A Systematic Review of Butterfly Pea Flower (Clitoria ternatea L.): Extraction and Application as a Food Freshness pH-Indicator for Polymer-Based Intelligent Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Strategy
2.2. Keyword Choices
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Study Selection and Data Extraction
2.6. Quality Assessment of the Included Studies
| Author/Year | Polymer Material | Composition of Polymer Film | Food Application | Conclusion |
|---|---|---|---|---|
| Hashim et al., 2021 [14] | Sugarcane wax/agar (SW/Agr) | Agr/BF/1% SW, Agr/BF/1.5% SW, Agr/BF/2% SW | Shrimp | Agr/BF/2%SW film was chosen as a sensor for shrimps’ freshness due to its overall performance and sensitivity to ammonia vapour. |
| Wu et al., 2021 [15] | Gellan gum/heat-treated soy protein isolate (G/HSPI) | G/CT, G/HSPI1%/CT, G/HSPI2%/CT | Shrimp | The incorporation of CT extract in G and G/HSPI films successfully reduced the release of CT anthocyanin content. |
| Ahmad et al., 2020 [16] | Sago (Metroxylon sagu) | SG, SG5/BPF, SG7/BPF, SG10/BPF, SG15/BPF | Chicken | The optimal concentration to formulate the SG film was 5% wt/vol and the surfaces of the films investigated were smooth (complete polymer gelatinisation). |
| Hidayati et al., 2021 [17] | Chitosan/polyvinyl alcohol (CH/PVA) | CH:PVA (20:80, 40:60, 60:40, and 80:20) | - | CH:PVA (40:60) had the best results for physical and mechanical properties and produced the clearest colour changes with different pH ranges. |
| Boonsiriwit et al., 2021 [18] | Hydroxypropyl methylcellulose/microcrystalline cellulose biocomposites (HMB) | HMB, 1.0BA-HMB, 1.5BA-HMB, and 2.0BA-HMB | Fish fillet (Scomber scombrus) | 1.0BA-HMB indicator exhibited the best physical properties; however, 1.5-HMB demonstrated a clear change in the colour response of quality fish (more sensitive). |
| Sai-Ut et al., 2021 [19] | Gelatin/methylcellulose (G/MC) | G, G/BPE, MC, MC/BPE | - | MC/BPE indicator had improved mechanical and physical properties. Meanwhile, G/BPE showed a clearer response to pH variation. |
| Koshy et al., 2022 [20] | Soy protein isolate/chitin nanowhisker (SPI/CNW) | SPI, SPI-CNW, SPI-CTE, and SPI-CNW-CTE | - | The addition of CTE to SPI enhanced the mechanical properties. However, the addition of CTE was found to decrease the tensile strength of SPI-CNW film and was found to make the film pH sensitive. |
| Mary et al., 2020 [21] | Potato starch/nanosized titanium dioxide (S/TiO2) | S, S/BPE, S/TiO2, S/TiO2/BPE | Shrimp | It was observed that the addition of BPE and TiO2 could greatly alter the physical properties of the film. The addition of TiO2 exhibited changes in colour during the spoilage of shrimp. |
| Yan et al., 2021 [22] | Chitosan (CH) | CH, CH-BP10%, CH-BP15%, CH-BP20% | Tilapia fish | The incorporation of BP extract increased the thickness, WVP, and mechanical properties of CH-BP films, while reducing their moisture content, swelling ratio, and water contact angle. |
| Kim et al., 2022 [23] | Gelatine/agar/zinc oxide nanoparticles (Gel/Agar/Zno) | Gel/Agar, Gel/Agar/ZnO, Gel/Agar/BA, Gel/Agar/ZnO/BA | Shrimp | The addition of BA and ZnO significantly increased the UV-blocking properties and surface hydrophobicity without significant changes in the film’s mechanical, thermal stability, and water vapour barrier properties. |
| Romruen et al., 2022 [24] | Alginate/agar/cellulose nanosphere (CN) | 0% CN, 5% CN, 10% CN, 20% CN, and 30% CN. | Shrimp | CN can improve the mechanical properties of smart bilayer films without affecting their chemical properties and proved it is effectively used to monitor shrimp freshness. |
