Parylene C as a Multipurpose Material for Electronics and Microfluidics
Abstract
:1. Introduction
2. Materials and Methods
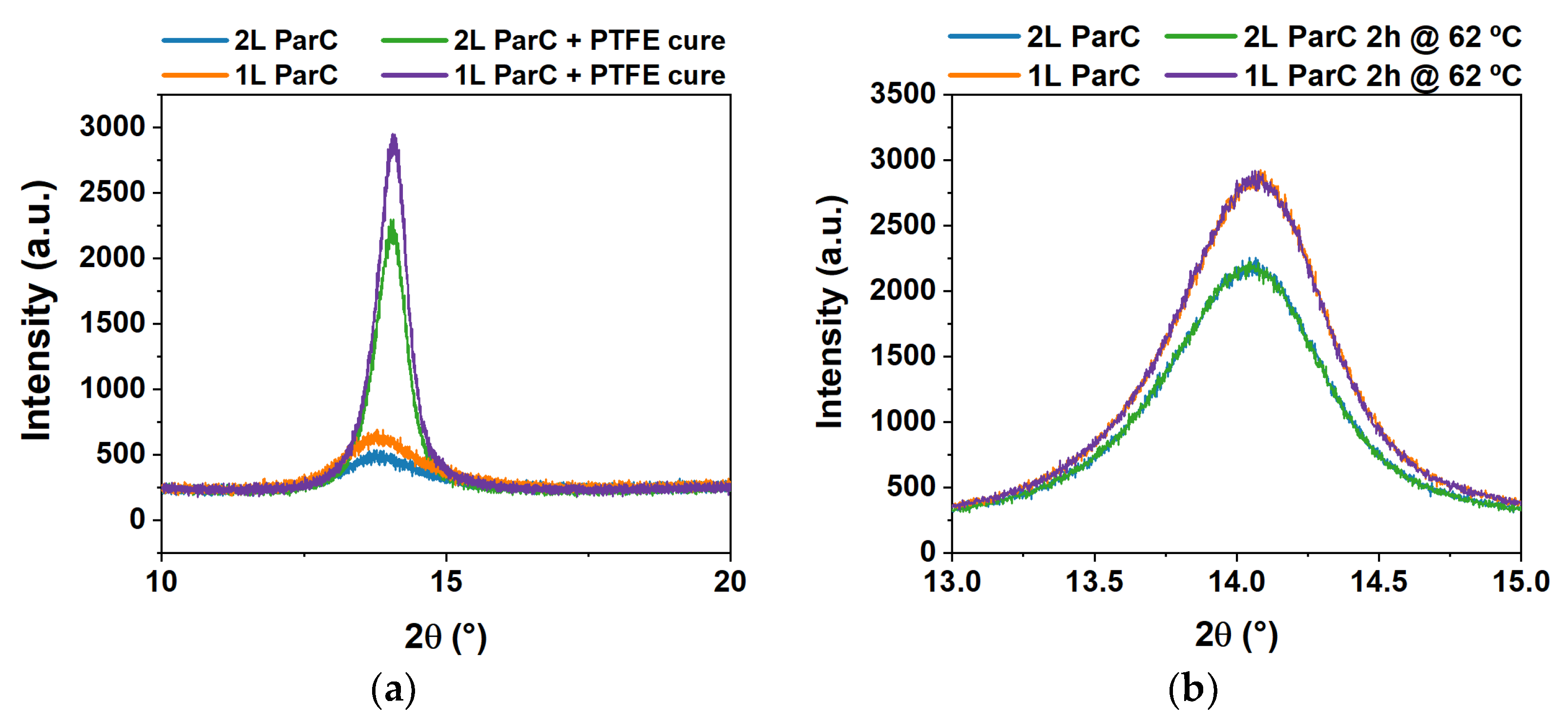
2.1. Parylene C Structural, Thermal, and Electrical Characterization
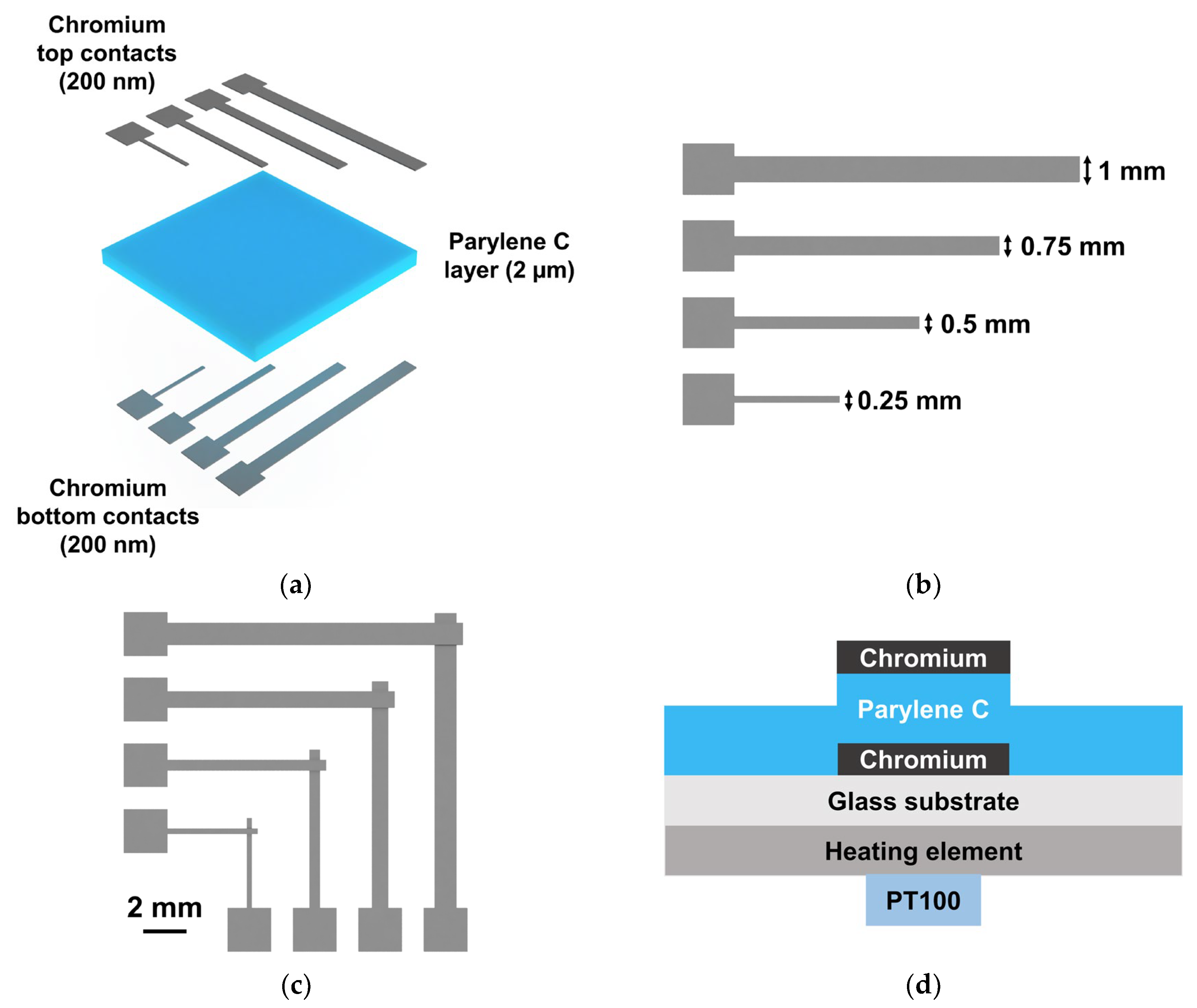
2.2. Parylene C-Based Devices
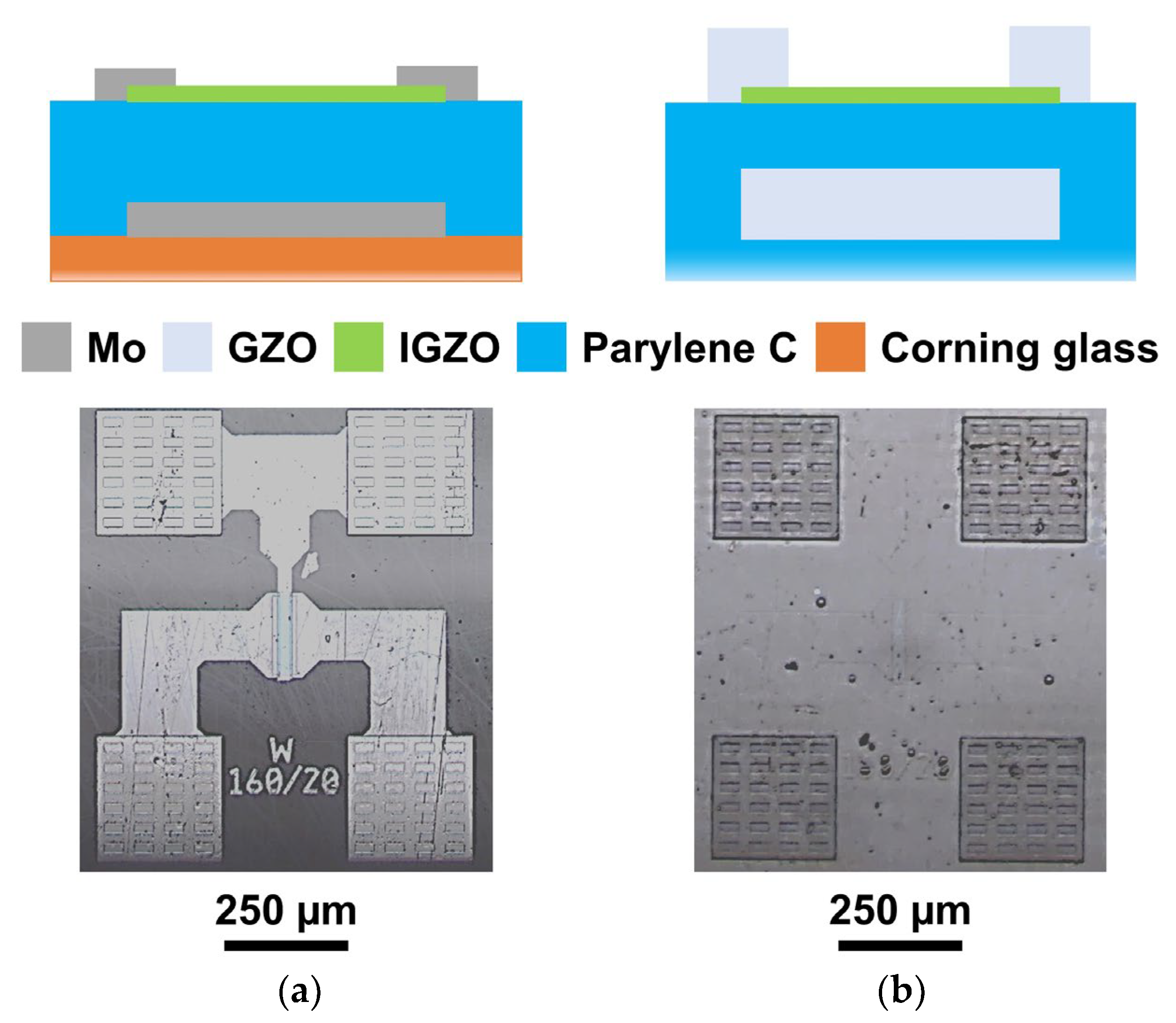
2.2.1. Thin-Film Transistors (TFT): Device Production
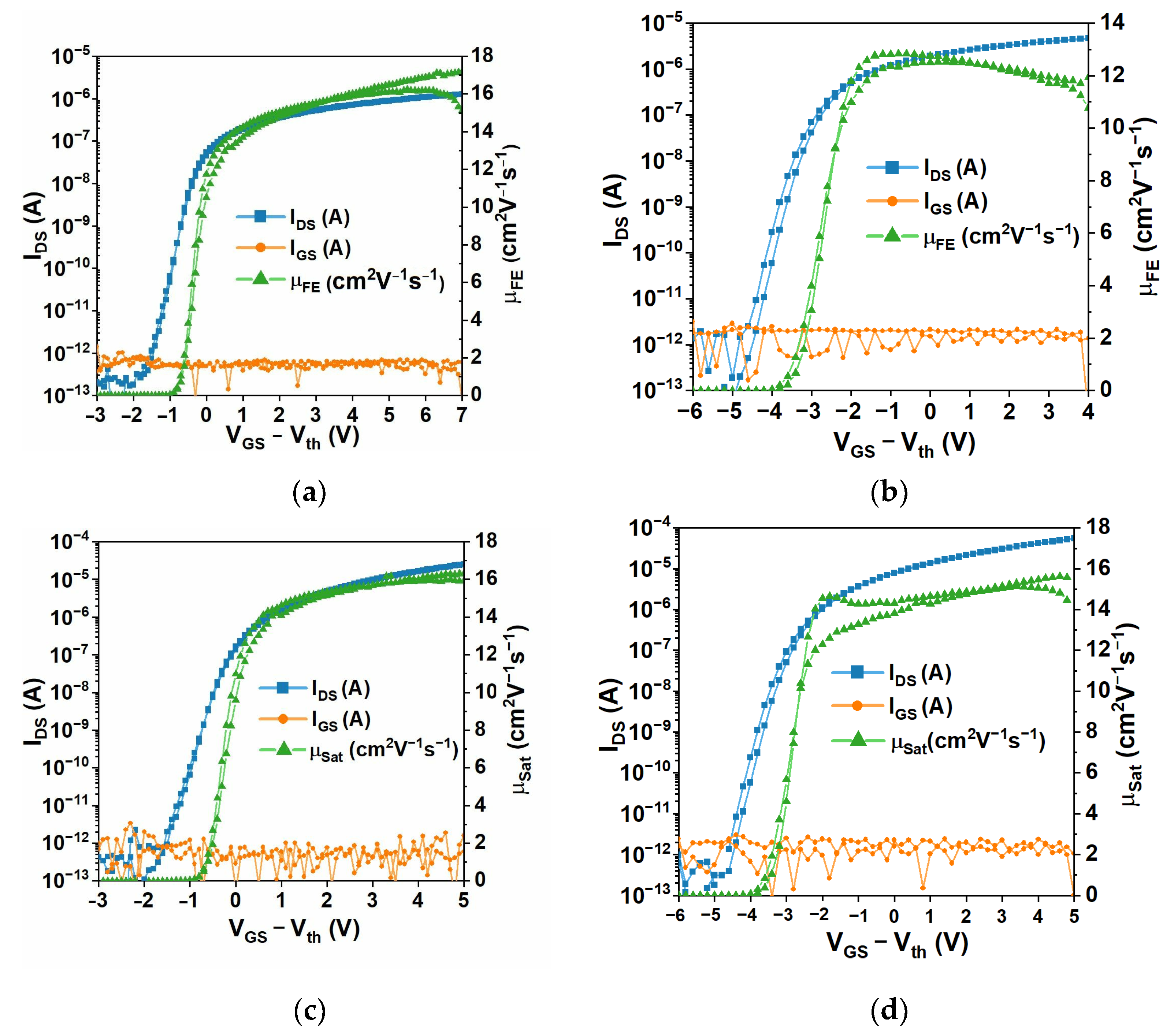
2.2.2. Thin-Film Transistors (TFT): Device Characterization
2.2.3. MIM Structures for Digital Microfluidic (DMF) Applications: Device Production
2.2.4. MIM Structures for Digital Microfluidic (DMF) Applications: Device Characterization
2.2.5. Digital Microfluidics: Device Production
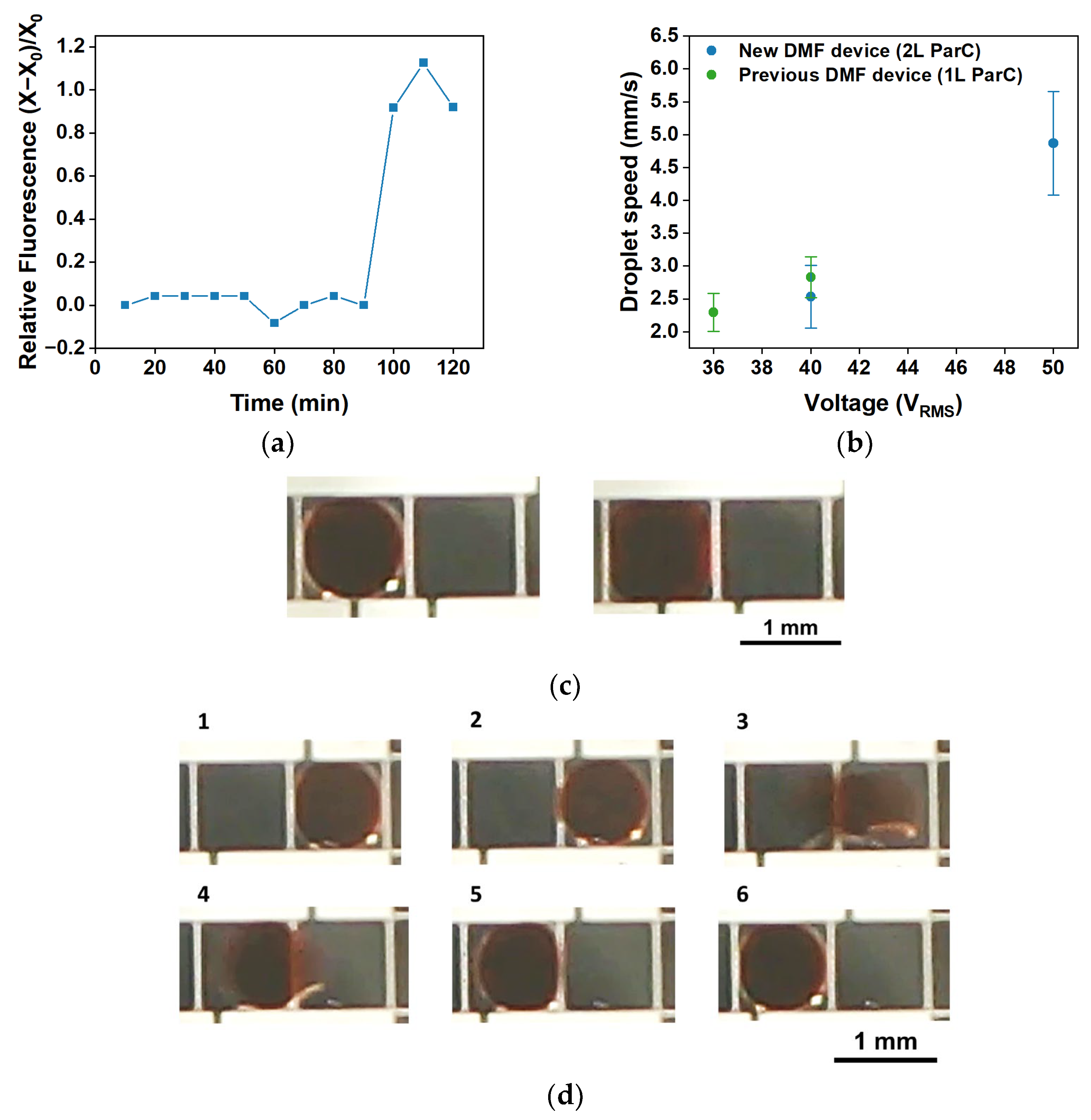
2.2.6. Digital Microfluidics: Device Characterization
3. Results and Discussion
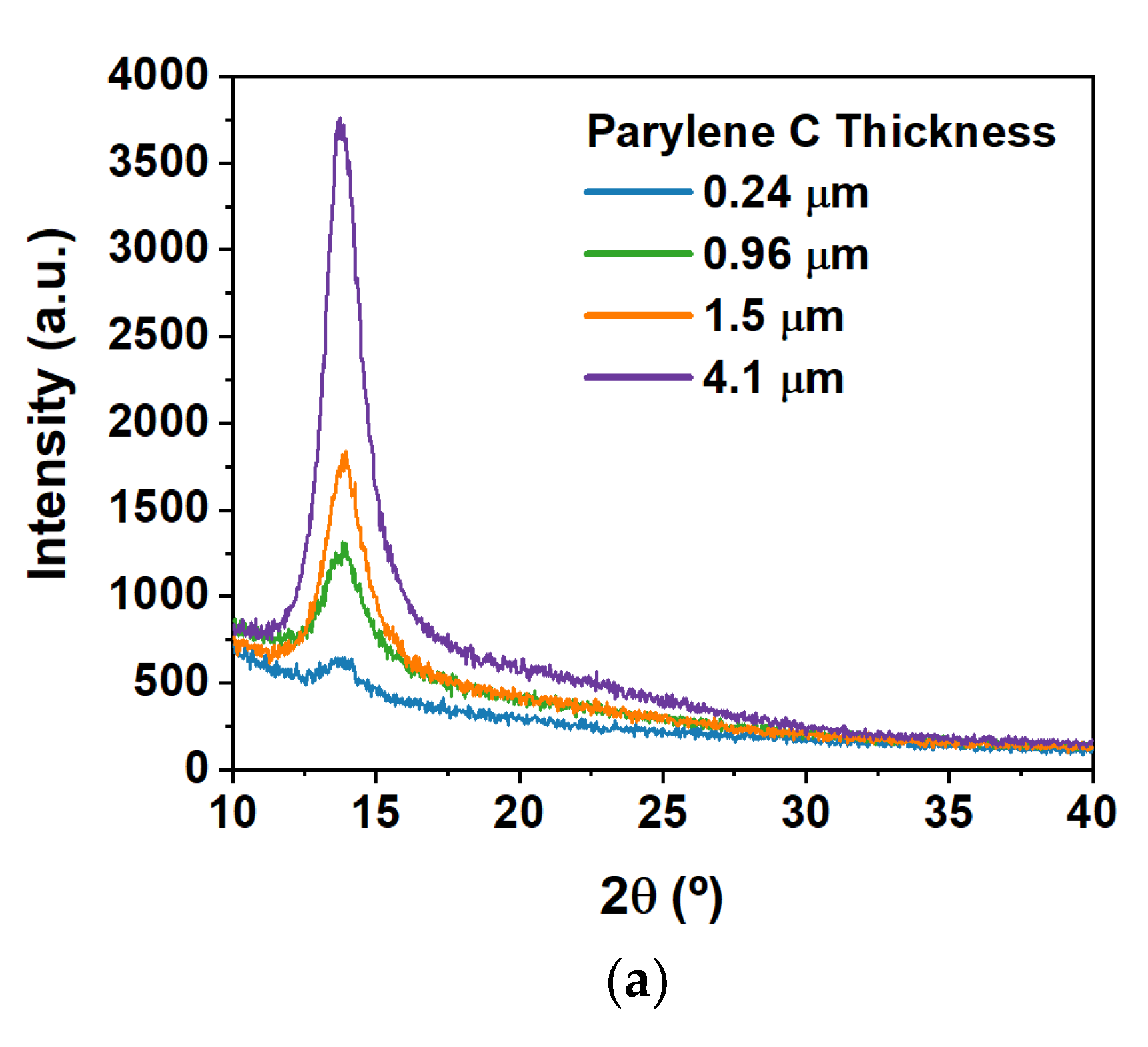
3.1. Parylene C Structural and Thermal Characterization
3.2. Electrical Properties of Parylene C
3.3. Parylene C-Powered Devices
3.3.1. TFT Oxide Electronics with Parylene C
3.3.2. MIM Structures Based on Parylene C for Mimicking Digital Microfluidic (DMF) Stress Conditions: Combined Thermal-and Electrically Stressed Devices
3.3.3. Digital Microfluidic Devices Based on Parylene C
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Khlyustova, A.; Cheng, Y.; Yang, R. Vapor-Deposited Functional Polymer Thin Films in Biological Applications. J. Mater. Chem. B 2020, 8, 6588–6609. [Google Scholar] [CrossRef] [PubMed]
- Marszalek, T.; Gazicki-Lipman, M.; Ulanski, J. Parylene C as a Versatile Dielectric Material for Organic Field-Effect Transistors. Beilstein J. Nanotechnol. 2017, 8, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Meng, E. Micromachining of Parylene C for BioMEMS: Micromachining of Parylene C for BioMEMS. Polym. Adv. Technol. 2016, 27, 564–576. [Google Scholar] [CrossRef]
- Kim, B.J.; Meng, E. Review of Polymer MEMS Micromachining. J. Micromechan. Microeng. 2016, 26, 13001. [Google Scholar] [CrossRef]
