Fermentation-Derived Albumin-Based Hydrogels for Tissue Adhesion Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Albumin Sample Preparation
2.3. Characterization of Mechanical Properties
2.3.1. Tensile Test
2.3.2. Rheological Test
2.4. Characterization of Adhesive Properties
2.5. Biological Properties
2.5.1. Cell Culture
2.5.2. Cell Viability
2.6. Statistical Analyses
3. Results and Discussion
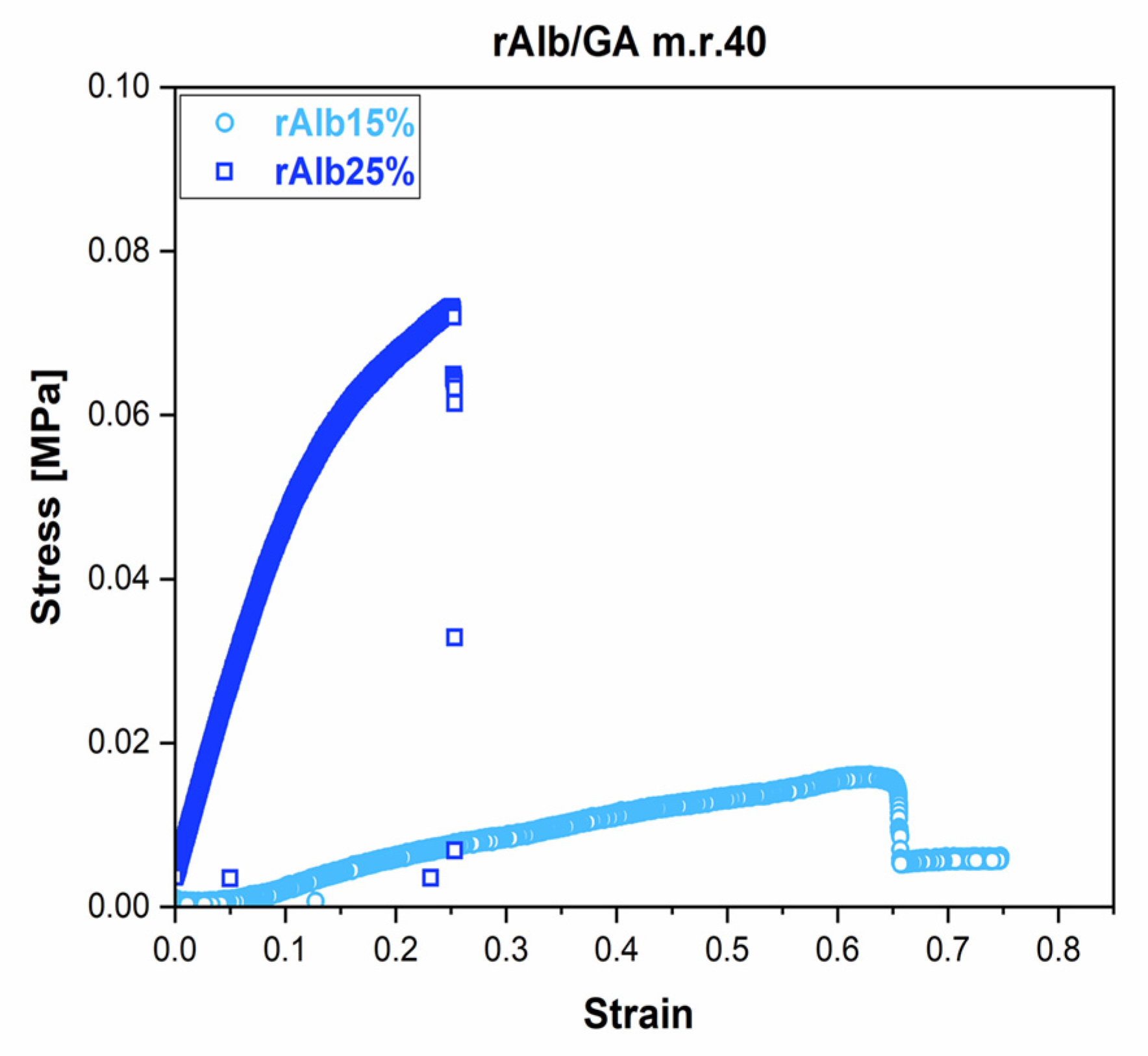
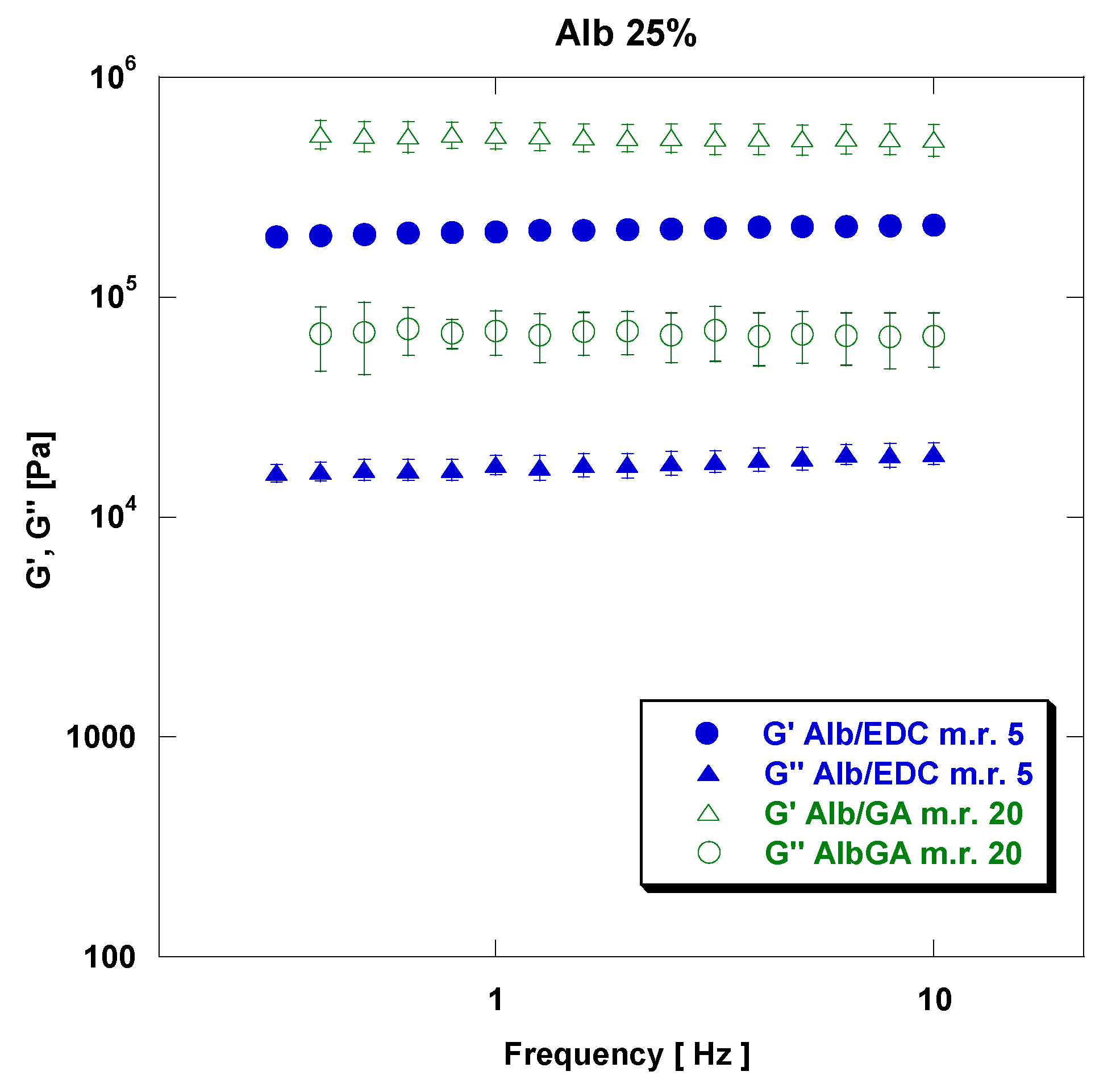
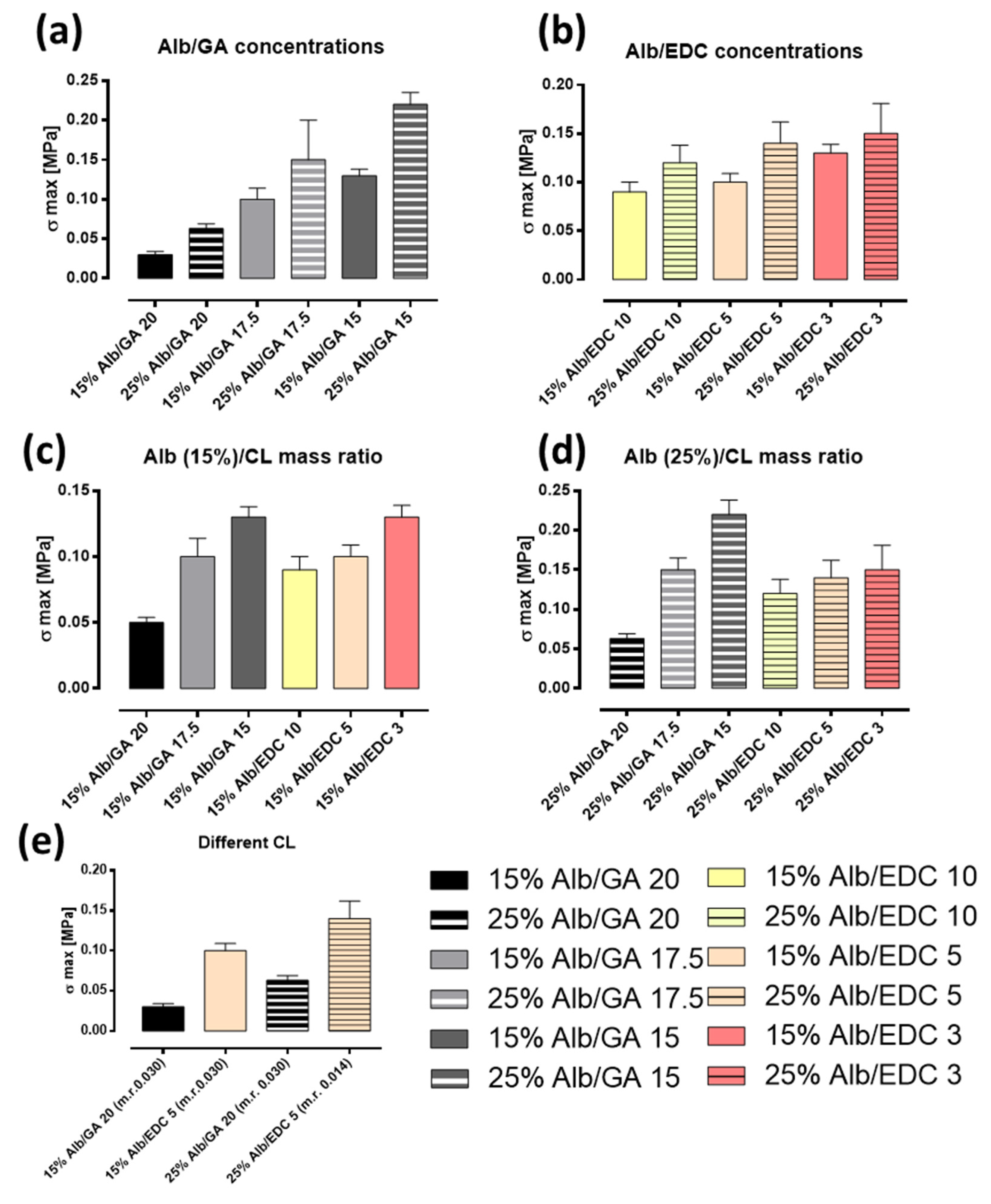
3.1. Mechanical Properties: Tensile and Rheological Analysis
3.2. Characterization of Adhesive Properties
3.3. Biological Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Heher, P.; Ferguson, J.; Redl, H.; Slezak, P. An overview of surgical sealant devices: Current approaches and future trends. Expert Rev. Med. Devices 2018, 15, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Wairkar, S. Recent developments and clinical applications of surgical glues: An overview. Int. J. Biol. Macromol. 2019, 137, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh-Narbat, M.; Annabi, N.; Khademhosseini, A. Surgical sealants and high strength adhesives. Mater. Today 2015, 18, 12. [Google Scholar] [CrossRef]
