Robust, Flame-Retardant, and Anti-Corrosive Waterborne Polyurethane Enabled by a PN Synergistic Flame-Retardant Containing Benzimidazole and Phosphinate Groups
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of WPU Dispersions
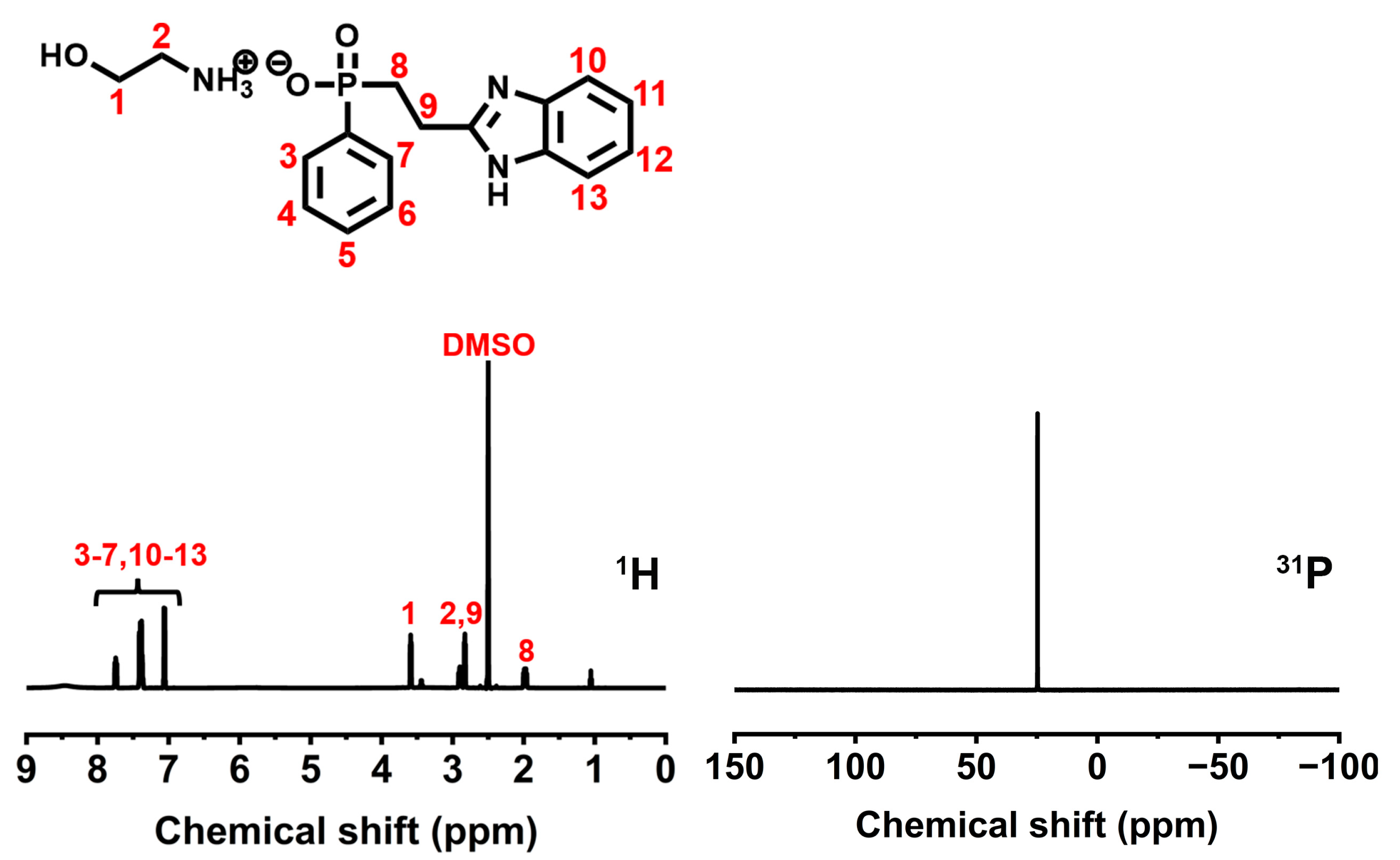
2.3. Synthesis of 2-Hydroxyethan-1-aminium (2-(1H-benzo[d]imidazol-2-yl)ethyl)(phenyl)phosphinate (BIEP-ETA)
2.4. Preparation of WPU/FRs
2.5. Characterization
3. Results and Discussion
3.1. Storage Stability
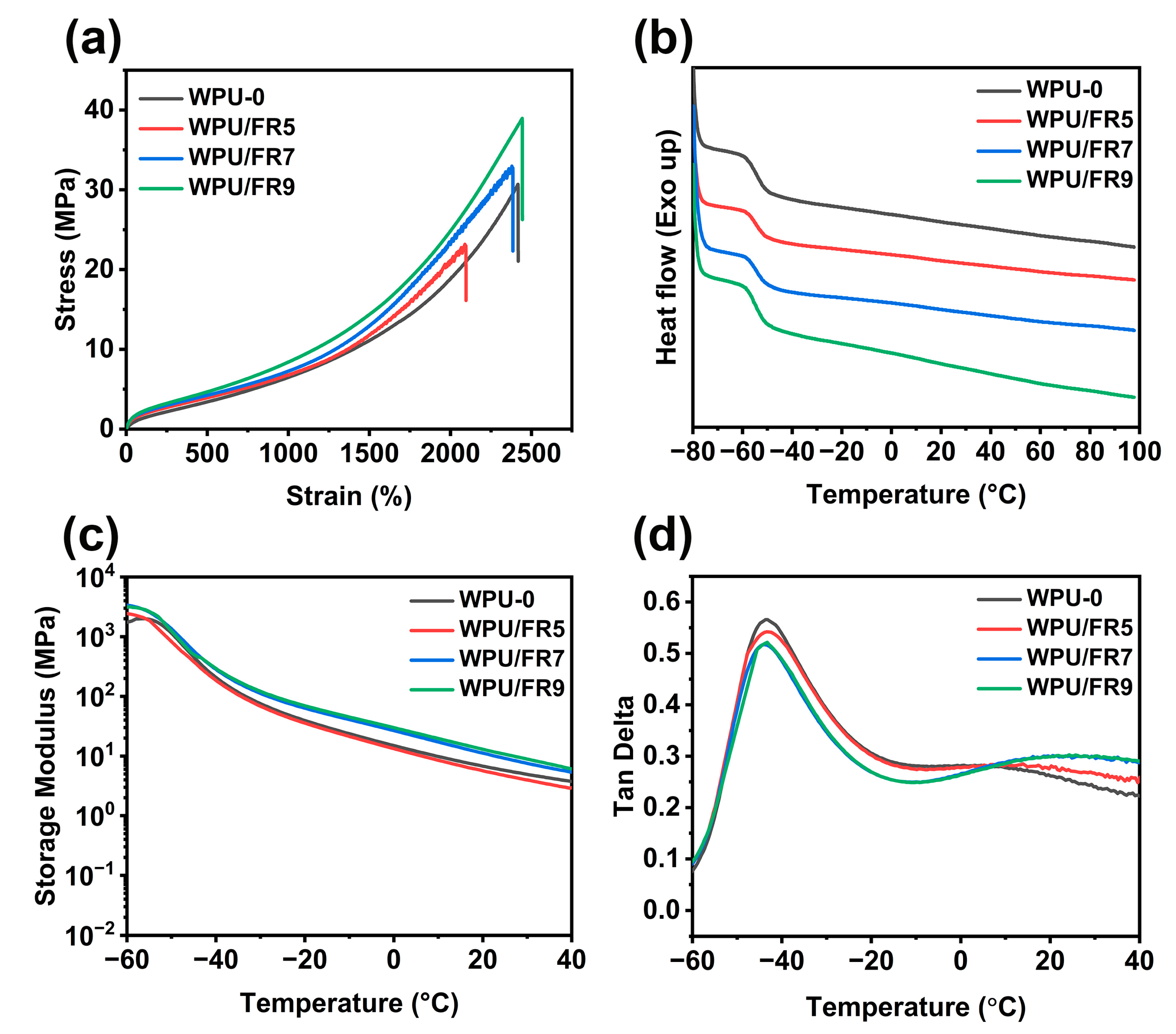
3.2. Thermal and Mechanical Properties
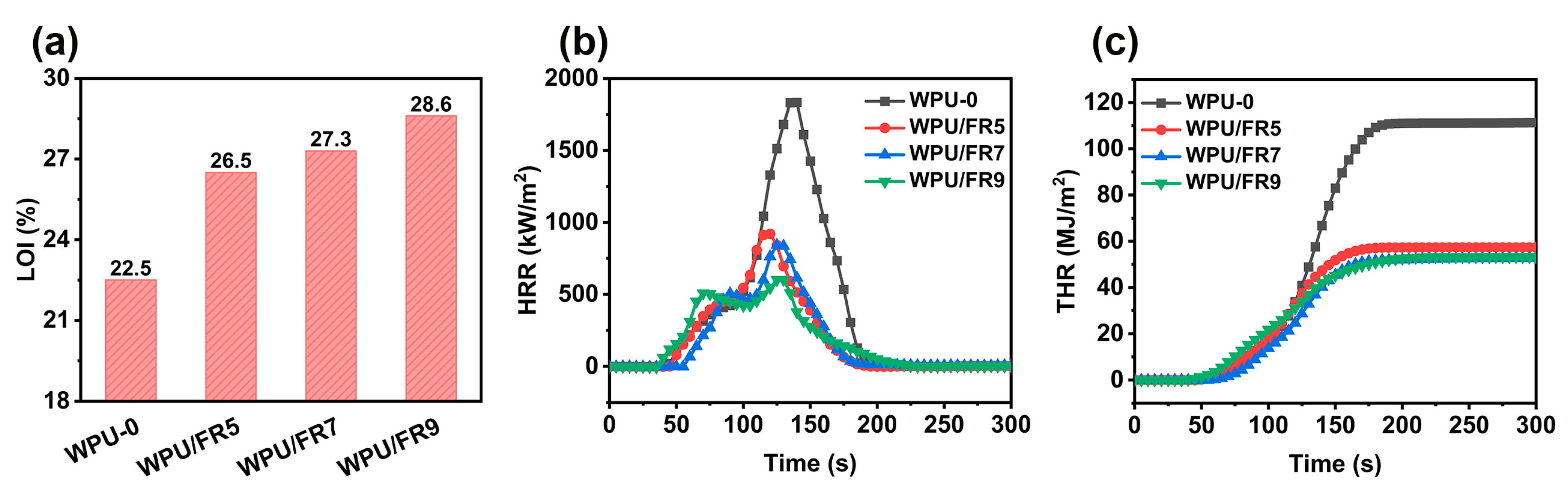
3.3. Flame-Retardancy and Burning Behaviors
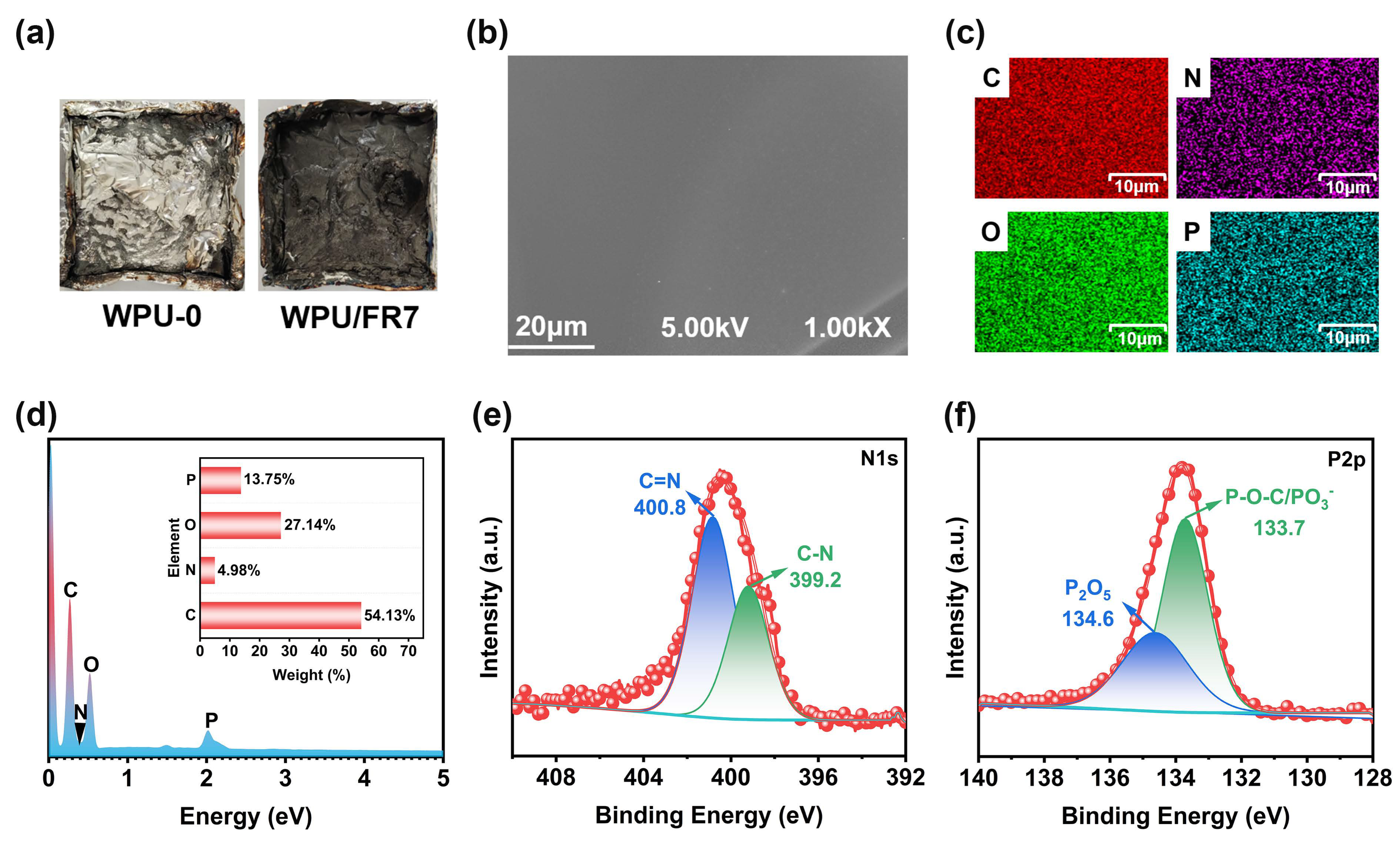
3.4. Flame-Retardant Mechanism Analysis of WPU/FRs
3.5. Thermal Stability and Transparency
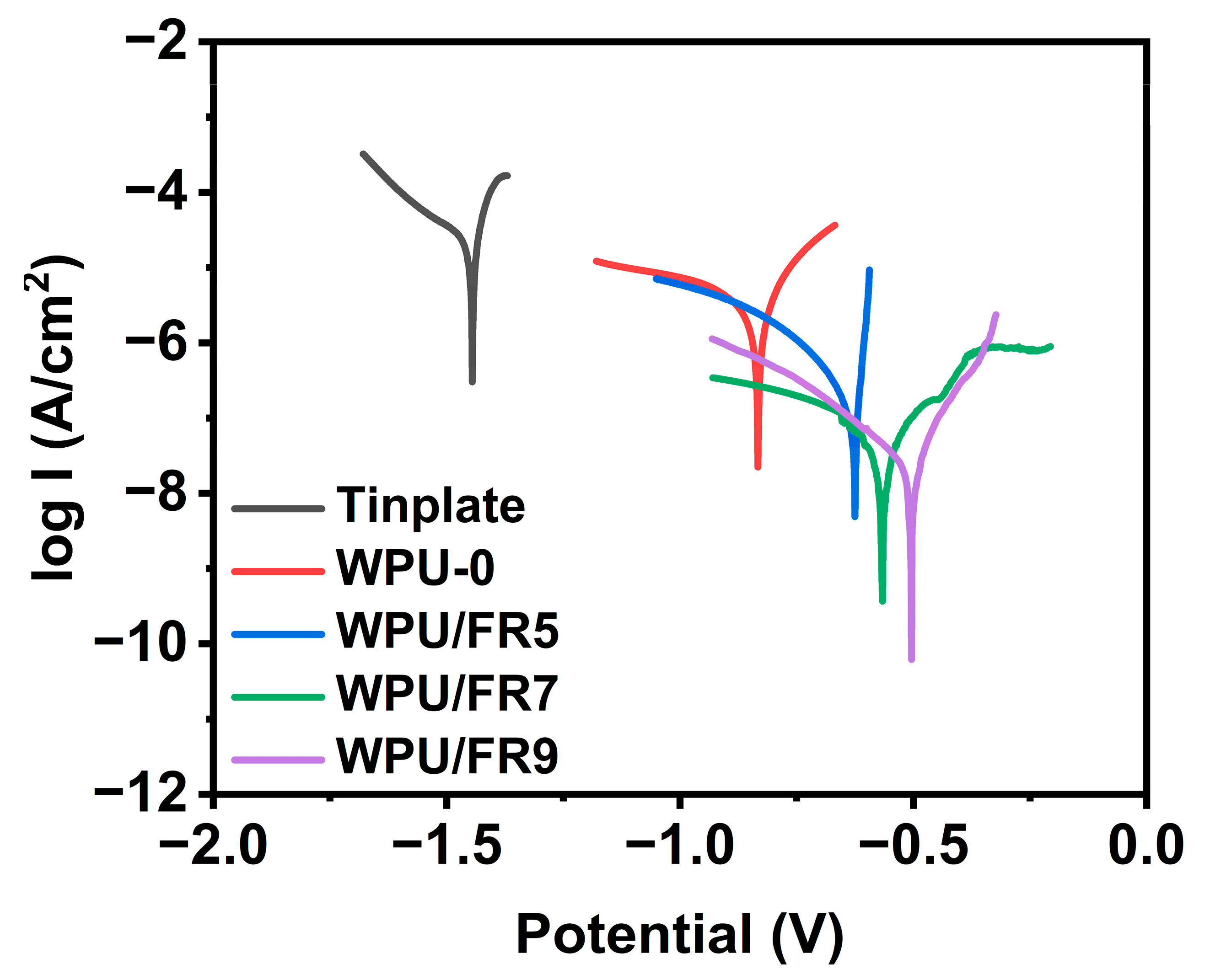
3.6. Anticorrosive Property of WPU/FRs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, X.; Liu, C.; Chen, Y.; Yin, F.; Bao, D.; Zhou, G. Waterborne polyurethane composites flame retardancy based on the bi-DOPO derivatives and dangling poly(dimethylsiloxane) chains. Prog. Org. Coat. 2023, 177, 107388–107398. [Google Scholar] [CrossRef]
- Shang, X.; Jin, Y.; Du, W.; Bai, L.; Zhou, R.; Zeng, W.; Lin, K. Flame-Retardant and Self-Healing Waterborne Polyurethane Based on Organic Selenium. ACS Appl. Mater. Interfaces 2023, 15, 16118–16131. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, C.; Hong, Y.; Liu, R.; Zhou, X. Synthesis and application of self-crosslinking and flame retardant waterborne polyurethane as fabric coating agent. Prog. Org. Coat. 2019, 137, 105323–105331. [Google Scholar] [CrossRef]