- Liu, X.; MacNaughton, S.; Shrekenhamer, D.B.; Tao, H.; Selvarasah, S.; Totachawattana, A.; Averitt, R.D.; Dokmeci, M.R.; Sonkusale, S.; Padilla, W.J. Metamaterials on Parylene Thin Film Substrates: Design, Fabrication, and Characterization at Terahertz Frequency. Appl. Phys. Lett. 2010, 96, 011906. [Google Scholar] [CrossRef]
- Sharifi, H.; Lahiji, R.R.; Lin, H.C.; Ye, P.D.; Katehi, L.P.B.; Mohammadi, S. Characterization of Parylene-N as Flexible Substrate and Passivation Layer for Microwave and Millimeter-Wave Integrated Circuits. IEEE Trans. Adv. Packag. 2009, 32, 84–92. [Google Scholar] [CrossRef]
- Specialty Coating Systems, Inc. SCS Parylene Properties; Specialty Coating Systems, Inc.: Indianapolis, IN, USA, 2018. [Google Scholar]
- Park, B.; Im, K.-J.; Cho, K.; Kim, S. Electrical Characteristics of Gold Nanoparticle-Embedded MIS Capacitors with Parylene Gate Dielectric. Org. Electron. 2008, 9, 878–882. [Google Scholar] [CrossRef]
- Gowrisanker, S.; Quevedo-Lopez, M.A.; Alshareef, H.N.; Gnade, B.E. Time Dependent Breakdown Characteristics of Parylene Dielectric in Metal–Insulator–Metal Capacitors. Org. Electron. 2009, 10, 1024–1027. [Google Scholar] [CrossRef]
- Khawaji, I.H.; Brigeman, A.N.; Awadelkarim, O.O.; Lakhtakia, A. Charge Buildup and Leakage Current in Gold/Parylene-C/Pentacene Capacitor under Constant-Voltage Stress. Flex. Print. Electron. 2020, 5, 035003. [Google Scholar] [CrossRef]
- Kim, M.; Mackenzie, D.M.A.; Kim, W.; Isakov, K.; Lipsanen, H. All-Parylene Flexible Wafer-Scale Graphene Thin Film Transistor. Appl. Surf. Sci. 2021, 551, 149410. [Google Scholar] [CrossRef]
- Kwon, H.; Ye, H.; An, T.K.; Hong, J.; Park, C.E.; Choi, Y.; Shin, S.; Lee, J.; Kim, S.H.; Li, X. Highly Stable Flexible Organic Field-Effect Transistors with Parylene-C Gate Dielectrics on a Flexible Substrate. Org. Electron. 2019, 75, 105391. [Google Scholar] [CrossRef]
