Effect of Solvent and Monomer Ratio on the Properties of Polyphenylene Sulphone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterisation and Methods
2.2. Chemicals
2.3. Synthesis of PPSU
3. Results and Discussion
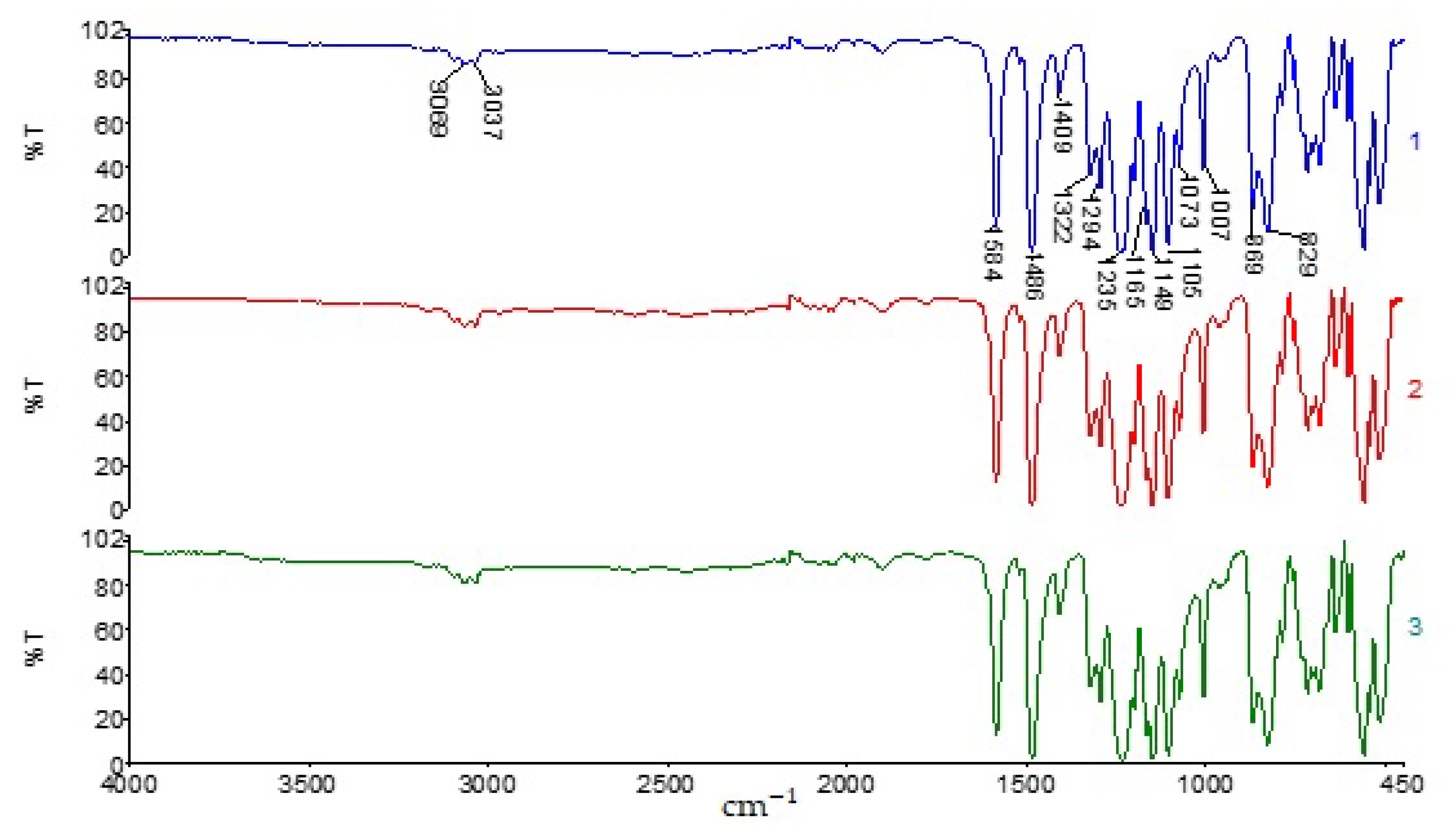
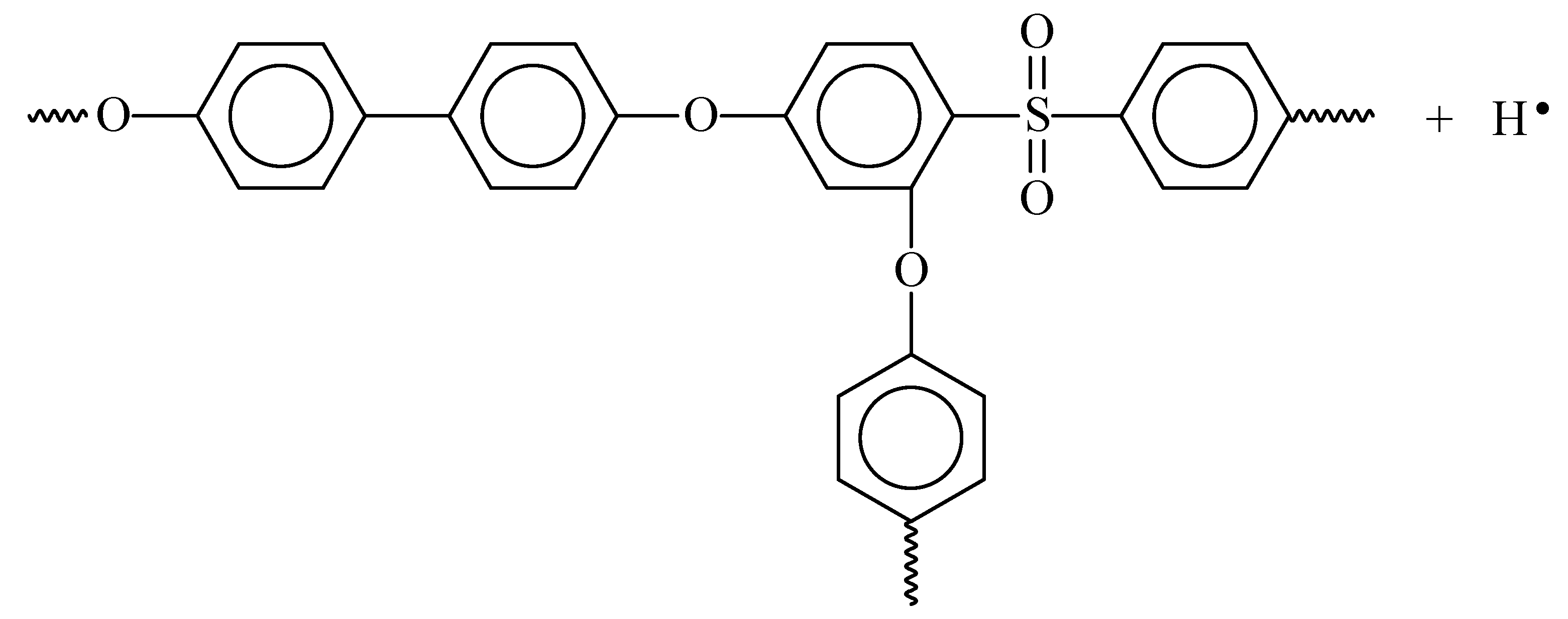