- He, L.; Zhou, X.; Cai, W.; Xiao, Y.; Chu, F.; Mu, X.; Fu, X.; Hu, Y.; Song, L. Electrochemical exfoliation and functionalization of black phosphorene to enhance mechanical properties and flame retardancy of waterborne polyurethane. Compos. Part B Eng. 2020, 202, 108446–108459. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, J.; Zhao, J.; Shi, H. Facile preparation of aluminum triphosphate-containing intumescence flame-retarding coatings using aliphatic waterborne polyurethane as the binder. Prog. Org. Coat. 2020, 147, 105758–105767. [Google Scholar] [CrossRef]
- Feng, J.; Ge, Z.; Chai, C.; Wang, S.; Yu, D.; Wu, G.; Luo, Y. Flame retardant modification of waterborne polyurethane fabric coating agent with high hydrostatic pressure resistance. Prog. Org. Coat. 2016, 97, 91–98. [Google Scholar] [CrossRef]
- Du, W.; Ge, X.; Huang, H.; Zhang, T.; Zhang, Z.; Shang, X. Fabrication of high transparent, mechanical strong, and flame retardant waterborne polyurethane composites by incorporating phosphorus-silicon functionalized cellulose nanocrystals. J. Appl. Polym. Sci. 2021, 139, 51496. [Google Scholar] [CrossRef]
- Yin, S.; Ren, X.; Zheng, R.; Li, Y.; Zhao, J.; Xie, D.; Mei, Y. Improving fire safety and mechanical properties of waterborne polyurethane by montmorillonite-passivated black phosphorus. Chem. Eng. J. 2023, 464, 142683–142695. [Google Scholar] [CrossRef]