- Ye, H.; Kwon, H.; Tang, X.; Park, C.E.; An, T.K.; Kim, S.H. Parylene-Based Polymeric Dielectric Top-Gate Organic Field-Effect Transistors Exposed to a UV/Ozone Environment. Org. Electron. 2020, 87, 105942. [Google Scholar] [CrossRef]
- Jang, S.; Kim, J.; Lee, K.J.; Kim, H.-S. Flexible Oxide Thin-Film Transistors Fabricated at Room Temperature By Introducing Polymer Dielectric and Microwave Annealing. ECS Meet. Abstr. 2020, MA2020-02, 1937. [Google Scholar] [CrossRef]
- Huang, S.; Liu, Y.; Zhao, Y.; Ren, Z.; Guo, C.F. Flexible Electronics: Stretchable Electrodes and Their Future. Adv. Funct. Mater. 2019, 29, 1805924. [Google Scholar] [CrossRef]
- Caironi, M.; Noh, Y.-Y. Large Area and Flexible Electronics, 1st ed.; Wiley-VCH: Weinheim, Germany, 2015; ISBN 978-3-527-33639-5. [Google Scholar]
- Trung, T.Q.; Lee, N.-E. Materials and Devices for Transparent Stretchable Electronics. J. Mater. Chem. C Mater. 2017, 5, 2202–2222. [Google Scholar] [CrossRef]
- Kuo, J.T.W.; Kim, B.J.; Hara, S.A.; Lee, C.D.; Gutierrez, C.A.; Hoang, T.Q.; Meng, E. Novel Flexible Parylene Neural Probe with 3D Sheath Structure for Enhancing Tissue Integration. Lab Chip 2013, 13, 554–561. [Google Scholar] [CrossRef]
- Wang, R.; Huang, X.; Liu, G.; Wang, W.; Dong, F.; Li, Z. Fabrication and Characterization of a Parylene-Based Three-Dimensional Microelectrode Array for Use in Retinal Prosthesis. J. Microelectromechanical Syst. 2010, 19, 367–374. [Google Scholar] [CrossRef]
- Iacovacci, V.; Naselli, I.; Salgarella, A.R.; Clemente, F.; Ricotti, L.; Cipriani, C. Stability and in Vivo Safety of Gold, Titanium Nitride and Parylene C Coatings on NdFeB Magnets Implanted in Muscles towards a New Generation of Myokinetic Prosthetic Limbs. RSC Adv. 2021, 11, 6766–6775. [Google Scholar] [CrossRef]
- Lecomte, A.; Degache, A.; Descamps, E.; Dahan, L.; Bergaud, C. In Vitro and in Vivo Biostability Assessment of Chronically-Implanted Parylene C Neural Sensors. Sens. Actuators B Chem. 2017, 251, 1001–1008. [Google Scholar] [CrossRef]