| Ahmad et al., 2019 [25] | t-carrageenan | Control, t-carrageenan/BPA | Shrimp and durian | The ability of the developed colourimetric pH sensor film from t-carrageenan shows colour changes on shrimp and durian, which provides a simple way to express the quality of food. |
| Rawdkuen et al., 2020 [26] | Gelatine | Control, Gelatine/BPA | - | The film with BPA extracts in gelatine films showed the highest antioxidant activity, improved water barrier properties, and showed greater pH sensitivity. |
| Roy et al., 2021 [27] | Carboxymethyl cellulose/agar (CMC/agar) | CMC/agar, CMCagar/ACN, CMC/agar/SKN | - | The incorporation of anthocyanin in CMC/agar-based films improved physical and functional properties without altering the thermal stability. |
| Sumiasih et al., 2022 [28] | Chitosan/polyvinyl alcohol (CH/PVA) | CH: PVA (20:80, 40:60, 60:40, and 80:20) | Beef | The best formulation was the composition of 20:80 PVA and chitosan 20:80 with the best thickness and total TVBN analysis |
| Cho et al., 2021 [29] | Corn starch (CS) | CS-BP (9% v/v, 13% v/v, 17% v/v, 20% v/v, and 23% v/v) | Pasteurised milk | The thickness of the films increased with the BP concentration added. Meanwhile, BP solutions incorporating 23% v/v exhibited the greatest ΔE values. |
| Polymer Film | Main Results after BPFA Incorporation | References | |||
|---|---|---|---|---|---|
| Physical Properties | Mechanical Properties | ||||
| Thickness | Water Permeability | Tensile Strength | Elongation at Break | ||
| Sugarcane wax and agar matrix | Increase | No significant difference | Low | No significant difference | [13] |
| Gellan gum and heat-treated soy protein isolate (HSPI) | - | Low | Low | Low | [14] |
| Chitosan and polyvinyl alcohol (PVA) | No effect | - | High | Low | [16] |
| Hydroxypropyl methylcellulose biocomposite (HMB) | No effect | - | High | Low | [17] |
| Gelatine and methylcellulose | No effect | High | Gelatine + BPFA (low) MC + BPFA (high) | Gelatine + BPFA (low) MC + BPFA (high) | [18] |
| Soy protein isolate (SPI) and chitin nanowhisker (CNW) | No effect | - | Low | Low | [19] |
| Nanosized TiO2 | Decrease | Low | - | - | [20] |
| Chitosan | Increase | High | High | Low | [21] |
| Zinc oxide nanoparticles (ZnO) + gelatine/agar | Increase | No significant difference | Low | High | [22] |
| Cellulose nanosphere (CN) and alginate/agar | Increase | No significant difference | High | Low | [23] |
| Gelatine | No effect | Low | Low | High | [25] |
| Carboxymethyl cellulose (CMC)/agar-based | No effect | Low | No significant difference | High | [26] |
| Film Matrix | Foods | Sample Size (g) | Storage (°C) | Visual Colour Change | Final Time (Day) | References |
|---|---|---|---|---|---|---|
| Sugarcane wax and agar matrix | Shrimp | 55 | 25 | Deep purple to bluish-green | 1.0 | [13] |
| Heat-treated soy protein isolate and gellan gum | Shrimp | - | 25 | Blue to bluish-green | 1.0 | [14] |
| Hydroxypropyl methylcellulose biocomposite (HMB) | Mackerel fish | 200 | 4 | Deep purple to violet | 6.0 | [17] |
| Nanosized TiO2 | Prawn | 20 | 4 | Pink to green | 6.0 | [20] |
| Chitosan | Tilapia fish | - | 4 | Purple-blue to dark green | 6.0 | [21] |
| t-carrageenan | Durian and shrimp | - | 28 | Shrimp: deep blue to greenish-blue Durian: deep blue to dark purple | Shrimp: 0.5 h Durian: 4.0 | [24] |
| Chitosan and polyvinyl alcohol (PVA) | Beef | 60 | 25 | Blue to bluish-green | 1.0 | [27] |
| Corn starch | Pasteurised milk | 250 (mL) | 25 | Deep blue to light blue | 3.0 | [28] |