- He, L.; Chu, F.; Zhou, X.; Song, L.; Hu, Y. Cactus-like structure of BP@MoS2 hybrids: An effective mechanical reinforcement and flame retardant additive for waterborne polyurethane. Polym. Degrad. Stab. 2022, 202, 110027–110038. [Google Scholar] [CrossRef]
- Patel, R.; Kapatel, P. Waterborne polyurethanes: A three step synthetic approach towards environmental friendly flame retardant coatings. Prog. Org. Coat. 2018, 125, 186–194. [Google Scholar] [CrossRef]
- Wang, S.; Du, X.; Jiang, Y.; Xu, J.; Zhou, M.; Wang, H.; Cheng, X.; Du, Z. Synergetic enhancement of mechanical and fire-resistance performance of waterborne polyurethane by introducing two kinds of phosphorus-nitrogen flame retardant. J. Colloid Interface Sci. 2019, 537, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Dong, C.; Chai, C.; Luo, Y. Thermostability and flame retardance of green functional two-component waterborne polyurethane coatings with nanoparticles. Prog. Org. Coat. 2018, 122, 119–128. [Google Scholar] [CrossRef]
- Wu, G.; Li, J.; Luo, Y. Flame retardancy and thermal degradation mechanism of a novel post-chain extension flame retardant waterborne polyurethane. Polym. Degrad. Stab. 2016, 123, 36–46. [Google Scholar] [CrossRef]
- Yin, X.; Luo, Y. The optical shielding performances and mechanisms of green flame-retardant two-component matte films under various application conditions. RSC Adv. 2021, 11, 12696–12702. [Google Scholar] [CrossRef]
- Ma, J.; Yang, N.; Li, W.; Zhou, Y.; Sun, X. Functional nanocomposite based on waterborne polyurethane and layered double hydroxide as a flame retardant for leather. J. Clean. Prod. 2022, 380, 134966–134975. [Google Scholar] [CrossRef]