- Palacio, M.L.; Bhushan, B. Bioadhesion: A review of concepts and applications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2012, 370, 2321–2347. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.T.; Shek, P.N. Novel wound sealants: Biomaterials and applications. Expert Rev. Med. Devices 2010, 7, 639–659. [Google Scholar] [CrossRef]
- Behrens, A.M.; Lee, N.G.; Casey, B.J.; Srinivasan, P.; Sikorski, M.J.; Daristotle, J.L.; Sandler, A.D.; Kofinas, P. Biodegradable-polymer-blend-based surgical sealant with body-temperature-mediated adhesion. Adv. Mater. 2015, 27, 8056–8061. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.-W.; Liu, C.; Wu, J.-H.; Lin, L.-X.; Fan, H.-M.; Zhao, D.-H.; Zhuang, Y.-Q.; Sun, Y.-L. In situ injectable hyaluronic acid/gelatin hydrogel for hemorrhage control. Mater. Sci. Eng. C 2019, 98, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Mobley, S.R.; Hilinski, J.; Toriumi, D.M. Surgical tissue adhesives. Facial Plast. Surg. Clin. 2002, 10, 147–154. [Google Scholar] [CrossRef]
- Scognamiglio, F.; Travan, A.; Rustighi, I.; Tarchi, P.; Palmisano, S.; Marsich, E.; Borgogna, M.; Donati, I.; de Manzini, N.; Paoletti, S. Adhesive and sealant interfaces for general surgery applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Yue, K.; Tamayol, A.; Khademhosseini, A. Elastic sealants for surgical applications. Eur. J. Pharm. Biopharm. 2015, 95, 27–39. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, Y.; Han, L.; Lu, X. Biodegradable polymer hydrogel-based tissue adhesives: A review. Biosurface Biotribology 2021, 7, 163–179. [Google Scholar] [CrossRef]
