Research on Properties of PBAT/CaCO3 Composite Films Modified with Titanate Coupling Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermal Processing of Composite Materials
2.2.1. CaCO3-Modified PBAT Films with Different Particle Sizes and Contents
2.2.2. PBAT Composite Filled with Modified CaCO3
2.3. Extrusion Blow-Molding Composite Film
2.4. Testing and Characterization
2.4.1. Tensile Properties
2.4.2. Hydrophilicity Testing
2.4.3. Water Absorption Test
- ω—the water absorption of the specimen, in %;
- M2—the wet mass of the specimen after water absorption, in g;
- M1—the dry mass of the specimen before water absorption, in g.
2.4.4. Water Vapor Transmission Performance Test
2.4.5. Microscopic Morphology Observation
2.4.6. Differential Scanning Calorimetry Analysis
- Xc—the degree of crystallinity of PBAT, in %;
- ΔHm—the enthalpy of melting of PBAT composites, in J/g;
- ΔH0—the enthalpy of melting of PBAT, in 114 J/g;
- Wf—the mass fraction of PBAT accounted for in PBAT composites, in %.
2.4.7. Thermogravimetric Analysis
2.4.8. Fourier Infrared Spectroscopy Analysis
3. Results and Discussion
3.1. Effect of CaCO3 Particle Size and Particle Content
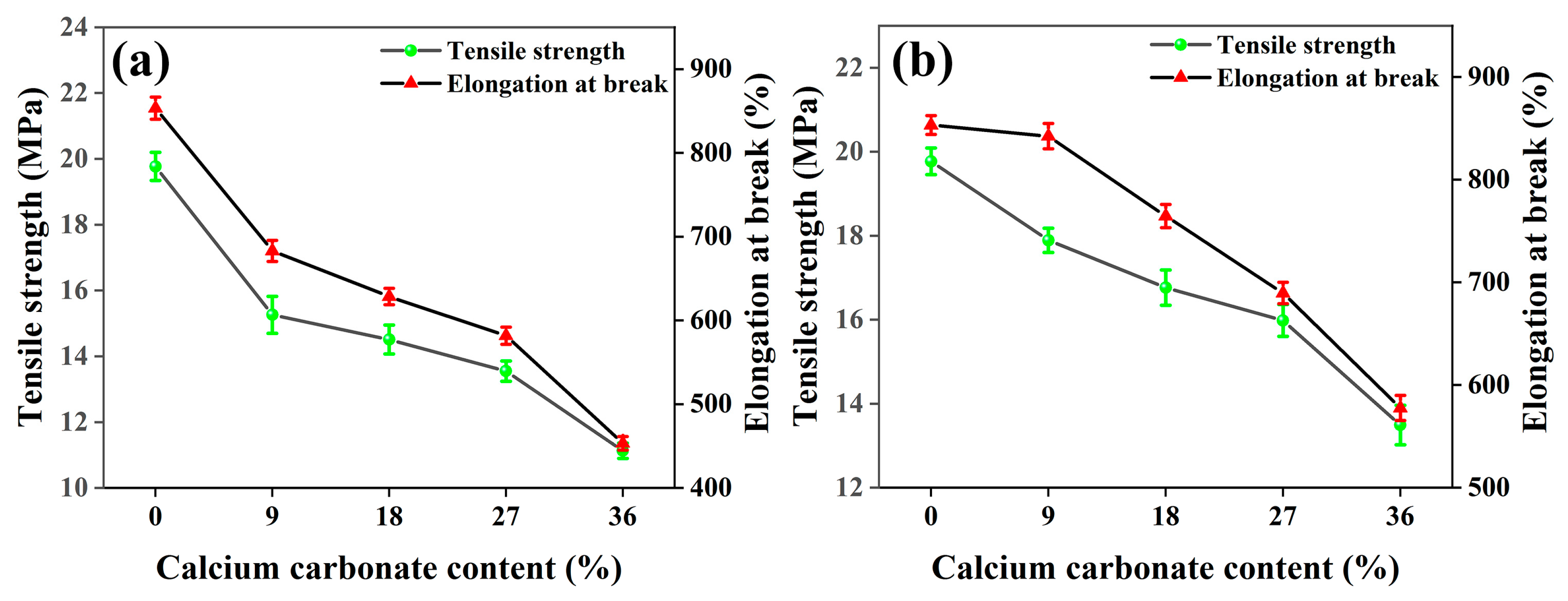
3.1.1. Effect of CaCO3 Particle Size and Content on Tensile Properties
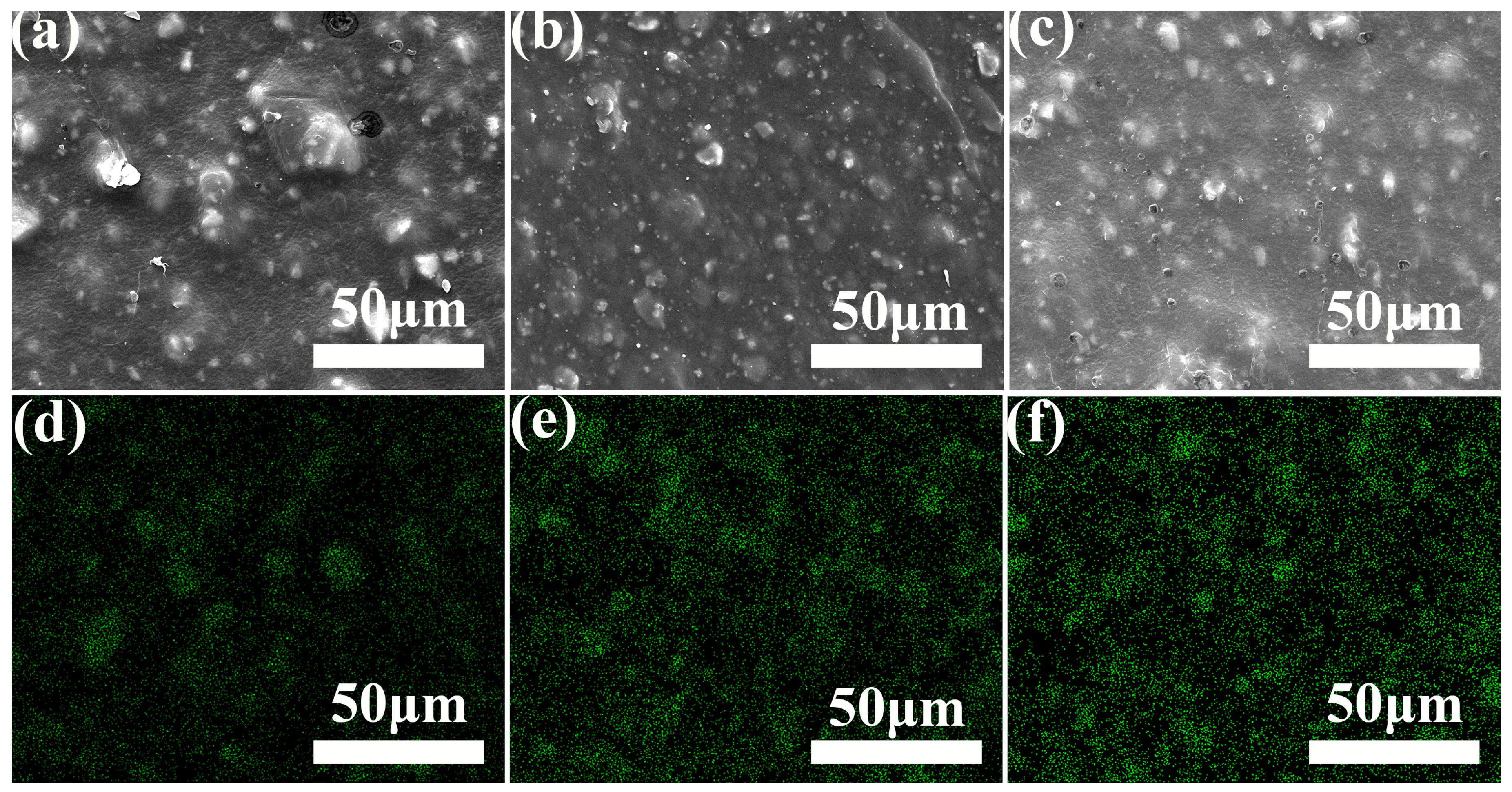
3.1.2. Effect of CaCO3 Particle Size and Content on Microscopic Morphology
3.2. Effect of TC-Modified CaCO3 on Properties of PBAT/CaCO3 Composite Film
3.2.1. FTIR Analysis of Modified CaCO3
3.2.2. Mechanical Properties
3.2.3. Micromorphological Analysis
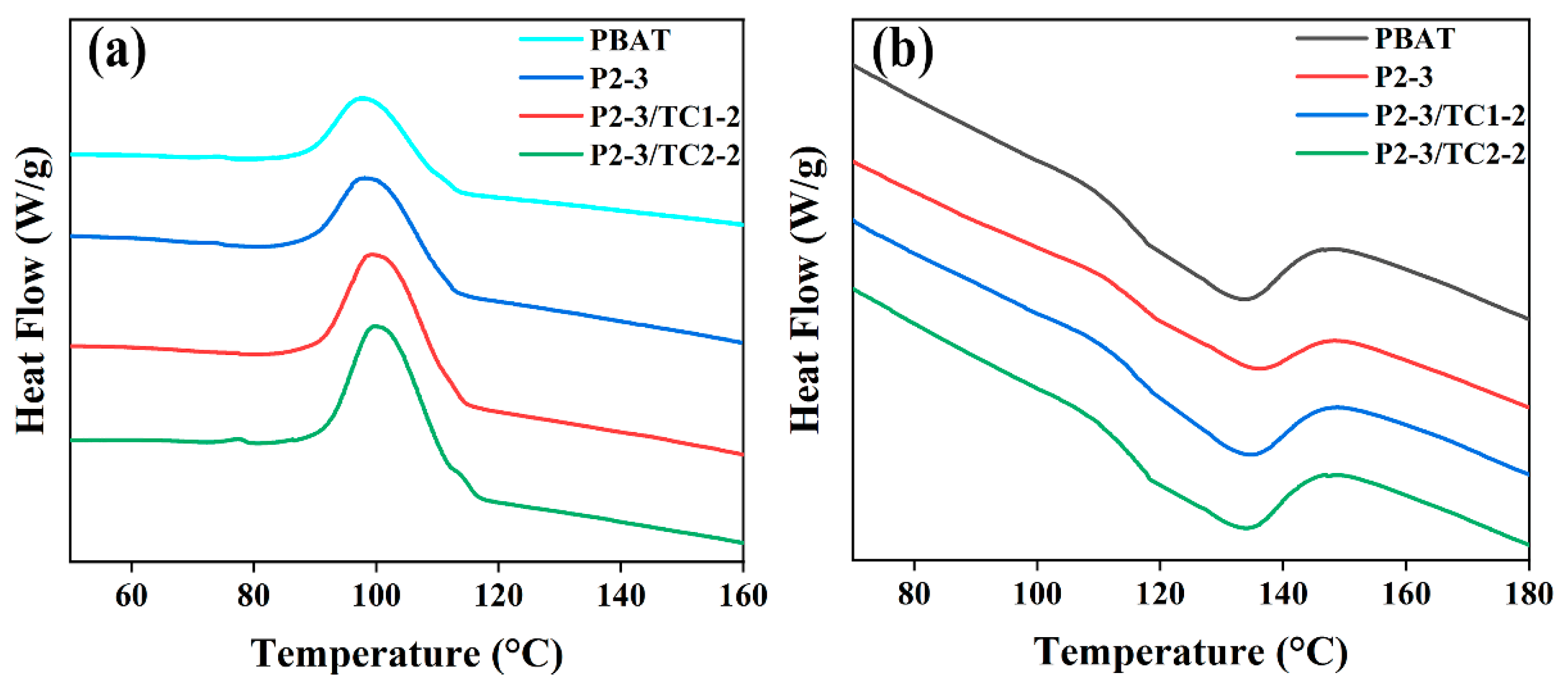
3.2.4. Melting and Crystallization Behavior
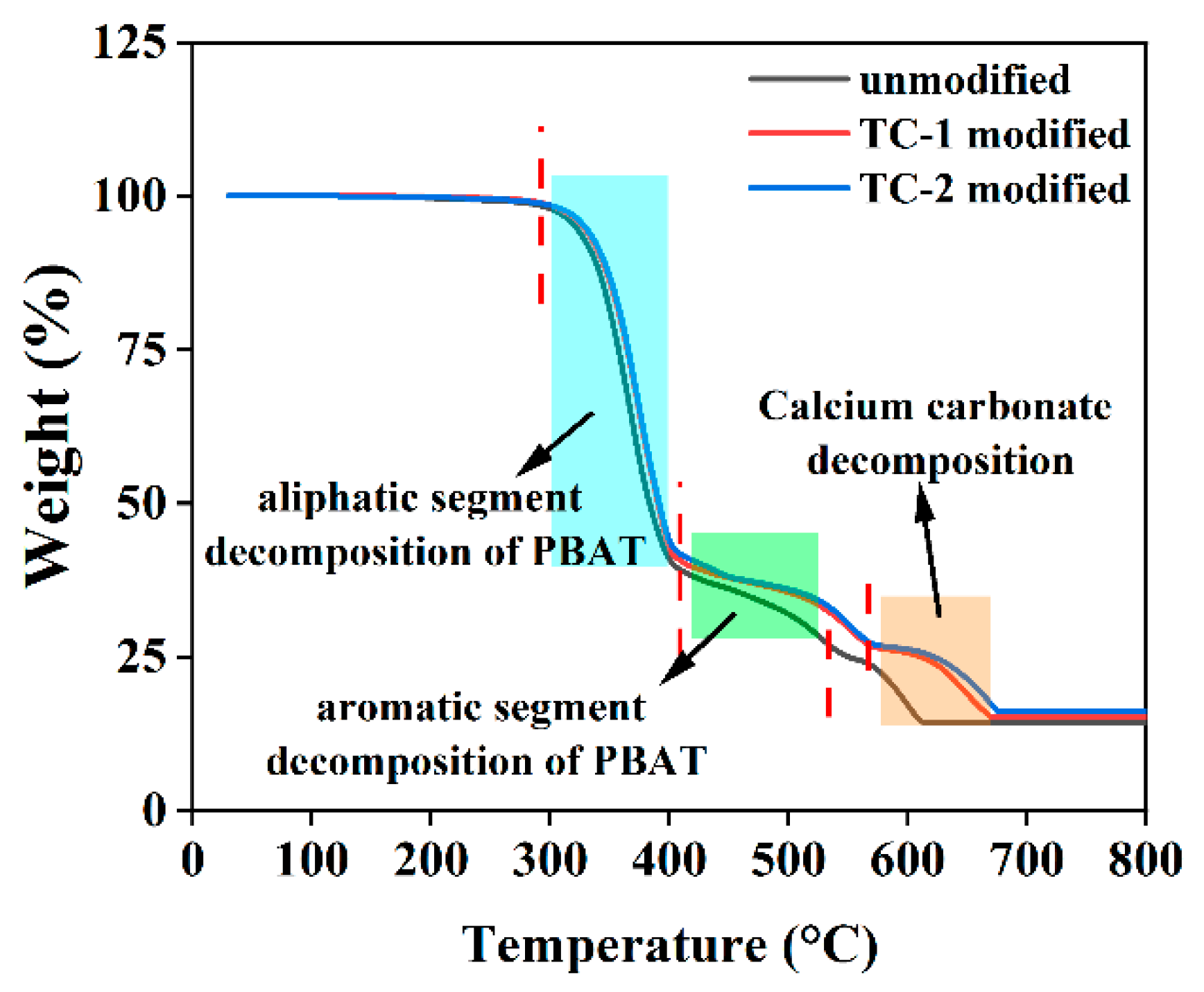
3.2.5. Thermal Stability
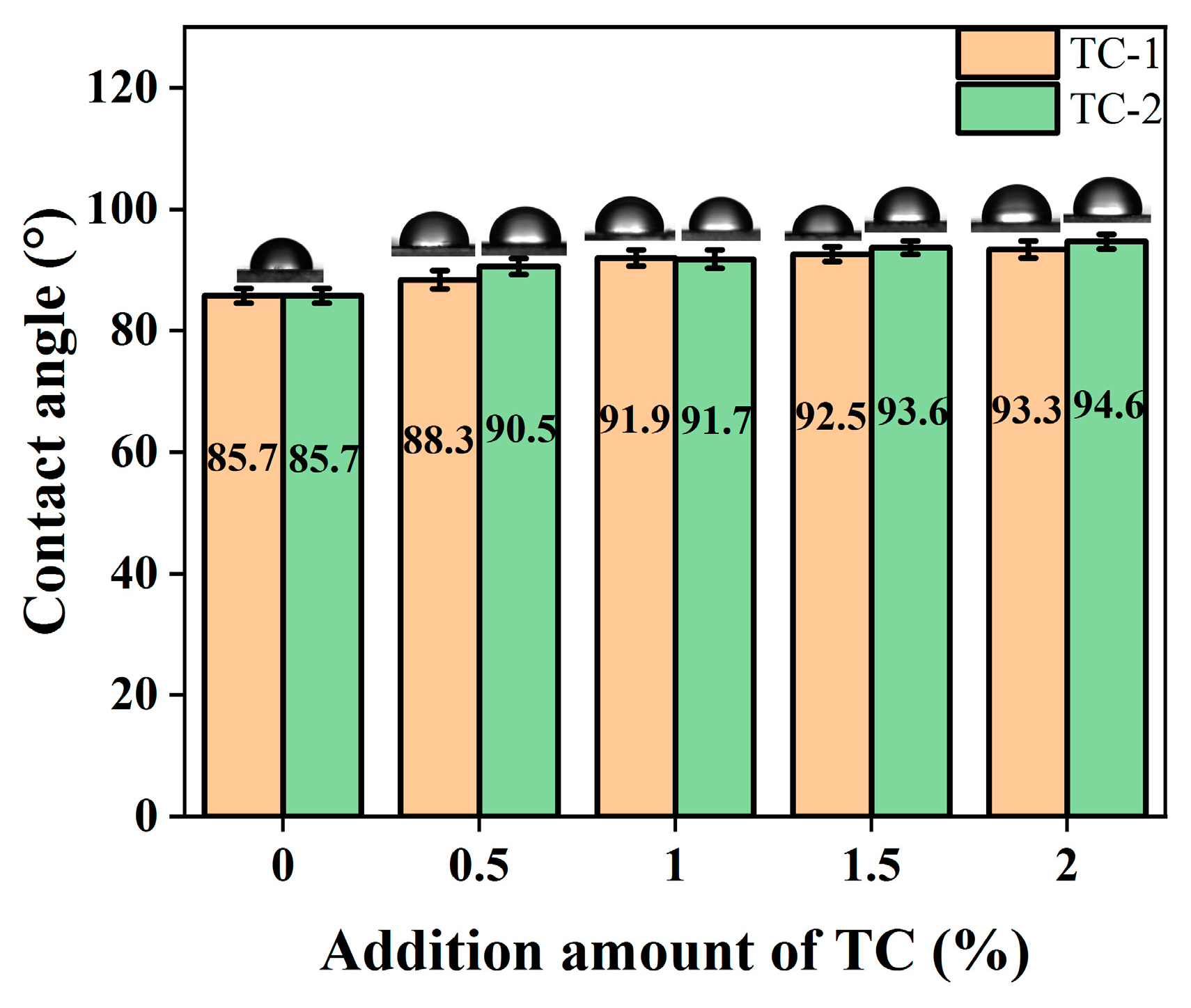
3.2.6. Hydrophilicity
3.2.7. Water Absorption
3.2.8. Water Vapor Transmission Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.-W.; Wang, G.-X.; Huang, D.; Lu, B.; Zhen, Z.-C.; Ding, Y.; Ren, Z.-L.; Wang, P.-L.; Zhang, W.; Ji, J.-H. Degradability comparison of poly(butylene adipate terephthalate) and its composites filled with starch and calcium carbonate in different aquatic environments. J. Appl. Polym. Sci. 2018, 136, 46916. [Google Scholar] [CrossRef]