- Zhao, Z.; Guo, D.-M.; Fu, T.; Wang, X.-L.; Wang, Y.-Z. A highly-effective ionic liquid flame retardant towards fire-safety waterborne polyurethane (WPU) with excellent comprehensive performance. Polymer 2020, 205, 122780–122788. [Google Scholar] [CrossRef]
- Tabatabaee, F.; Khorasani, M.; Ebrahimi, M.; González, A.; Irusta, L.; Sardon, H. Synthesis and comprehensive study on industrially relevant flame retardant waterborne polyurethanes based on phosphorus chemistry. Prog. Org. Coat. 2019, 131, 397–406. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Chen, L.; Rao, W.-H.; Qi, M.; Guo, D.-M.; Liao, W.; Wang, Y.-Z. Latent curing epoxy system with excellent thermal stability, flame retardance and dielectric property. Chem. Eng. J. 2018, 347, 223–232. [Google Scholar] [CrossRef]
- Zhu, X.; Han, K.; Li, C.; Wang, J.; Yuan, J.; Pan, Z.; Pan, M. Tough, Photoluminescent, Self-Healing Waterborne Polyurethane Elastomers Resulting from Synergistic Action of Multiple Dynamic Bonds. ACS Appl. Mater. Interfaces 2023, 15, 19414–19426. [Google Scholar] [CrossRef]
- Xi, P.; Quan, F.; Yao, J.; Xia, Y.; Fang, K.; Jiang, Y. Strategy to Fabricate a Strong and Supertough Bio-Inspired Fiber with Organic-Inorganic Networks in a Green and Scalable Way. ACS Nano 2021, 15, 16478–16487. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Zhao, J.-J.; Chen, H.-B.; He, M.-X.; Ni, Y.-P.; Zhai, J.-Q.; Wang, X.-L.; Wang, Y.-Z. A phosphorus-containing PET ionomer: From ionic aggregates to flame retardance and restricted melt-dripping. Polym. Chem. 2014, 5, 1982–1991. [Google Scholar] [CrossRef]