- Lu, B.; Zhu, D.; Hinton, D.; Humayun, M.S.; Tai, Y.C. Mesh-Supported Submicron Parylene-C Membranes for Culturing Retinal Pigment Epithelial Cells. Biomed. Microdevices 2012, 14, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Park, D.-W.; Kim, H.; Bong, J.; Mikael, S.; Kim, T.J.; Williams, J.C.; Ma, Z. Flexible Bottom-Gate Graphene Transistors on Parylene C Substrate and the Effect of Current Annealing. Appl. Phys. Lett. 2016, 109, 152105. [Google Scholar] [CrossRef]
- Chamlagain, B.; Li, Q.; Ghimire, N.J.; Chuang, H.-J.; Perera, M.M.; Tu, H.; Xu, Y.; Pan, M.; Xaio, D.; Yan, J.; et al. Mobility Improvement and Temperature Dependence in MoSe2 Field-Effect Transistors on Parylene-C Substrate. ACS Nano 2014, 8, 5079–5088. [Google Scholar] [CrossRef]
- Wei, T.; Fujiwara, K.; Kanki, T.; Tanaka, H. Impact of Parylene-C Thickness on Performance of KTaO3 Field-Effect Transistors with High-k Oxide/Parylene-C Hybrid Gate Dielectric. J. Appl. Phys. 2016, 119, 34502. [Google Scholar] [CrossRef]
- Jakabovič, J.; Kováč, J.; Weis, M.; Haško, D.; Srnánek, R.; Valent, P.; Resel, R. Preparation and Properties of Thin Parylene Layers as the Gate Dielectrics for Organic Field Effect Transistors. Microelectron. J. 2009, 40, 595–597. [Google Scholar] [CrossRef]
- Wang, M.L.; Chen, J.; Amer, M.; Cronin, S.; Bushmaker, A. Effects of Parylene Coating on Electron Transport in Pristine Suspended Carbon Nanotube Field-Effect-Transistors. IEEE Trans. Electron. Devices 2014, 61, 3539–3545. [Google Scholar] [CrossRef]
- Nair, S.; Kathiresan, M.; Mukundan, T.; Natarajan, V. Passivation of Organic Field Effect Transistor with Photopatterned Parylene to Improve Environmental Stability. Microelectron. Eng. 2016, 163, 36–42. [Google Scholar] [CrossRef]
- Kiazadeh, A.; Gomes, H.L.; Barquinha, P.; Martins, J.; Rovisco, A.; Pinto, J.V.; Martins, R.; Fortunato, E. Improving Positive and Negative Bias Illumination Stress Stability in Parylene Passivated IGZO Transistors. Appl. Phys. Lett. 2016, 109, 51606. [Google Scholar] [CrossRef]