3. Results and Discussion
3.1. Study Characteristics
3.2. Butterfly Pea Flower (Clitoria ternatea L.)
3.2.1. Plant Morphology
3.2.2. Plant Pigment (Anthocyanin)
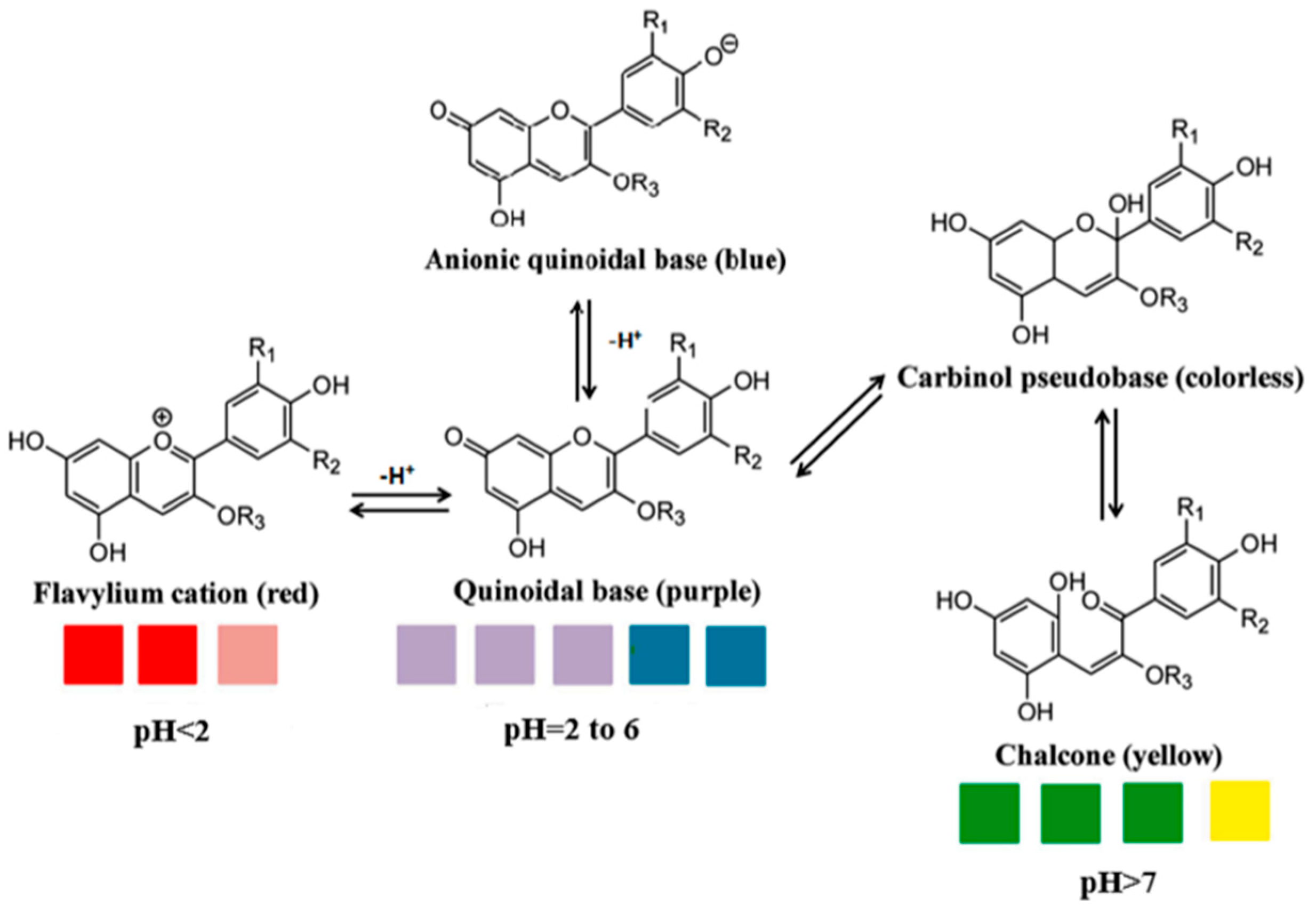
3.2.3. pH Sensitivity of Anthocyanin
3.3. Anthocyanin Extraction from BPFs
3.3.1. Extraction Solvent
3.3.2. Conventional and Non-Conventional Extraction Methods

3.3.3. Ultrasonic-Assisted Extraction (UAE)
3.3.4. Microwave-Assisted Extraction (MAE)
3.4. Intelligent Packaging
3.4.1. Time-Temperature Indicators
3.4.2. Integrity Indicators
3.4.3. Freshness Indicators
3.5. Application of BPF Anthocyanin (BPFA) as a Polymer-Based pH Film Indicator
3.5.1. Effect of BPFA on the Physical Properties of Films
Thickness
Water Vapour Permeability
3.5.2. Effect of BPFA on the Mechanical Properties of Films
3.6. Evaluation of BPFA pH Indicator Potential Tested on Food
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thuy, N.M.; Minh, V.Q.; Ben, T.C.; Thi Nguyen, M.T.; Ha, H.T.N.; Tai, N.V. Identification of anthocyanin compounds in butterfly pea flowers (Clitoria ternatea L.) by ultra-performance liquid chromatography/ultraviolet coupled to mass spectrometry. Molecules 2021, 26, 4539. [Google Scholar] [CrossRef] [PubMed]
- Jamil, N.; Pa’ee, F. Antimicrobial activity from leaf, flower, stem, and root of Clitoria ternatea–A review. In Proceedings of the AIP Conference, Yogyakarta, Indonesia, 15 August 2018; Volume 2002, p. 020044. [Google Scholar]
- Marpaung, A.M.; Lee, M.; Kartawiria, I.S. The development of butterfly pea (Clitoria ternatea) flower powder drink by co-crystallization. Indones. Food Sci. Technol. J. 2020, 3, 34–37. [Google Scholar] [CrossRef]
- Suarna, I.W.; Wijaya, I.M.S. Butterfly pea (Clitoria ternatea L.: Fabaceae) and its morphological variations in Bali. J. Trop. Biodivers. Biotechnol. 2021, 6, 63013. [Google Scholar] [CrossRef]