- Gu, L.; Luo, Y. Flame Retardancy and Thermal Decomposition of Phosphorus-Containing Waterborne Polyurethanes Modified by Halogen-Free Flame Retardants. Ind. Eng. Chem. Res. 2015, 54, 2431–2438. [Google Scholar] [CrossRef]
- Cui, M.; Li, J.; Chen, X.; Hong, W.; Chen, Y.; Xiang, J.; Yan, J.; Fan, H. A halogen-free, flame retardant, waterborne polyurethane coating based on the synergistic effect of phosphorus and silicon. Prog. Org. Coat. 2021, 158, 106359–106370. [Google Scholar] [CrossRef]
- Lee, J.K.; Lee, K.-R.; Kang, Y.T. Development of binary nanoemulsion to apply for diffusion absorption refrigerator as a new refrigerant. Energy 2014, 78, 693–700. [Google Scholar] [CrossRef]
- Deng, Y.J.; Zhou, C.; Zhang, M.Y.; Zhang, H.X. Effects of the reagent ratio on the properties of waterborne polyurethanes-acrylate for application in damping coating. Prog. Org. Coat. 2018, 122, 239–247. [Google Scholar] [CrossRef]
- Yin, X.; Dong, C.; Luo, Y. Effects of hydrophilic groups of curing agents on the properties of flame-retardant two-component waterborne coatings. Colloid Polym. Sci. 2017, 295, 2423–2431. [Google Scholar] [CrossRef]
- Liu, G.-C.; He, Y.-S.; Zeng, J.-B.; Xu, Y.; Wang, Y.-Z. In situ formed crosslinked polyurethane toughened polylactide. Polym. Chem. 2014, 5, 2530–2539. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, Q.; Wu, Y.; Chen, H.; Liu, Y.; Wang, J.; Duan, X.; Chen, Q.; Ge, Z.; Zhang, Y. Extremely Strong and Tough Biodegradable Poly(urethane) Elastomers with Unprecedented Crack Tolerance via Hierarchical Hydrogen-Bonding Interactions. Adv. Mater. 2023, 35, 2212130–2212140. [Google Scholar] [CrossRef]