- Yasuda, T.; Fujita, K.; Nakashima, H.; Tsutsui, T. Organic Field-Effect Transistors with Gate Dielectric Films of Poly-p-Xylylene Derivatives Prepared by Chemical Vapor Deposition. Jpn. J. Appl. Phys. 2003, 42, 6614–6618. [Google Scholar] [CrossRef]
- Coelho, B.J.; Veigas, B.; Fortunato, E.; Martins, R.; Águas, H.; Igreja, R.; Baptista, P.V. Digital Microfluidics for Nucleic Acid Amplification. Sensors 2017, 17, 1495. [Google Scholar] [CrossRef]
- Tan, M.K.; Friend, J.R.; Yeo, L.Y. Microparticle Collection and Concentration via a Miniature Surface Acoustic Wave Device. Lab Chip 2007, 7, 618–625. [Google Scholar] [CrossRef]
- Min, X.; Kim, W.S. Beyond High Voltage in the Digital Microfluidic Devices for an Integrated Portable Sensing System. Microfluid. Nanofluidics 2019, 23, 127. [Google Scholar] [CrossRef]
- Paul, G.; Das, P.K.; Manna, I. Motion, Deformation and Pearling of Ferrofluid Droplets Due to a Tunable Moving Magnetic Field. Soft Matter 2020, 16, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Samiei, E.; Tabrizian, M.; Hoorfar, M. A Review of Digital Microfluidics as Portable Platforms for Lab-on a-Chip Applications. Lab Chip 2016, 16, 2376–2396. [Google Scholar] [CrossRef] [PubMed]
- Coelho, B.J.; Veigas, B.; Bettencourt, L.; Águas, H.; Fortunato, E.; Martins, R.; Baptista, P.V.; Igreja, R. Digital Microfluidics-Powered Real-Time Monitoring of Isothermal DNA Amplification of Cancer Biomarker. Biosensors 2022, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Coelho, B.J.; Veigas, B.; Águas, H.; Fortunato, E.; Martins, R.; Baptista, P.P.V.; Igreja, R. A Digital Microfluidics Platform for Loop-Mediated Isothermal Amplification Detection. Sensors 2017, 17, 2616. [Google Scholar] [CrossRef]