- Chao, H.H.; Torchiana, D.F. BioGlue: Albumin/glutaraldehyde sealant in cardiac surgery. J. Card. Surg. 2003, 18, 500–503. [Google Scholar] [CrossRef]
- Zehr, K.J. Use of bovine albumin-glutaraldehyde glue in cardiovascular surgery. Ann. Thorac. Surg. 2007, 84, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Bahouth, Z.; Halachmi, S.; Shprits, S.; Burbara, Y.; Avitan, O.; Masarwa, I.; Moskovitz, B.; Nativ, O. The use of bovine serum albumin-glutaraldehyde tissue adhesive (BioGlue®) for tumor bed closure following open partial nephrectomy. Actas Urológicas Españolas Engl. Ed. 2017, 41, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Fürst, W.; Banerjee, A. Release of glutaraldehyde from an albumin-glutaraldehyde tissue adhesive causes significant in vitro and in vivo toxicity. Ann. Thorac. Surg. 2005, 79, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, J.; Li, B.; Li, D.; Meng, Z.; Sun, S.-K. Biocompatible therapeutic albumin/genipin bioglue for postoperative wound adhesion and residual tumor ablation. Biomaterials 2021, 279, 121179. [Google Scholar] [CrossRef]
- Berchane, N.; Andrews, M.; Kerr, S.; Slater, N.; Jebrail, F. On the mechanical properties of bovine serum albumin (BSA) adhesives. J. Mater. Sci. Mater. Med. 2008, 19, 1831–1838. [Google Scholar] [CrossRef]
- Zhu, W.; Chuah, Y.J.; Wang, D.-A. Bioadhesives for internal medical applications: A review. Acta Biomater. 2018, 74, 1–16. [Google Scholar] [CrossRef]
- Makvandi, P.; Caccavale, C.; Della Sala, F.; Zeppetelli, S.; Veneziano, R.; Borzacchiello, A. Natural Formulations Provide Antioxidant Complement to Hyaluronic Acid-Based Topical Applications Used in Wound Healing. Polymers 2020, 12, 1847. [Google Scholar] [CrossRef]
- Makvandi, P.; Della Sala, F.; di Gennaro, M.; Solimando, N.; Pagliuca, M.; Borzacchiello, A. A Hyaluronic Acid-Based Formulation with Simultaneous Local Drug Delivery and Antioxidant Ability for Active Viscosupplementation. ACS Omega 2022, 7, 10039–10048. [Google Scholar] [CrossRef]
- Guarino, V.; Altobelli, R.; Della Sala, F.; Borzacchiello, A.; Ambrosio, L. Alginate processing routes to fabricate bioinspired platforms for tissue engineering and drug delivery. In Alginates and Their Biomedical Applications; Rehm, B.H.A., Moradali, M.F., Eds.; Springer: Singapore, 2018; pp. 101–120. [Google Scholar]
- ISO 10993-5: 2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro cytotoxicity. ISO: Geneva, Switzerland, 2009; German Version.