3.1. Investigation of the Influence of the Solvent on the Properties of PPSU
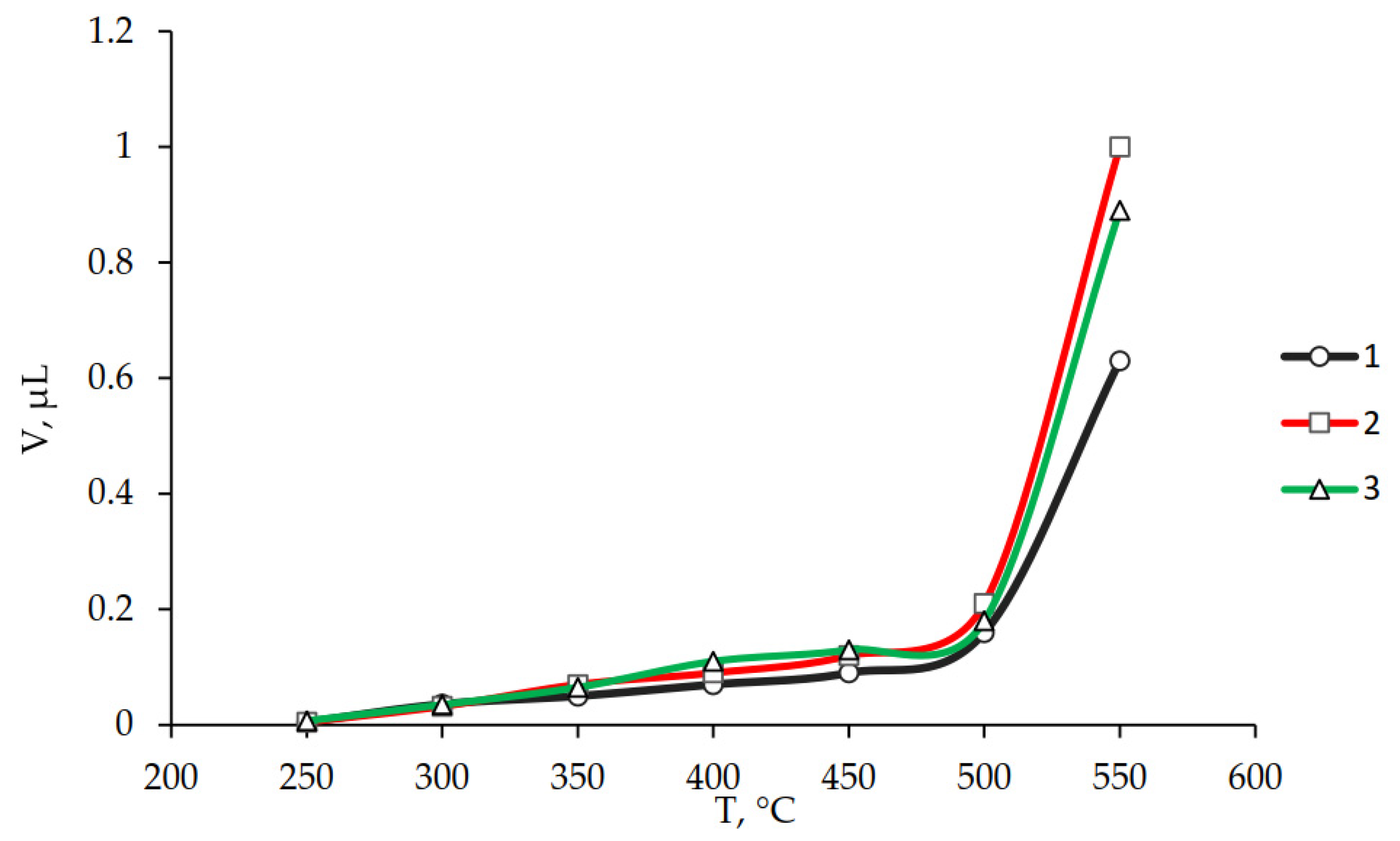
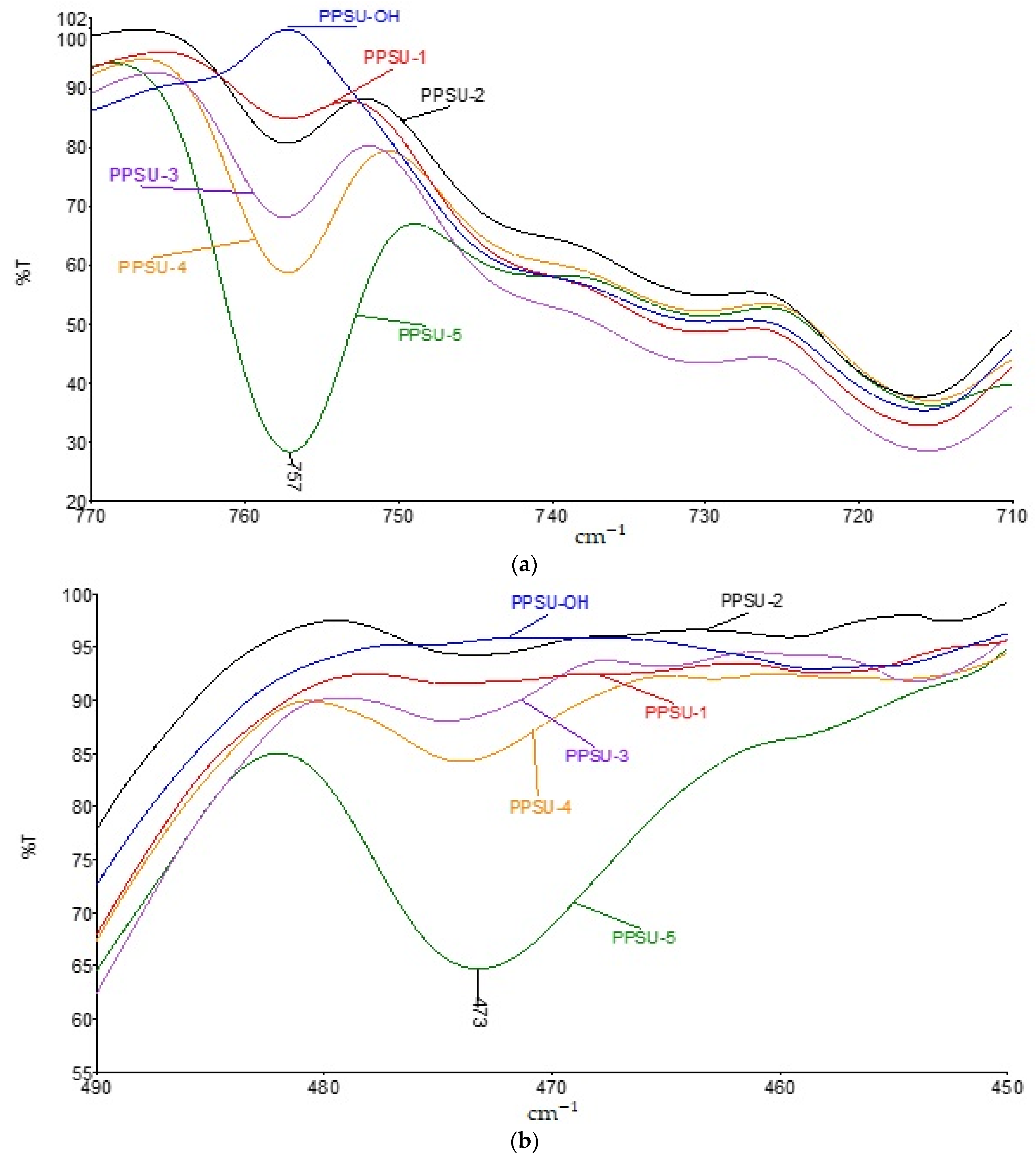
3.2. Investigation of the Influence of the Ratio of Components on the Properties of Polyphenylene Sulfone
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Parker, D.; Bussink, J.; van de Grampel, H.T.; Wheatley, G.W.; Dorf, E.-U.; Ostlinning, E.; Reinking, K.; Schubert, F.; Jünger, O.; Wagener, R. Polymers, High-Temperature. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinham, Germany, 2012. [Google Scholar]
- El-Hibri, M.; Jamal; Weinberg, S.A. Polysulfones. In Encyclopedia of Polymer Science and Technology, 4th ed.; Mark, H.F., Ed.; Wiley: New York, NY, USA, 2014; Volume 11, pp. 179–204. [Google Scholar]
- Available online: https://products.basf.com/de/en/Ultrason.html (accessed on 2 April 2023).