- Rahim, M.Z.A.; Husin, N.; Mohd Noor, M.A.; Yet, Z.R.; Ismail-Fitry, M.R. Screening of natural colours from various natural resources as potential reusable visual indicators for monitoring food freshness. Malaysian J. Anal. Sci. 2020, 24, 288–299. [Google Scholar]
- Abedi-Firoozjah, R.; Yousefi, S.; Heydari, M.; Seyedfatehi, F.; Jafarzadeh, S.; Mohammadi, R.; Rouhi, M.; Garavand, F. Application of red cabbage anthocyanins as pH-sensitive pigments in smart food packaging and sensors. Polymers 2022, 14, 1629. [Google Scholar] [CrossRef]
- Ghaani, M.; Cozzolino, C.A.; Castelli, G.; Farris, S. An overview of the intelligent packaging technologies in the food sector. Trends Food Sci. Technol. 2016, 51, 1–11. [Google Scholar] [CrossRef]
- Poh, N.A.B.A. Investigation of the Colour Changes of Clitoria ternatea in Different pH Conditions; School of Science and Technology: Singapore, 2019. [Google Scholar]
- Roy, S.; Rhim, J.W. Anthocyanin food colorant and its application in pH-responsive color change indicator films. Crit. Rev. Food Sci. Nutr. 2021, 61, 2297–2325. [Google Scholar] [CrossRef]
- Omar, S.R.; Noh, A.A.; Rohaizan, A.N. The Potential Use of Anthocyanin in Butterfly Pea (Clinoteria ternatea) Petals as Colorimetric Indicator in Intelligent Food Packaging Article. Malaysian J. Sci. Health Technol. 2022, 8, 71–76. [Google Scholar] [CrossRef]
- Oladzadabbasabadi, N.; Nafchi, A.M.; Ghasemlou, M.; Ariffin, F.; Singh, Z.; Al-Hassan, A.A. Natural anthocyanins: Sources, extraction, characterization, and suitability for smart packaging. Food Packag. Shelf Life 2022, 33, 100872. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Critical Appraisal Skills Programme. CASP (Qualitative Studies) Checklist. 2018. Available online: https://casp-uk.net (accessed on 6 May 2023).