- Shang, J.; Li, C.; Song, Y.; Yan, M.; Li, L.; Hu, C. Gas-solid fluidization modification of calcium carbonate for high-performance poly (butylene adipate-co-terephthalate) (PBAT) composites. Front. Chem. 2023, 10, 1119978. [Google Scholar] [CrossRef] [PubMed]
- Nampoothiri, K.M.; Nair, N.R.; John, R.P. Biological degradation of plastics: A comprehensive review. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar]
- Kasirajan, S.; Ngouajio, M. Polyethylene and biodegradable mulches for agricultural applications: A review. Agron. Sustain. Dev. 2012, 32, 501–529. [Google Scholar] [CrossRef]
- Yin, G.-Z.; Yang, X.-M. Biodegradable polymers: A cure for the planet, but a long way to go. J. Polym. Res. 2020, 27, 38. [Google Scholar] [CrossRef]
- Khan, H.; Kaur, S.; Baldwin, T.C.; Radecka, I.; Jiang, G.; Bretz, I.; Duale, K.; Adamus, G.; Kowalczuk, M. Effective Control against Broadleaf Weed Species Provided by Biodegradable PBAT/PLA Mulch Film Embedded with the Herbicide 2-Methyl-4-Chlorophenoxyacetic Acid (MCPA). ACS Sustain. Chem. Eng. 2020, 8, 5360–5370. [Google Scholar] [CrossRef]
- Wang, L.; He, J.; Wang, Q.; Zhang, J.; Feng, J. Lignin reinforced, water resistant, and biodegradable cassava starch/PBAT sandwich composite pieces. J. Polym. Eng. 2021, 41, 818–826. [Google Scholar] [CrossRef]
- Wei, X.Y.; Ren, L.; Sun, Y.N.; Zhang, X.Y.; Guan, X.F.; Zhang, H.X. Sustainable composites from biodegradable poly(butylene succinate) modified with thermoplastic starch and poly(butylene adipate-co-terephthalate): Preparation and performance. New J. Chem. 2021, 45, 17384–17397. [Google Scholar] [CrossRef]
- Liu, J.; Wang, P.; Wang, Y.; Zhang, Y.; Xu, T.; Zhang, Y.; Xi, J.; Hou, L.; Li, L.; Zhang, Z.; et al. Negative effects of poly(butylene adipate-co-terephthalate) microplastics on Arabidopsis and its root-associated microbiome. J. Hazard. Mater. 2022, 437, 129294. [Google Scholar] [CrossRef]