- Zhansitov, A.A.; Khashirova, S.Y.; Slonov, A.L.; Kurdanova, Z.I.; Shabaev, A.S.; Khashirov, A.A.; Mikitaev, A.K. Development of technology of polysulfone production for 3D printing. High Perform. Polym. 2017, 29, 724–729. [Google Scholar] [CrossRef]
- Slonov, A.L.; Khashirov, A.A.; Zhansitov, A.A.; Rzhevskaya, E.V.; Khashirova, S.Y. The influence of the 3D-printing technology on the physical and mechanical properties of polyphenylene sulfone. Rapid Prototyp. J. 2018, 24, 1124–1130. [Google Scholar] [CrossRef]
- Praneeth, K.; Tardio, J.; Sridhar, S. Design of novel ultrafiltration systems based on robust polyphenylsulfone hollow fiber membranes for treatment of contaminated surface water. Chem. Eng. J. 2014, 248, 297–306. [Google Scholar] [CrossRef]
- Darvishmanesh, S.; Jansen, J.; Tasselli, F.; Tocci, E.; Luis, P.; Degrève, J.; Drioli, E.; Van der Bruggen, B. Novel polyphenylsulfone membrane for potential use in solvent nanofiltration. J. Membr. Sci. 2011, 379, 60–68. [Google Scholar] [CrossRef]
- Darvishmanesh, S.; Tasselli, F.; Jansen, J.; Tocci, E.; Bazzarelli, F.; Bernardo, P.; Luis, P.; Degrève, J.; Drioli, E.; Van der Bruggen, B. Preparation of solvent stable polyphenylsulfone hollow fiber nanofiltration membranes. J. Membr. Sci. 2011, 384, 89–96. [Google Scholar] [CrossRef]
- Anokhina, T.; Raeva, A.; Sokolov, S.; Storchun, A.; Filatova, M.; Zhansitov, A.; Kurdanova, Z.; Shakhmurzova, K.; Khashirova, S.; Borisov, I. Effect of Composition and Viscosity of Spinning Solution on Ultrafiltration Properties of Polyphenylene Sulfone Hollow-Fiber Membranes. Membranes 2022, 12, 1113. [Google Scholar] [CrossRef] [PubMed]
- Gavalyan, V.B. On the effect of terminal halogen atoms on the thermal properties of poly-p-phenylene sulfides. Vysokomolek. Soyed. 1978, 10, 768–771. [Google Scholar]
- Danilina, L.; Teleshov, E.; Pravednikov, A. Thermal degradation of aromatic polysulphones. Vysokomolek. Soyed. 1974, 16, 581. [Google Scholar] [CrossRef]
- Garner, S.M.; Cites, J.S.; He, M.; Wang, J. Polysulfone as an electro-optic polymer host material. Appl. Phys. Lett. 2004, 8, 1049–1051. [Google Scholar] [CrossRef]
- Lakshmana, R.V. Polyether Sulfones. J. Macromol. Sci. Part C Polym. Rev. 1999, 39, 655–711. [Google Scholar] [CrossRef]
- Kurdanova, Z.I. Synthesis and Properties of Polyphenylene Sulfone and Its Copolymers for Use in Additive Technologies. Ph.D. Thesis, Kabardino-Balkarian State University, Nalchik, Russia, 2017. [Google Scholar]
- Kricheldorf, H.R.; Jahnke, P. Synthesis of aromatic polyethers from silylated diphenols and activated dichloro-substituted aromatics. Macromol. Chem. 1990, 191, 2027–2035. [Google Scholar] [CrossRef]
- Labadie, J.W.; Hedrick, J.L. New activating groups for poly(aryl ether) synthesis. J. Macromol. Chem. 1992, 54, 313–330. [Google Scholar] [CrossRef]
- Production of Aromatic Polyethers with Infusible Particulate Substance. Patent US 4331798A, 20 September 1979.