- Hashim, S.B.; Tahir, H.E.; Liu, L.; Zhang, J.; Zhai, X.; Mahdi, A.A.; Awad, F.N.; Haassan, M.M.; Xiaobo, Z.; Jiyong, S. Intelligent colorimetric pH sensoring packaging films based on sugarcane wax/agar integrated with butterfly pea flower extract for optical tracking of shrimp freshness. Food Chem. 2022, 373, 131514. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.T.; Tsai, I.L.; Ho, Y.C.; Hang, Y.H.; Lin, C.; Tsai, M.L.; Mi, F.L. Active and intelligent gellan gum-based packaging films for controlling anthocyanins release and monitoring food freshness. Carbohydr. Polym. 2021, 254, 117410. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.N.; Abdullah Lim, S.; Navaranjan, N. Development of sago (metroxylon sagu)-based colorimetric indicator incorporated with butterfly pea (Clitoria ternatea) anthocyanin for intelligent food packaging. J. Food Saf. 2020, 40, e12807. [Google Scholar] [CrossRef]
- Hidayati, N.A.; Wijaya, M.W.; Bintoro, V.P.; Mulyani, S.; Pratama, Y. Development of biodegradable smart packaging from chitosan, polyvinyl alcohol (PVA) and butterfly pea flower’s (Clitoria ternatea L.) anthocyanin extract. Food Res. 2021, 5, 307–314. [Google Scholar] [CrossRef]
- Boonsiriwit, A.; Lee, M.; Kim, M.; Inthamat, P.; Siripatrawan, U.; Lee, Y.S. Hydroxypropyl methylcellulose/microcrystalline cellulose biocomposite film incorporated with butterfly pea anthocyanin as a sustainable pH-responsive indicator for intelligent food-packaging applications. Food Biosci. 2021, 44, 101392. [Google Scholar] [CrossRef]
- Sai-Ut, S.; Suthiluk, P.; Tongdeesoontorn, W.; Rawdkuen, S.; Kaewprachu, P.; Karbowiak, T.; Degraeve, P.; Degraeve, P. Using anthocyanin extracts from butterfly pea as pH indicator for intelligent gelatin film and methylcellulose film. Curr. Appl. Sci. Technol. 2021, 21, 652–661. [Google Scholar]
- Koshy, R.R.; Reghunadhan, A.; Mary, S.K.; Pillai, P.S.; Joseph, S.; Pothen, L.A. pH indicator films fabricated from soy protein isolate modified with chitin nanowhisker and Clitoria ternatea flower extract. Curr. Res. Food Sci. 2022, 5, 743–751. [Google Scholar] [CrossRef]
- Mary, S.K.; Koshy, R.R.; Daniel, J.; Koshy, J.T.; Pothen, L.A.; Thomas, S. Development of starch based intelligent films by incorporating anthocyanins of butterfly pea flower and TiO2 and their applicability as freshness sensors for prawns during storage. RSC Adv. 2020, 10, 39822–39830. [Google Scholar] [CrossRef]
- Yan, J.; Cui, R.; Qin, Y.; Li, L.; Yuan, M. A pH indicator film based on chitosan and butterfly pudding extract for monitoring fish freshness. Int. J. Biol. Macromol. 2021, 177, 328–336. [Google Scholar] [CrossRef]