- Venkatesan, R.; Rajeswari, N. Nanosilica-reinforced poly(butylene adipate-co-terephthalate) nanocomposites: Preparation, characterization and properties. Polym. Bull. 2018, 76, 4785–4801. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, Y.; Tang, Y.; Li, Q.; Zhong, B.; Zeng, X.; Xie, D.; Jia, Z.; Jia, D. A novel nanosilica-supported ultraviolet absorber for the preparation of robust biodegradable plastic film with high ultraviolet aging resistance. Polym. Compos. 2019, 40, 4154–4161. [Google Scholar] [CrossRef]
- Phetwarotai, W.; Aht-Ong, D. Isothermal crystallization behaviors and kinetics of nucleated polylactide/poly(butylene adipate-co-terephthalate) blend films with talc. J. Therm. Anal. Calorim. 2016, 126, 1797–1808. [Google Scholar] [CrossRef]
- Helanto, K.; Talja, R.; Rojas, O.J. Effects of talc, kaolin and calcium carbonate as fillers in biopolymer packaging materials. J. Polym. Eng. 2021, 41, 746–758. [Google Scholar] [CrossRef]
- Xie, J.; Yan, Y.; Fan, S.; Min, X.; Wang, L.; You, X.; Jia, X.; Waterhouse, G.I.N.; Wang, J.; Xu, J. Prediction Model of Photodegradation for PBAT/PLA Mulch Films: Strategy to Fast Evaluate Service Life. Environ. Sci. Technol. 2022, 56, 9041–9051. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhang, L.; Li, C. Modified cellulose nanocrystals based on SI-ATRP for enhancing interfacial compatibility and mechanical performance of biodegradable PLA/PBAT blend. Polym. Compos. 2022, 43, 3753–3764. [Google Scholar] [CrossRef]
- Maldonado, L.F.; Muñoz, P.A.R.; Fechine, G.J.M. Transfer of Graphene CVD to Surface of Low Density Polyethylene (LDPE) and Poly(butylene adipate-co-terephthalate) (PBAT) Films: Effect on Biodegradation Process. J. Polym. Environ. 2018, 26, 3187–3196. [Google Scholar] [CrossRef]
- Zolali, A.M.; Favis, B.D. Partial to complete wetting transitions in immiscible ternary blends with PLA: The influence of interfacial confinement. Soft Matter 2017, 13, 2844–2856. [Google Scholar] [CrossRef]
- Oliveira, T.A.; Oliveira, R.R.; Barbosa, R.; Azevedo, J.B.; Alves, T.S. Effect of reprocessing cycles on the degradation of PP/PBAT-thermoplastic starch blends. Carbohydr. Polym. 2017, 168, 52–60. [Google Scholar] [CrossRef]