- Kurdanova, Z.; Zhansitov, A.; Shakhmurzova, K.; Slonov, A.; Baykaziev, A.; Khashirova, S. Synthesis and Properties of Copolyphenylene Sulphones with Cardo Fragments. Polymers 2021, 13, 3689. [Google Scholar] [CrossRef]
- Kazitsyna, L.A.; Kupletskaya, N.B. Application of UF, IR, NMR and Mass-Spectroscopy in Organic Chemistry; Publishing House of the Moscow State University: Moscow, Russia, 1979. [Google Scholar]
- Shelgaev, V.; Gazaev, M.; Zaikov, G.; Shabaev, A.; Gokzhaev, M.; Mikitaev, A. Competing processes occurring at different stages of the thermolysis of poly(arylate-arylene sulphonoxide) block copolymers. Plasticheskie Massy 2011, 2, 25–30. [Google Scholar] [CrossRef]
- Danilina, L.I.; Muromtsev, V.I.; Pravednikov, A.N. Phenol formation during thermal breakdown of aromatic polysulphones. Polym. Sci. USSR 1975, 17, 2984–2990. [Google Scholar] [CrossRef]
- Kalugina, E.V. Thermal Transformations and Stabilization of Some Heat-Resistant Heterochain Polymers. Ph.D. Thesis, Institute of Chemical Physics RAS, Russian Federation, Moscow, Russia, 2003. [Google Scholar]
- Shabaev, A.S.; Zhansitov, A.A.; Kurdanova, Z.I.; Khashirova, S.Y.; Mikitaev, A.K. New Method of Investigation of Polysulfone Thermal Destruction. Polym. Sci. Ser. B 2017, 59, 216–224. [Google Scholar] [CrossRef]


| Solvent | η (dL g−1) |
|---|---|
| DMAA | 0.47 |
| N-MP | 0.45 |
| DMSO | 0.48 |
| Solvent | MFI (g min−1) | |
|---|---|---|
| 5 min | 20 min | |
| DMAA | 20.2 | 20.0 |
| N-MP | 23.0 | 23.4 |
| DMSO | 4.0 | 1.1 |
| Sample | MP × 10−3 (g/mole) | MW × 10−3 (g/mole) | MN × 10−3 (g/mole) | MW/MN | MFI (g min−1) |
|---|---|---|---|---|---|
| PPSU-1 | 84 | 102 | 38 | 2.7 | 0.3 |
| PPSU-2 | 48.5 | 61.9 | 26.7 | 2.3 | 4 |