- Coelho, B.J.; Veigas, B.; Bettencourt, L.; Águas, H.; Fortunato, E.; Martins, R.; Baptista, P.V.; Igreja, R. Digital Microfluidics for Amplification Monitoring of Cancer Biomarkers. Mater. Proc. 2022, 8, 8103. [Google Scholar] [CrossRef]
- Chen, L.; Bonaccurso, E. Electrowetting—From Statics to Dynamics. Adv. Colloid Interface Sci. 2014, 210, 2–12. [Google Scholar] [CrossRef]
- Gao, J.; Chen, T.; Dong, C.; Jia, Y.; Mak, P.-I.; Vai, M.-I.; Martins, R.P. Adhesion Promoter for a Multi-Dielectric-Layer on a Digital Microfluidic Chip. RSC Adv. 2015, 5, 48626–48630. [Google Scholar] [CrossRef]
- Charmet, J.; Bitterli, J.; Sereda, O.; Liley, M.; Renaud, P.; Keppner, H. Optimizing Parylene C Adhesion for MEMS Processes: Potassium Hydroxide Wet Etching. J. Microelectromechanical Syst. 2013, 22, 855–864. [Google Scholar] [CrossRef]
- Martins, J.; Kiazadeh, A.; Pinto, J.V.; Rovisco, A.; Gonçalves, T.; Deuermeier, J.; Alves, E.; Martins, R.; Fortunato, E.; Barquinha, P. Ta2O5/SiO2 Multicomponent Dielectrics for Amorphous Oxide TFTs. Electron. Mater. 2020, 2, 1. [Google Scholar] [CrossRef]
- Fortunato, E.; Barquinha, P.; Martins, R. Oxide Semiconductor Thin-Film Transistors: A Review of Recent Advances. Adv. Mater. 2012, 24, 2945–2986. [Google Scholar] [CrossRef]
- Senkevich, J.J.; Desu, S.B. Morphology of Poly(Chloro-p-Xylylene) CVD Thin Films. Polymer 1999, 40, 5751–5759. [Google Scholar] [CrossRef]
- Senkevich, J.J.; Desu, S.B. Poly(Chloro-p-Xylylene)/SiO2 Multilayer Thin Films Deposited near-Room Temperature by Thermal CVD. Thin Solid Films 1998, 322, 148–157. [Google Scholar] [CrossRef]
- Isoda, S.; Ichida, T.; Kawaguchi, A.; Katayama, K. Crystal structure of poly(2-chloro-p-xylylene). Bull. Inst. Chem. Res. Kyoto Univ. 1983, 61, 222–228. [Google Scholar]
- Advanced Coatings Parylene C Specifications. Available online: https://www.advancedcoating.com/typical-specification-parylene-c (accessed on 3 August 2022).
- VSI Parylene Parylene Properties. Available online: https://vsiparylene.com/parylene-properties/ (accessed on 3 August 2022).