- Bang, Y.; Shankar, S.; Rhim, J. Preparation of polypropylene/poly (butylene adipate-co-terephthalate) composite films incorporated with melanin for prevention of greening of potatoes. Packag. Technol. Sci. 2020, 33, 433–441. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, C.; Ma, Z.; Weng, Y. In Situ Formation of Microfibrillar PBAT in PGA Films: An Effective Way to Robust Barrier and Mechanical Properties for Fully Biodegradable Packaging Films. ACS Omega 2022, 7, 21280–21290. [Google Scholar] [CrossRef]
- Samantaray, P.K.; Ellingford, C.; Farris, S.; O’sullivan, D.; Tan, B.; Sun, Z.; McNally, T.; Wan, C. Electron Beam-Mediated Cross-Linking of Blown Film-Extruded Biodegradable PGA/PBAT Blends toward High Toughness and Low Oxygen Permeation. ACS Sustain. Chem. Eng. 2022, 10, 1267–1276. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, C.; Yang, Y.; Yang, F.; Zhao, M.; Weng, Y. Improved properties of poly(butylene adipate-co-terephthalate)/calcium carbonate films through silane modification. J. Appl. Polym. Sci. 2021, 138, 50970. [Google Scholar] [CrossRef]
- Rapisarda, M.; Mistretta, M.C.; Scopelliti, M.; Leanza, M.; La Mantia, F.P.; Rizzarelli, P. Influence of Calcium Carbonate Nanoparticles on the Soil Burial Degradation of Polybutyleneadipate-Co-Butylenetherephthalate Films. Nanomaterials 2022, 12, 2275. [Google Scholar] [CrossRef] [PubMed]
- Diao, X.; Zhang, C.; Weng, Y. Properties and Degradability of Poly(Butylene Adipate-Co-Terephthalate)/Calcium Carbonate Films Modified by Polyethylene Glycol. Polymers 2022, 14, 484. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A. Cutting-edge Shape Memory Polymer/Fullerene Nanocomposite: Design and Contemporary Status. Polym. Technol. Mater. 2022, 62, 604–617. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Liu, Y.; Wang, X.; Yang, B. A novel surface modification of carbon fiber for high-performance thermoplastic polyurethane composites. Appl. Surf. Sci. 2016, 382, 144–154. [Google Scholar] [CrossRef]