- Ni, Y.-P.; Li, Q.-T.; Chen, L.; Wu, W.-S.; Qin, Z.-H.; Zhang, Y.; Chen, L.; Wang, X.-L.; Wang, Y.-Z. Semi-aromatic copolyesters with high strength and fire safety via hydrogen bonds and π-π stacking. Chem. Eng. J. 2019, 374, 694–705. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, P.; Liu, Y.; Zhu, P. Green flame-retardant flexible polyurethane foam based on polyphenol-iron-phytic acid network to improve the fire safety. Compos. Part B Eng. 2022, 239, 109958–109970. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, P.; Wang, B.; She, J.-H.; Liu, Y.; Zhu, P. Inorganic-organic hybrid coatings from tea polyphenols and laponite to improve the fire safety of flexible polyurethane foams. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130336–130348. [Google Scholar] [CrossRef]
- Wang, H.; Du, X.; Wang, S.; Du, Z.; Wang, H.; Cheng, X. Improving the flame retardancy of waterborne polyurethanes based on the synergistic effect of P-N flame retardants and a Schiff base. RSC Adv. 2020, 10, 12078–12088. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zeng, J.; Liu, W.; Qiu, X.; Qian, Y.; Zhang, H.; Yang, Y.; Liu, M.; Yang, D. Pristine lignin as a flame retardant in flexible PU foam. Green Chem. 2021, 23, 5972–5980. [Google Scholar] [CrossRef]