- Della Sala, F.; Longobardo, G.; Lista, G.; Messina, F.; Borzacchiello, A. Effect of Hyaluronic Acid and Mesenchymal Stem Cells Secretome Combination in Promoting Alveolar Regeneration. Int. J. Mol. Sci. 2023, 24, 3642. [Google Scholar] [CrossRef] [PubMed]
- Borzacchiello, A.; Della Sala, F.; Ambrosio, L. Rheometry of polymeric biomaterials. In Characterization of Polymeric Biomaterials; Elsevier: Amsterdam, The Netherlands, 2017; pp. 233–253. [Google Scholar]
- Azadani, A.N.; Matthews, P.B.; Ge, L.; Shen, Y.; Jhun, C.-S.; Guy, T.S.; Tseng, E.E. Mechanical properties of surgical glues used in aortic root replacement. Ann. Thorac. Surg. 2009, 87, 1154–1160. [Google Scholar] [CrossRef]
- Della Sala, F.; Biondi, M.; Guarnieri, D.; Borzacchiello, A.; Ambrosio, L.; Mayol, L. Mechanical behavior of bioactive poly (ethylene glycol) diacrylate matrices for biomedical application. J. Mech. Behav. Biomed. Mater. 2020, 110, 103885. [Google Scholar] [CrossRef]
- Della Sala, F.; di Gennaro, M.; Lista, G.; Messina, F.; Valente, T.; Borzacchiello, A. Effect of Composition of Lung Biomimetic Niche on The Mesenchymal Stem Cell Differentiation Toward Alveolar Type II Pneumocytes. Macromol. Biosci. 2023, e2300035. [Google Scholar] [CrossRef] [PubMed]
- Vakalopoulos, K.A.; Wu, Z.; Kroese, L.; Kleinrensink, G.-J.; Jeekel, J.; Vendamme, R.; Dodou, D.; Lange, J.F. Mechanical strength and rheological properties of tissue adhesives with regard to colorectal anastomosis: An ex vivo study. Ann. Surg. 2015, 261, 323–331. [Google Scholar] [CrossRef]
- Serrero, A.; Trombotto, S.; Bayon, Y.; Gravagna, P.; Montanari, S.; David, L. Polysaccharide-based adhesive for biomedical applications: Correlation between rheological behavior and adhesion. Biomacromolecules 2011, 12, 1556–1566. [Google Scholar] [CrossRef]
- Della Sala, F.; Silvestri, T.; Borzacchiello, A.; Mayol, L.; Ambrosio, L.; Biondi, M. Hyaluronan-coated nanoparticles for active tumor targeting: Influence of polysaccharide molecular weight on cell uptake. Colloids Surf. B Biointerfaces 2022, 210, 112240. [Google Scholar] [CrossRef] [PubMed]
- Courtois, J.; Baroudi, I.; Nouvel, N.; Degrandi, E.; Pensec, S.; Ducouret, G.; Chanéac, C.; Bouteiller, L.; Creton, C. Supramolecular soft adhesive materials. Adv. Funct. Mater. 2010, 20, 1803–1811. [Google Scholar] [CrossRef]
- Yang, J.; Bai, R.; Chen, B.; Suo, Z. Hydrogel adhesion: A supramolecular synergy of chemistry, topology, and mechanics. Adv. Funct. Mater. 2020, 30, 1901693. [Google Scholar] [CrossRef]