- Yang, B.; Li, P.; Luo, Z.; Zhong, J.; Yin, L. Influence of thermal oxidation and maleinized liquid polybutadiene on dynamic and static performance of short aramid fiber-reinforced carbon black-ethylene propylene diene monomer composites. Polym. Compos. 2020, 41, 2036–2045. [Google Scholar] [CrossRef]
- Li, C.-Q.; Liang, C.; Chen, Z.-M.; Di, Y.-H.; Zheng, S.-L.; Wei, S.; Sun, Z.-M. Surface modification of calcium carbonate: A review of theories, methods and applications. J. Central South Univ. 2021, 28, 2589–2611. [Google Scholar] [CrossRef]
- Shahrajabian, H.; Bagherzadeh, S.A.; Moghri, M.; Karimi, V.; Jamali, M. Estimation of mechanical properties of LDPE/LLDPE/SEBS nanocomposite reinforced with calcium carbonate nanoparticles by Ch mathematical model. J. Therm. Anal. Calorim. 2021, 144, 2099–2107. [Google Scholar] [CrossRef]
- Murtaja, Y.; Lapčík, L.; Sepetcioglu, H.; Vlček, J.; Lapčíková, B.; Ovsík, M.; Staněk, M. Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior. Nanotechnol. Rev. 2022, 11, 312–320. [Google Scholar] [CrossRef]
- Barletta, M.; Puopolo, M. Thermoforming of compostable PLA/PBS blends reinforced with highly hygroscopic calcium carbonate. J. Manuf. Process. 2020, 56, 1185–1192. [Google Scholar] [CrossRef]
- Wang, X.; Zhai, S.; Wang, K.; Xie, T. Modification of bonding properties and microstructure of resin-cement interface by coupling agents. Compos. Interfaces 2017, 25, 27–37. [Google Scholar] [CrossRef]
- Cui, Y.-H.; Jin, X.; Shen, F.; Han, C.; Wang, J. Fabrication and characterization of in situ synthesized nano-CaCo3-reinforced polypropylene composite materials. J. Vinyl Addit. Technol. 2009, 15, 178–183. [Google Scholar] [CrossRef]
- Kemal, I.; Whittle, A.; Burford, R.; Vodenitcharova, T.; Hoffman, M. Toughening of unmodified polyvinylchloride through the addition of nanoparticulate calcium carbonate and titanate coupling agent. J. Appl. Polym. Sci. 2012, 127, 2339–2353. [Google Scholar] [CrossRef]
- Li, J.; Yang, S.; Liu, Y.; Muhammad, Y.; Su, Z.; Yang, J. Studies on the properties of modified heavy calcium carbonate and SBS composite modified asphalt. Constr. Build. Mater. 2019, 218, 413–423. [Google Scholar] [CrossRef]