- Mugele, F.; Baret, J. Electrowetting: From Basics to Applications. J. Phys. Condens. Matter 2005, 17, R705–R774. [Google Scholar] [CrossRef]
- Hassler, C.; von Metzen, R.P.; Ruther, P.; Stieglitz, T. Characterization of Parylene C as an Encapsulation Material for Implanted Neural Prostheses. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 93B, 266–274. [Google Scholar] [CrossRef]
- Hsu, J.-M.; Rieth, L.; Kammer, S.; Orthner, M.; Solzbacher, F. Effect of Thermal and Deposition Processes on Surface Morphology, Crystallinity, and Adhesion of Parylene-C. Sens. Mater. 2008, 20, 87–102. [Google Scholar] [CrossRef]
- Surendran, G.; Gazicki, M.; James, W.J.; Yasuda, H. Polymerization of Para-Xylylene Derivatives. V. Effects of the Sublimation Rate of Di-p-Xylylene on the Crystallinity of Parylene C Deposited at Different Temperatures. J. Polym. Sci. A Polym. Chem. 1987, 25, 2089–2106. [Google Scholar] [CrossRef]
- Rao, V.K.P.; Sagar, T. Numerical Evaluation of the Influence of AC and DC Electric Field on the Response of the Droplet. In Proceedings of the 3rd International Conference on Condensed Matter & Applied Physics (ICC 2019), Bikaner, India, 14–15 October 2019; AIP Publishing: Melville, NY, USA, 2020; Volume 2220, p. 130006. [Google Scholar] [CrossRef]
- Fair, R.B. Digital Microfluidics: Is a True Lab-on-a-Chip Possible? Microfluid. Nanofluidics 2007, 3, 245–281. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Garrell, R.L. Preventing Biomolecular Adsorption in Electrowetting-Based Biofluidic Chips. Anal. Chem. 2003, 75, 5097–5102. [Google Scholar] [CrossRef]
- García-Sánchez, P.; Ramos, A.; Mugele, F. Electrothermally Driven Flows in Ac Electrowetting. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2010, 81, 015303. [Google Scholar] [CrossRef] [PubMed]
- Samad, M.F.; Kouzani, A.Z.; Hossain, M.F.; Mohammed, M.I.; Alam, M.N.H. Reducing Electrowetting-on-Dielectric Actuation Voltage Using a Novel Electrode Shape and a Multi-Layer Dielectric Coating. Microsyst. Technol. 2017, 23, 3005–3013. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Evans, R.D.; Welch, E.; Hsu, B.N.; Madison, A.C.; Fair, R.B. Low Voltage Electrowetting-on-Dielectric Platform Using Multi-Layer Insulators. Sens. Actuators B Chem. 2010, 150, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Cho, S.K.; Garrell, R.L.; Kim, C.-J.; Garrell, R.L. Low Voltage Electrowetting-on-Dielectric. J. Appl. Phys. 2002, 92, 4080–4087. [Google Scholar] [CrossRef]
- Zou, F.; Ruan, Q.; Lin, X.; Zhang, M.; Song, Y.; Zhou, L.; Zhu, Z.; Lin, S.; Wang, W.; Yang, C.J. Rapid, Real-Time Chemiluminescent Detection of DNA Mutation Based on Digital Microfluidics and Pyrosequencing. Biosens. Bioelectron. 2019, 126, 551–557. [Google Scholar] [CrossRef]
- Chemorus. TeflonTM AF Amorphous Fluoroplastic Resins—Product Information; Chemorus: Wilmington, DE, USA, 2016. [Google Scholar]
- 3M Science. 3MTM FluorinertTM Electronic Liquid FC-40 Product Description; 3M Science: Saint Paul, MN, USA, 2019. [Google Scholar]
- Nelson, W.C.; Kim, C.-J. ‘Cj’ Droplet Actuation by Electrowetting-on-Dielectric (EWOD): A Review. J. Adhes. Sci. Technol. 2012, 26, 1747–1771. [Google Scholar] [CrossRef]
- Norian, H.S.; Shepard, K.; Kymissis, J.; Field, R. An Integrated CMOS Quantitative-Polymerase-Chain-Reaction Lab-on-Chip for Point-of-Care Diagnostics. Lab Chip 2014, 14, 4076–4084. [Google Scholar] [CrossRef]
- Kühnemund, M.; Witters, D.; Nilsson, M.; Lammertyn, J. Circle-to-Circle Amplification on a Digital Microfluidic Chip for Amplified Single Molecule Detection. Lab Chip 2014, 14, 2983–2992. [Google Scholar] [CrossRef]
- Kaneko, H.; Kawana, T.; Fukushima, E.; Suzutani, T. Tolerance of Loop-Mediated Isothermal Amplification to a Culture Medium and Biological Substances. J. Biochem. Biophys. Methods 2007, 70, 499–501. [Google Scholar] [CrossRef]
- Francois, P.; Tangomo, M.; Hibbs, J.; Bonetti, E.J.; Boehme, C.C.; Notomi, T.; Perkins, M.D.; Schrenzel, J. Robustness of a Loop-Mediated Isothermal Amplification Reaction for Diagnostic Applications. FEMS Immunol. Med. Microbiol. 2011, 62, 41–48. [Google Scholar] [CrossRef]