- Liu, C.; Li, P.; Xu, Y.J.; Liu, Y.; Zhu, P. Nickel alginate-enhanced fire safety of aluminum diethylphosphinate on epoxy resin. J. Appl. Polym. Sci. 2022, 140, 53552–53563. [Google Scholar] [CrossRef]
- Ding, H.; Huang, K.; Li, S.; Xu, L.; Xia, J.; Li, M. Flame retardancy and thermal degradation of halogen-free flame-retardant biobased polyurethane composites based on ammonium polyphosphate and aluminium hypophosphite. Polym. Test. 2017, 62, 325–334. [Google Scholar] [CrossRef]
- Wang, S.; Du, X.; Fu, X.; Du, Z.; Wang, H.; Cheng, X. Highly effective flame-retarded polyester diol with synergistic effects for waterborne polyurethane application. J. Appl. Polym. Sci. 2019, 137, 48444–48456. [Google Scholar] [CrossRef]
- Kuznetsov, Y.I.; Redkina, G.V. Thin Protective Coatings on Metals Formed by Organic Corrosion Inhibitors in Neutral Media. Coatings 2022, 12, 149. [Google Scholar] [CrossRef]
- Solomon, M.M.; Onyeachu, I.B.; Njoku, D.I.; Nwanonenyi, S.C.; Oguzie, E.E. Adsorption and corrosion inhibition characteristics of 2–(chloromethyl)benzimidazole for C1018 carbon steel in a typical sweet corrosion environment: Effect of chloride ion concentration and temperature. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125638–125684. [Google Scholar] [CrossRef]
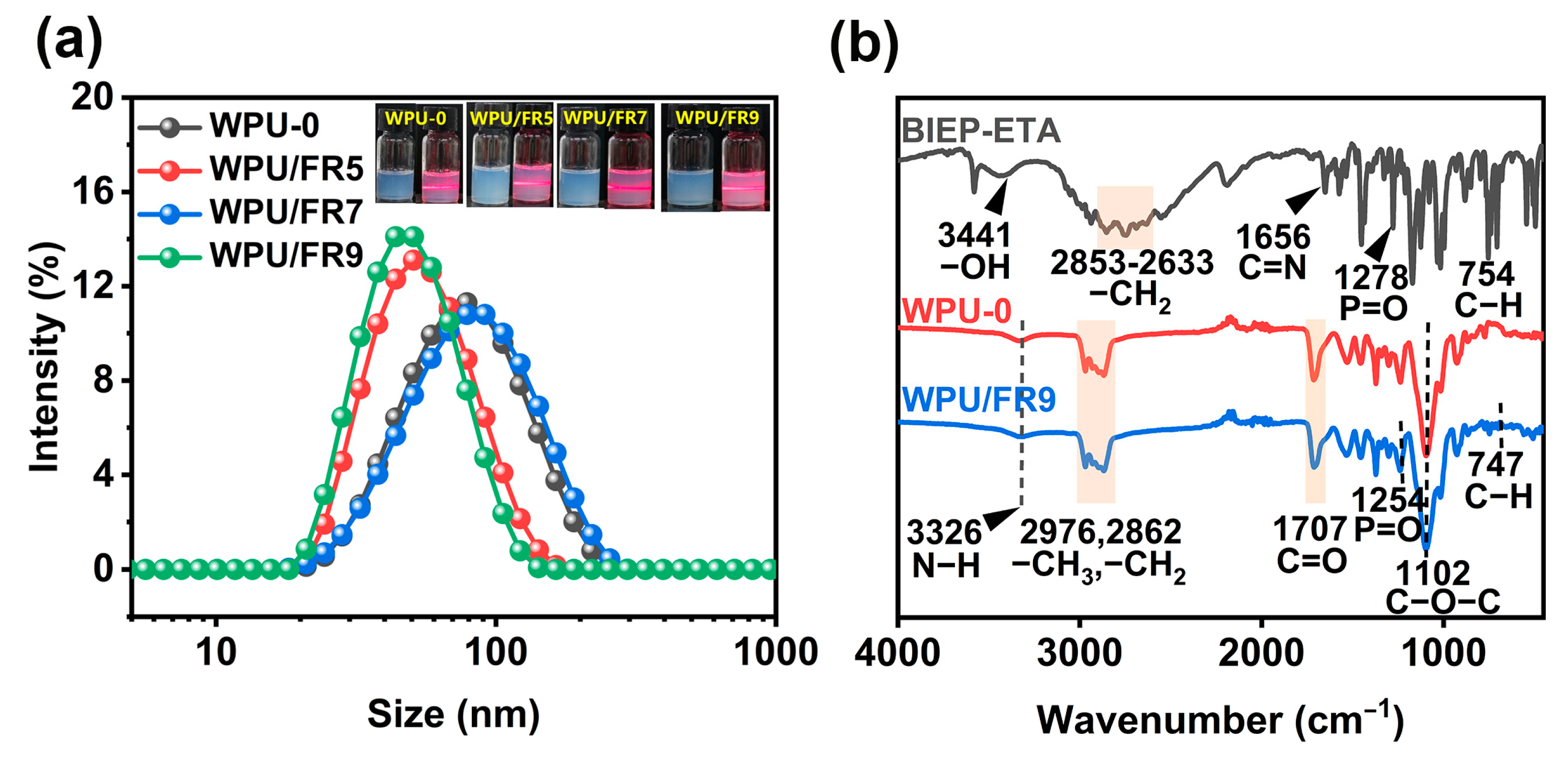


| Sample | Z-Average Size (nm) | PDI | Zeta Potential (mV) | Centrifugal Stability |
|---|---|---|---|---|
| WPU-0 | 74.66 | 0.257 | −37.7 | No precipitate |
| WPU/FR5 | 53.25 | 0.193 | −36.7 | No precipitate |
| WPU/FR7 | 77.69 | 0.226 | −41.0 | No precipitate |