- Doufnoune, R.; Chebira, F.; Haddaoui, N. Effect of titanate coupling agent on the mechanical properties of calcium carbonate filled polypropylene. Int. J. Polym. Mater. Polym. Biomater. 2003, 52, 967–984. [Google Scholar] [CrossRef]
- Hasan, M.R.M.; You, Z.; Satar, M.K.I.M.; Warid, M.N.M.; Kamaruddin, N.H.M.; Ge, D.; Zhang, R. Effects of Titanate Coupling Agent on Engineering Properties of Asphalt Binders and Mixtures Incorporating LLDPE-CaCO3 Pellet. Appl. Sci. 2018, 8, 1029. [Google Scholar] [CrossRef]
- Wang, N.; She, Q.; Xu, H.; Yao, Y.; Zhang, L.; Qu, X.; Zhang, L. Preparation and characterization of nano-CaCO3 encapsulated with polyacrylic and its application in PVC toughness. J. Appl. Polym. Sci. 2009, 115, 1336–1346. [Google Scholar] [CrossRef]
- Sahebian, S.; Zebarjad, S.M.; Sajjadi, S.A.; Sherafat, Z.; Lazzeri, A. Effect of both uncoated and coated calcium carbonate on fracture toughness of HDPE/CaCO3 nanocomposites. J. Appl. Polym. Sci. 2007, 104, 3688–3694. [Google Scholar] [CrossRef]
- Zapata, P.A.; Palza, H.; Díaz, B.; Armijo, A.; Sepúlveda, F.; Ortiz, J.A.; Ramírez, M.P.; Oyarzún, C. Effect of CaCO3 Nanoparticles on the Mechanical and Photo-Degradation Properties of LDPE. Molecules 2018, 24, 126. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Morreale, M.; Scaffaro, R.; Tulone, S. Rheological and mechanical behavior of LDPE/calcium carbonate nanocomposites and microcomposites. J. Appl. Polym. Sci. 2012, 127, 2544–2552. [Google Scholar] [CrossRef]
- Li, M.; Jia, Y.; Shen, X.; Shen, T.; Tan, Z.; Zhuang, W.; Zhao, G.; Zhu, C.; Ying, H. Investigation into lignin modified PBAT/thermoplastic starch composites: Thermal, mechanical, rheological and water absorption properties. Ind. Crop. Prod. 2021, 171, 113916. [Google Scholar] [CrossRef]
- Qiao, L.; Yan, X.; Tan, H.; Dong, S.; Ju, G.; Shen, H.; Ren, Z. Mechanical Properties, Melting and Crystallization Behaviors, and Morphology of Carbon Nanotubes/Continuous Carbon Fiber Reinforced Polyethylene Terephthalate Composites. Polymers 2022, 14, 2892. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.J.; Shukla, A.; Bose, A. Effect of particle size and surface treatment on constitutive properties of polyester-cenosphere composites. J. Mater. Sci. 2002, 37, 603–613. [Google Scholar] [CrossRef]