| PPSU-3 | 34.5 | 39.5 | 20.7 | 1.9 | 21 |
| PPSU-4 | 21.2 | 23.5 | 11.6 | 2.0 | 218 |
| PPSU-5 | 9.4 | 13.4 | 4.0 | 3.3 | Leaked |
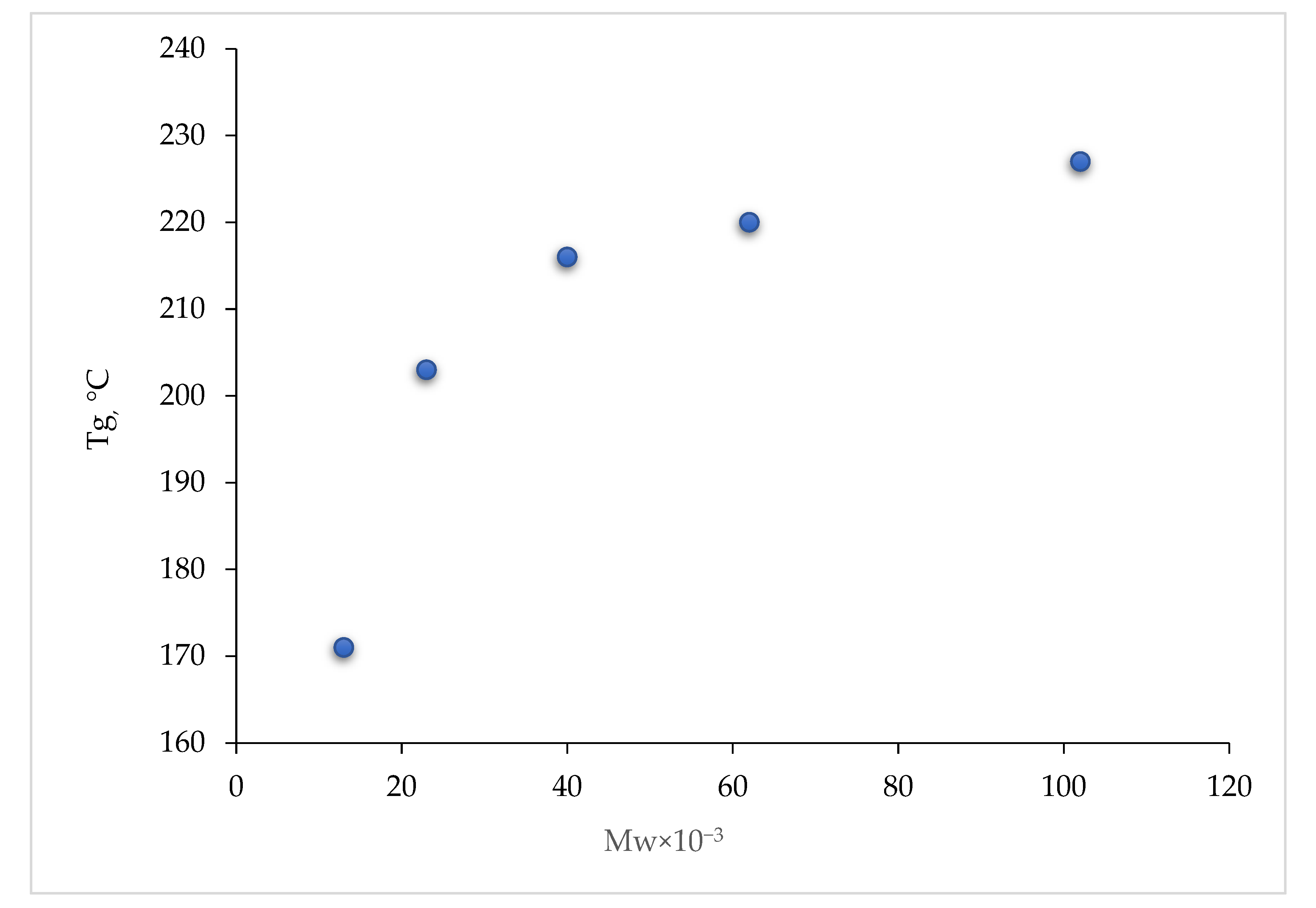
| Sample | T2% | T5% | T10% | Tg, °C |
|---|---|---|---|---|
| PPSU-1 | 472 | 502 | 520 | 227 |
| PPSU-2 | 486 | 527 | 549 | 220 |
| PPSU-3 | 471 | 506 | 519 | 216 |
| PPSU-4 | 485 | 511 | 533 | 203 |
| PPSU-5 | 477 | 500 | 522 | 171 |
| Sample | MW × 10−3 (g/mole) | Impact Strength, kJ/m² | Efl, | Eten, | σyield, | σten, | ε, | |
|---|---|---|---|---|---|---|---|---|
| Unnotched | Notched | GPa | GPa | MPa | MPa | % | ||
| PPSU-1 | 102 | - | - | - | - | - | - | - |
| PPSU-2 | 61.9 | n/b | 20.1 | 2.49 | 2.22 | 72 | 87 | 17.0 |
| PPSU-3 | 39.5 | n/b | 12 | 2.52 | 2.44 | 70 | 91 | 11.5 |
| PPSU-4 | 23.5 | 1.7 | 2.59 | - | - | - | - | - |
| PPSU-5 | 13.4 | - | - | - | - | - | - | - |
| Ultrason-P [3] | 60 | - | - | - | 2.2 | - | 75 | 7.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhansitov, A.; Kurdanova, Z.; Shakhmurzova, K.; Slonov, A.; Borisov, I.; Khashirova, S. Effect of Solvent and Monomer Ratio on the Properties of Polyphenylene Sulphone. Polymers 2023, 15, 2279. https://doi.org/10.3390/polym15102279
Zhansitov A, Kurdanova Z, Shakhmurzova K, Slonov A, Borisov I, Khashirova S. Effect of Solvent and Monomer Ratio on the Properties of Polyphenylene Sulphone. Polymers. 2023; 15(10):2279. https://doi.org/10.3390/polym15102279
Chicago/Turabian StyleZhansitov, Azamat, Zhanna Kurdanova, Kamila Shakhmurzova, Azamat Slonov, Ilya Borisov, and Svetlana Khashirova. 2023. "Effect of Solvent and Monomer Ratio on the Properties of Polyphenylene Sulphone" Polymers 15, no. 10: 2279. https://doi.org/10.3390/polym15102279