- Park, J.-Y.; Jung, I.-H.; Kim, Y.-K.; Lim, H.-C.; Lee, J.-S.; Jung, U.-W.; Choi, S.-H. Guided bone regeneration using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC)-cross-linked type-I collagen membrane with biphasic calcium phosphate at rabbit calvarial defects. Biomater. Res. 2015, 19, 15. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.H.; Lim, H.-G.; Lim, C.; Kim, Y.J. The anti-calcification effect of dithiobispropionimidate, carbodiimide and ultraviolet irradiation cross-linking compared to glutaraldehyde in rabbit implantation models. Korean J. Thorac. Cardiovasc. Surg. 2013, 46, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Murabayashi, S.; Mitamura, Y.; Taguchi, T. Characterization of alkali-treated collagen gels prepared by different crosslinkers. J. Mater. Sci. Mater. Med. 2008, 19, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Scheffel, D.L.S.; Bianchi, L.; Soares, D.G.; Basso, F.G.; Sabatini, C.; de Souza Costa, C.; Pashley, D.H.; Hebling, J. Transdentinal cytotoxicity of carbodiimide (EDC) and glutaraldehyde on odontoblast-like cells. Oper. Dent. 2015, 40, 44–54. [Google Scholar] [CrossRef] [PubMed]






| Albumin Concentration (w/v) | Crosslinker (CL) | Albumin/CL Mass Ratio (Molar Ratio) |
|---|---|---|
| 15% | EDC | 10 (0.030) |
| 5 (0.015) | ||
| 3 (0.010) | ||
| GA | 40 (0.060) | |
| 20 (0.030) | ||
| 15 (0.020) | ||
| 25% | EDC | 10 (0.030) |
| 5 (0.015) | ||
| 3 (0.010) | ||
| GA | 40 (0.060) | |
| 20 (0.030) | ||
| 15 (0.020) |
| Albumin Concentration (w/v%) | Crosslinker | Albumin/CL Mass Ratio | σmax [MPa] |
|---|---|---|---|
| 15% | EDC | 10 | 4 × 10−2 ± 1 × 10−2 |
| 25% | EDC | 10 | 6 × 10−2 ± 2 × 10−2 |
| 15% | EDC | 5 | 1 × 10−1 ± 1 × 10−2 |
| 25% | EDC | 5 | 3 × 10−1 ± 2 × 10−2 |
| 15% | GA | 40 | 2 × 10−2 ± 1 × 10−2 |
| 25% | GA | 40 | 7 × 10−2 ± 1 × 10−2 |
| 15% | GA | 20 | 1 × 10−1 ± 2 × 10−3 |
| 25% | GA | 20 | 2 × 10−1 ± 3 × 10−2 |
| Albumin Concentration (w/v%) | Crosslinker | rAlb/CL Mass Ratio | G′, Pa at 5 Hz | G″, Pa at 5 Hz |
|---|---|---|---|---|
| 15% | EDC | 10 | 6.06 × 103 | 1.27 × 103 |
| 25% | EDC | 10 | 8.71 × 104 | 5.36 × 103 |
| 15% | EDC | 5 | 1.85 × 104 | 1.58 × 103 |
| 25% | EDC | 5 | 2.09 × 105 | 1.83 × 104 |
| 15% | GA | 40 | 7.22 × 103 | 3.29 × 102 |
| 25% | GA | 40 | 1.89 × 105 | 1.35 × 104 |
| 15% | GA | 20 | 2.44 × 104 | 2.13 × 103 |
| 25% | GA | 20 | 5.22 × 105 | 6.84 × 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Sala, F.; Malle, B.M.; Ambrosio, L.; Borzacchiello, A. Fermentation-Derived Albumin-Based Hydrogels for Tissue Adhesion Applications. Polymers 2023, 15, 2530. https://doi.org/10.3390/polym15112530
Della Sala F, Malle BM, Ambrosio L, Borzacchiello A. Fermentation-Derived Albumin-Based Hydrogels for Tissue Adhesion Applications. Polymers. 2023; 15(11):2530. https://doi.org/10.3390/polym15112530
Chicago/Turabian StyleDella Sala, Francesca, Birgitte Mølholm Malle, Luigi Ambrosio, and Assunta Borzacchiello. 2023. "Fermentation-Derived Albumin-Based Hydrogels for Tissue Adhesion Applications" Polymers 15, no. 11: 2530. https://doi.org/10.3390/polym15112530