- Han, Z.; Hu, J.; Huang, H.; Han, X.; Ke, Y.; Li, Z.; Wang, Y.; Song, D.; Xu, W. Effect of in situ deposition of calcium carbonate in cotton fiber on its mechanical properties. J. Appl. Polym. Sci. 2022, 140, e53344. [Google Scholar] [CrossRef]
- Du, B.; Chen, F.; Luo, R.; Zhou, S.; Wu, Z. Synthesis and Characterization of Nano-TiO2/SiO2-Acrylic Composite Resin. Adv. Mater. Sci. Eng. 2019, 2019, 6318623. [Google Scholar] [CrossRef]
- Tang, Z.; Cheng, G.; Chen, Y.; Yu, X.; Wang, H. Characteristics evaluation of calcium carbonate particles modified by surface functionalization. Adv. Powder Technol. 2014, 25, 1618–1623. [Google Scholar] [CrossRef]
- Montes-Zavala, I.; Pérez-González, M.J.; Castrejón-González, E.O.; Santamaría-Razo, D.A.; Almendárez-Camarillo, A.; Pérez, E.; Gonzalez-Calderon, J.A. Thermal and mechanical properties of poly(lactic acid) filled with modified silicon dioxide: Importance of the surface area. Polym. Bull. 2021, 79, 1409–1435. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, H.; Li, G.; Mai, K. Role of interfacial interaction on the crystallization behavior and melting characteristics of PP/Nano-CaCO3 composites modified with different compatibilizers. E-Polymers 2010, 10, 053. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, Y.; Zhang, N.; Wang, X.; Wei, Q.; Zhang, Y. Impact of titanate coupling agent on properties of high density polyethylene composite filled with coal gangue. Surf. Interface Anal. 2020, 52, 645–655. [Google Scholar] [CrossRef]
- Shuai, C.; Shuai, C.; Feng, P.; Yang, Y.; Xu, Y.; Qin, T.; Yang, S.; Gao, C.; Peng, S. Silane Modified Diopside for Improved Interfacial Adhesion and Bioactivity of Composite Scaffolds. Molecules 2017, 22, 511. [Google Scholar] [CrossRef] [PubMed]