| WPU/FR9 | 46.35 | 0.111 | −39.1 | No precipitate |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|
| WPU-0 | 31.5 ± 4.7 | 2439 ± 107 | 3.5 ± 0.7 |
| WPU/FR5 | 24.4 ± 0.7 | 2138 ± 64 | 2.2 ± 0.1 |
| WPU/FR7 | 32.7 ± 1.7 | 2349 ± 60 | 2.5 ± 0.3 |
| WPU/FR9 | 39.1 ± 0.7 | 2442 ± 13 | 3.5 ± 0.1 |
| Sample | TTI (s) | pHRR (kW/m2) | THR (MJ/m2) | Av-EHC (MJ/kg) | MARHE (kW/m2) | Char Residue (wt%) |
|---|---|---|---|---|---|---|
| WPU-0 | 39 | 1834 | 111 | 33 | 612 | 0 |
| WPU/FR5 | 40 | 920 | 57 | 27 | 345 | 2.5 |
| WPU/FR7 | 52 | 842 | 52 | 25 | 306 | 15.0 |
| WPU/FR9 | 33 | 602 | 53 | 25 | 301 | 9.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.-P.; Zhao, Z.-G.; Huang, Y.-Y.; Zhu, C.-J.; Cao, X.; Ni, Y.-P. Robust, Flame-Retardant, and Anti-Corrosive Waterborne Polyurethane Enabled by a PN Synergistic Flame-Retardant Containing Benzimidazole and Phosphinate Groups. Polymers 2023, 15, 2400. https://doi.org/10.3390/polym15102400
Zhang L-P, Zhao Z-G, Huang Y-Y, Zhu C-J, Cao X, Ni Y-P. Robust, Flame-Retardant, and Anti-Corrosive Waterborne Polyurethane Enabled by a PN Synergistic Flame-Retardant Containing Benzimidazole and Phosphinate Groups. Polymers. 2023; 15(10):2400. https://doi.org/10.3390/polym15102400
Chicago/Turabian StyleZhang, Li-Ping, Zhen-Guo Zhao, Yuan-Yuan Huang, Chang-Jian Zhu, Xing Cao, and Yan-Peng Ni. 2023. "Robust, Flame-Retardant, and Anti-Corrosive Waterborne Polyurethane Enabled by a PN Synergistic Flame-Retardant Containing Benzimidazole and Phosphinate Groups" Polymers 15, no. 10: 2400. https://doi.org/10.3390/polym15102400




