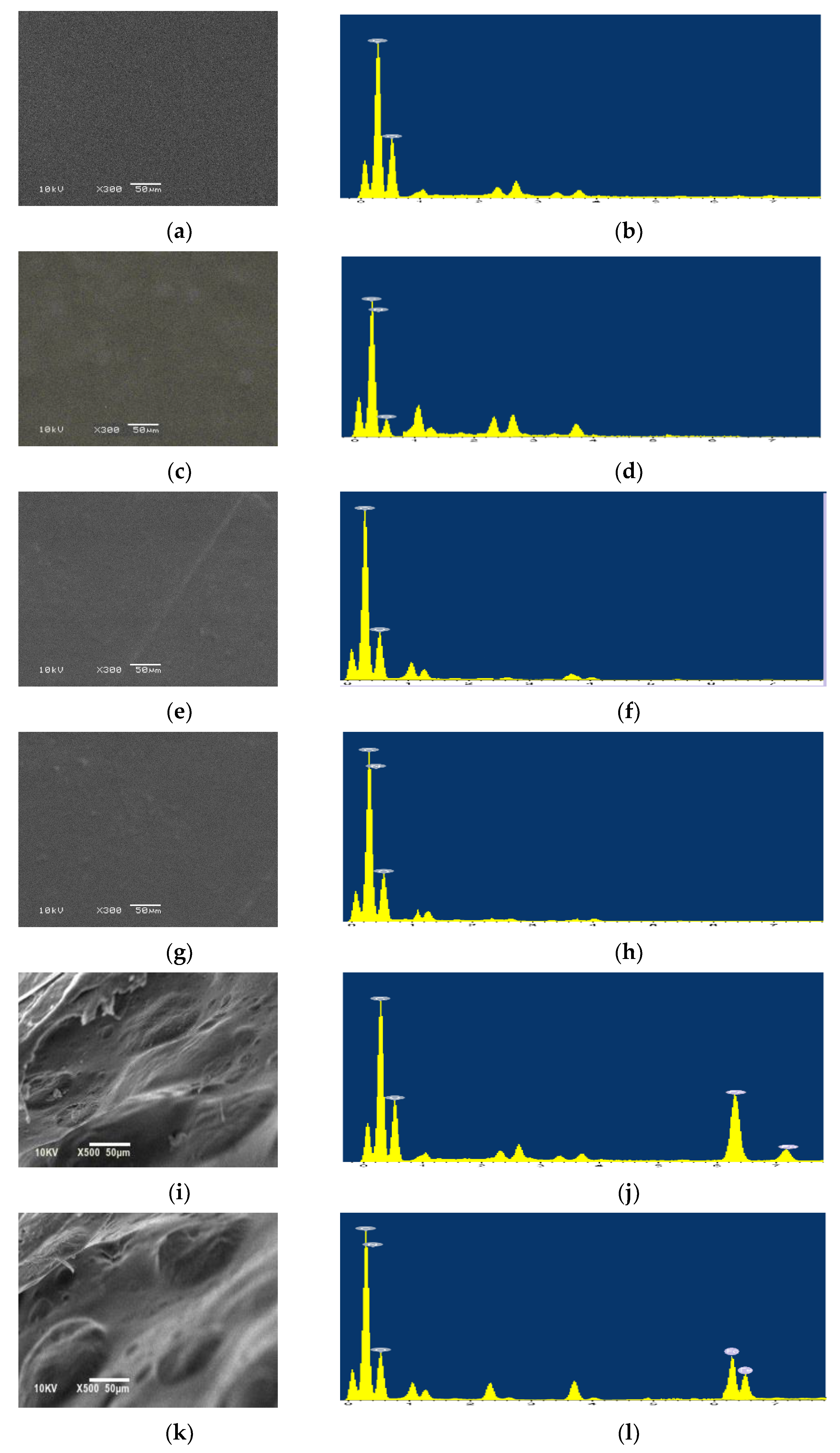
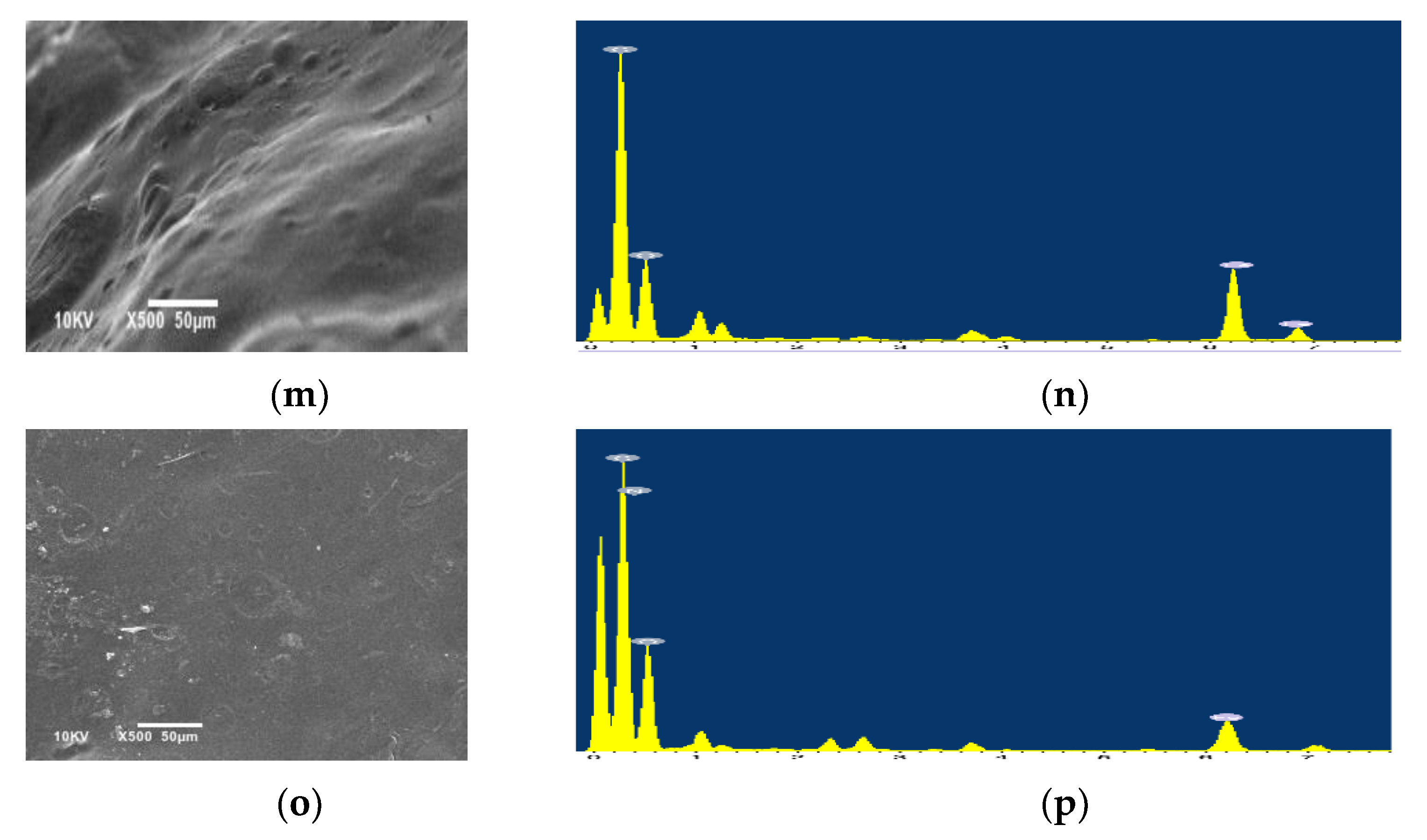
| Element | Composition, wt.% | |||||
|---|---|---|---|---|---|---|
| C | Si | Mn | S | P | Fe | |
| 3.17 | 2.84 | 0.37 | 0.12 | 0.09 | 93.41 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elessawy, N.A.; Gouda, M.H.; Elnouby, M.; Taha, N.A.; Youssef, M.E.; Santos, D.M.F. Polyvinyl Alcohol/Polyaniline/Carboxylated Graphene Oxide Nanocomposites for Coating Protection of Cast Iron in Simulated Seawater. Polymers 2022, 14, 1791. https://doi.org/10.3390/polym14091791
Elessawy NA, Gouda MH, Elnouby M, Taha NA, Youssef ME, Santos DMF. Polyvinyl Alcohol/Polyaniline/Carboxylated Graphene Oxide Nanocomposites for Coating Protection of Cast Iron in Simulated Seawater. Polymers. 2022; 14(9):1791. https://doi.org/10.3390/polym14091791
Chicago/Turabian StyleElessawy, Noha A., Marwa H. Gouda, Mohamed Elnouby, Nahla A. Taha, M. Elsayed Youssef, and Diogo M. F. Santos. 2022. "Polyvinyl Alcohol/Polyaniline/Carboxylated Graphene Oxide Nanocomposites for Coating Protection of Cast Iron in Simulated Seawater" Polymers 14, no. 9: 1791. https://doi.org/10.3390/polym14091791