- Rizal, S.; Alfatah, T.; Khalil, H.P.S.A.; Yahya, E.B.; Abdullah, C.K.; Mistar, E.M.; Ikramullah, I.; Kurniawan, R.; Bairwan, R.D. Enhanced Functional Properties of Bioplastic Films Using Lignin Nanoparticles from Oil Palm-Processing Residue. Polymers 2022, 14, 5126. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Cui, L.; Liu, Y.; Fan, S. Improvement of Gas Barrier Properties for Biodegradable Poly(butylene adipate-co-terephthalate) Nanocomposites with MXene Nanosheets via Biaxial Stretching. Polymers 2022, 14, 480. [Google Scholar] [CrossRef] [PubMed]





| Samples | PBAT (wt%) | CaCO3 (wt%) | |
|---|---|---|---|
| 1250 Mesh | 2000 Mesh | ||
| PBAT | 100 | - | - |
| P1-1 | 91 | 9 | |
| P1-2 | 82 | 18 | |
| P1-3 | 73 | 27 | |
| P1-4 | 64 | 36 | |
| P2-1 | 91 | 9 | |
| P2-2 | 82 | 18 | |
| P2-3 | 73 | 27 | |
| P2-4 | 64 | 36 | |
| Samples | PBAT (wt%) | CaCO3 (wt%) | TC-1 (wt%) | TC-2 (wt%) |
|---|---|---|---|---|
| P2-3 | 73 | 27 | - | - |
| P2-3/TC1-1 | 73 | 27 | 0.5 | |
| P2-3/TC1-2 | 73 | 27 | 1 | |
| P2-3/TC1-3 | 73 | 27 | 1.5 | |
| P2-3/TC1-4 | 73 | 27 | 2 | |
| P2-3/TC2-1 | 73 | 27 | 0.5 | |
| P2-3/TC2-2 | 73 | 27 | 1 | |
| P2-3/TC2-3 | 73 | 27 | 1.5 | |
| P2-3/TC2-4 | 73 | 27 | 2 |
| Samples | Tc (°C) | Tm (°C) | ΔHm (J/g) | Xc (%) |
|---|---|---|---|---|
| PBAT | 97.51 | 133.49 | 8.08 | 7.09 |
| P2-3 | 97.82 | 136.21 | 11.20 | 13.46 |
| P2-3/TC1-2 | 99.32 | 134.82 | 12.14 | 14.59 |
| P2-3/TC2-2 | 99.67 | 133.79 | 12.34 | 14.83 |
| Samples | WVTR [g/(m2·24 h)] | WVP [g·cm/(Pa·s·cm2)] |
|---|---|---|
| PBAT | 600.37 | 3.45 × 10−13 |
| P2-3 | 549.10 | 2.39 × 10−13 |
| P2-3/TC1-2 | 487.82 | 2.06 × 10−13 |
| P2-3/TC2-2 | 432.30 | 1.96 × 10−13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Meng, F.; Tang, X.; Su, C.; Mu, Q.; Ju, G. Research on Properties of PBAT/CaCO3 Composite Films Modified with Titanate Coupling Agent. Polymers 2023, 15, 2379. https://doi.org/10.3390/polym15102379
Liu Z, Meng F, Tang X, Su C, Mu Q, Ju G. Research on Properties of PBAT/CaCO3 Composite Films Modified with Titanate Coupling Agent. Polymers. 2023; 15(10):2379. https://doi.org/10.3390/polym15102379
Chicago/Turabian StyleLiu, Zhekun, Fantao Meng, Xianggang Tang, Chengzhuang Su, Qinglin Mu, and Guannan Ju. 2023. "Research on Properties of PBAT/CaCO3 Composite Films Modified with Titanate Coupling Agent" Polymers 15, no. 10: 2379. https://doi.org/10.3390/polym15102379