- Li, W.; Rodger, D.; Menon, P.; Tai, Y.-C. Corrosion Behavior of Parylene-Metal-Parylene Thin Films in Saline. ECS Trans. 2008, 11, 18. [Google Scholar] [CrossRef]
- Von Metzen, R.P.; Stieglitz, T. The Effects of Annealing on Mechanical, Chemical, and Physical Properties and Structural Stability of Parylene C. Biomed. Microdevices 2013, 15, 727–735. [Google Scholar] [CrossRef]
- Li, W.; Rodger, D.C.; Meng, E.; Weiland, J.D.; Humayun, M.S.; Tai, Y.-C. Flexible Parylene Packaged Intraocular Coil for Retinal Prostheses. In Proceedings of the 2006 International Conference on Microtechnologies in Medicine and Biology, Okinawa, Japan, 9–12 May 2006; IEEE: Okinawa, Japan, 2006; pp. 105–108. [Google Scholar]
- Hsu, J.-M.; Rieth, L.; Normann, R.A.; Tathireddy, P.; Solzbacher, F. Encapsulation of an Integrated Neural Interface Device with Parylene C. IEEE Trans. Biomed. Eng. 2009, 56, 23–29. [Google Scholar] [CrossRef]
- Schultz, A.; Chevalliot, S.; Kuiper, S.; Heikenfeld, J. Detailed Analysis of Defect Reduction in Electrowetting Dielectrics through a Two-Layer “barrier” Approach. Thin Solid Films 2013, 534, 348–355. [Google Scholar] [CrossRef]
- Papathanasiou, A.G.; Boudouvis, A.G. Manifestation of the Connection between Dielectric Breakdown Strength and Contact Angle Saturation in Electrowetting. Appl. Phys. Lett. 2005, 86, 164102. [Google Scholar] [CrossRef]
- Drygiannakis, A.I.; Papathanasiou, A.G.; Boudouvis, A.G. On the Connection between Dielectric Breakdown Strength, Trapping of Charge, and Contact Angle Saturation in Electrowetting. Langmuir 2009, 25, 147–152. [Google Scholar] [CrossRef]
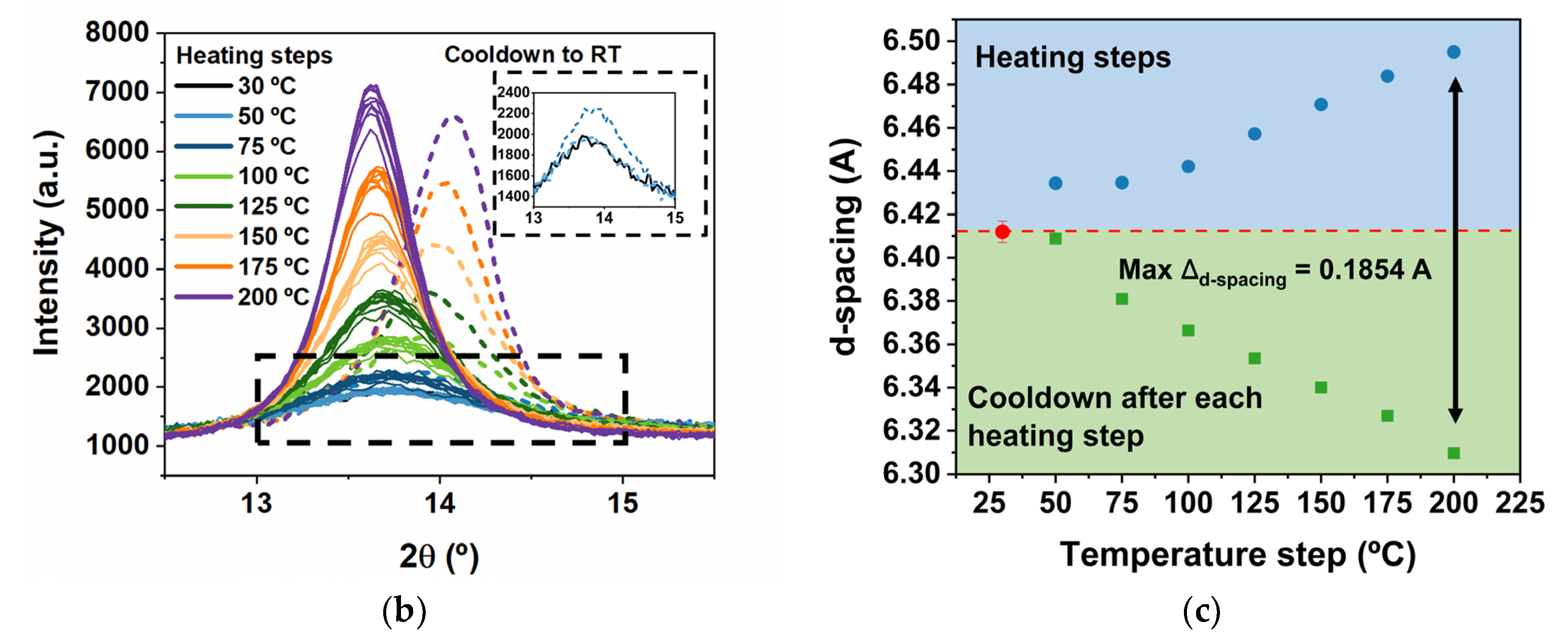





| Temperature (°C) | 2θ | d-Spacing (Å) | 2θ (RT) | d-Spacing (Å) RT | Δd-Spacing | Crystallite Size RT (Å) |
|---|---|---|---|---|---|---|
| RT | - | - | 13.80 | 6.41 | - | 53 |
| 50 | 13.75 | 6.43 | 13.81 | 6.41 | 0.02 | 54 |
| 75 | 13.75 | 6.43 | 13.87 | 6.38 | 0.05 | 59 |
| 100 | 13.73 | 6.44 | 13.90 | 6.37 | 0.07 | 76 |
| 125 | 13.70 | 6.46 | 13.93 | 6.35 | 0.11 | 86 |
| 150 | 13.67 | 6.47 | 13.96 | 6.34 | 0.13 | 102 |
| 175 | 13.65 | 6.48 | 13.99 | 6.33 | 0.15 | 116 |
| 200 | 13.62 | 6.50 | 14.02 | 6.31 | 0.19 | 130 |
| Thickness (μm) | Capacitance per Unit Area at 100 kHz (nF/cm2) | Dielectric Constant at 100 kHz | tan δ at 100 kHz | Maximum E Field Tested (MV/cm) |
|---|---|---|---|---|
| 0.24 | 9.48 | 2.60 | 0.028 | 4.11 |
| 0.46 | 6.21 | 3.24 | 0.030 | 2.16 |
| 0.96 | 3.00 | 3.25 | 0.027 | 1.04 |
| 1.14 | 2.48 | 3.19 | 0.035 | 0.88 |
| 1.45 | 1.83 | 3.20 | 0.024 | 0.69 |
| Peak Height (a.u.) | Peak Position (2θ) | FWHM (2θ) | d-Spacing (Å) | Crystallite Size (Å) | |
|---|---|---|---|---|---|
| Single layer | 252.15 | 13.84° | 1.54 | 6.39 | 43 |
| Double layer | 164.27 | 13.85° | 1.70 | 6.39 | 40 |
| Single layer and cure | 1691.37 | 14.03° | 0.66 | 6.31 | 116 |
| Double layer and cure | 1277.65 | 14.01° | 0.67 | 6.32 | 110 |
| Peak Height (a.u.) | Peak Position (2θ) | FWHM (2θ) | d-Spacing (Å) | Crystallite Size (Å) | |
|---|---|---|---|---|---|
| Single layer and cure | 1691.37 | 14.03° | 0.66 | 6.31 | 116 |
| Double layer and cure | 1277.65 | 14.01° | 0.67 | 6.32 | 110 |
| Single layer, cure, and 62 °C for 2 h | 1700.28 | 14.03° | 0.65 | 6.31 | 116 |
| Double layer, cure and 62 °C for 2 h | 1259.11 | 14.00° | 0.68 | 6.32 | 112 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, B.J.; Pinto, J.V.; Martins, J.; Rovisco, A.; Barquinha, P.; Fortunato, E.; Baptista, P.V.; Martins, R.; Igreja, R. Parylene C as a Multipurpose Material for Electronics and Microfluidics. Polymers 2023, 15, 2277. https://doi.org/10.3390/polym15102277
Coelho BJ, Pinto JV, Martins J, Rovisco A, Barquinha P, Fortunato E, Baptista PV, Martins R, Igreja R. Parylene C as a Multipurpose Material for Electronics and Microfluidics. Polymers. 2023; 15(10):2277. https://doi.org/10.3390/polym15102277
Chicago/Turabian StyleCoelho, Beatriz J., Joana V. Pinto, Jorge Martins, Ana Rovisco, Pedro Barquinha, Elvira Fortunato, Pedro V. Baptista, Rodrigo Martins, and Rui Igreja. 2023. "Parylene C as a Multipurpose Material for Electronics and Microfluidics" Polymers 15, no. 10: 2277. https://doi.org/10.3390/polym15102277