- Kim, H.J.; Roy, S.; Rhim, J.W. Gelatin/agar-based color-indicator film integrated with Clitoria ternatea flower anthocyanin and zinc oxide nanoparticles for monitoring freshness of shrimp. Food Hydrocoll. 2022, 124, 107294. [Google Scholar] [CrossRef]
- Romruen, O.; Kaewprachu, P.; Karbowiak, T.; Rawdkuen, S. Development of smart bilayer alginate/agar film containing anthocyanin and catechin-lysozyme. Polymers 2022, 14, 5042. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Yook Heng, L.; Salam, F.; Mat Zaid, M.H.; Abu Hanifah, S. A colorimetric pH sensor based on Clitoria sp and Brassica sp for monitoring of food spoilage using chromametry. Sensors 2019, 19, 4813. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Faseha, A.; Benjakul, S.; Kaewprachu, P. Application of anthocyanin as a color indicator in gelatin films. Food Biosci. 2020, 36, 100603. [Google Scholar] [CrossRef]
- Roy, S.; Kim, H.J.; Rhim, J.W. Effect of blended colorants of anthocyanin and shikonin on carboxymethyl cellulose/agar-based smart packaging film. Int. J. Biol. Macromol. 2021, 183, 305–315. [Google Scholar] [CrossRef]
- Sumiasih, I.H. Indicator film of natural coloring of butterfly pea (Clitoria ternatea L.) as detection of beef damage: Indicator film of natural coloring of butterfly pea (Clitoria ternatea L.) as detection of beef damage. Int. J. Appl. Biol. 2022, 6, 79–92. [Google Scholar]
- Cho, T.F.; Yassoralipour, A.; Lee, Y.Y.; Tang, T.K.; Lai, O.M.; Chong, L.C.; Kuan, C.-H.; Phuah, E.T. Evaluation of milk deterioration using simple biosensor. J. Food Meas. Charact. 2021, 16, 258–268. [Google Scholar] [CrossRef]
- Chauhan, N.; Rajvaidhya, S.; Dubey, B.K. Pharmacognostical, phytochemical and pharmacological review on Clitoria ternatea for antiasthmatic activity. Int. J. Pharm. Sci. 2012, 3, 398. [Google Scholar]
- Havananda, T.; Luengwilai, K. Variation in floral antioxidant activities and phytochemical properties among butterfly pea (Clitoria ternatea L.) germplasm. Genet. Resour. Crop. Evol. 2019, 66, 645–658. [Google Scholar] [CrossRef]
- Jamil, N.; Zairi, M.N.M.; Nasim, N.A.I.M.; Pa’ee, F. Influences of environmental conditions to phytoconstituents in Clitoria ternatea (butterfly pea flower)—A review. J. Sci. Technol. 2018, 10, 208–228. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Jeyaraj, E.J.; Lim, Y.Y.; Choo, W.S. Extraction methods of butterfly pea (Clitoria ternatea) flower and biological activities of its phytochemicals. J. Sci. Technol. 2021, 58, 2054–2067. [Google Scholar] [CrossRef] [PubMed]
- Madhu, K. Phytochemical screening and antioxidant activity of in vitro grown plants Clitoria ternatea L., using DPPH assay. Asian J. Pharm. Clin. Res. 2013, 6, 38–42. [Google Scholar]
- Vidana-Gamage, G.C.; Lim, Y.Y.; Choo, W.S. Anthocyanins from Clitoria ternatea flower: Biosynthesis, extraction, stability, antioxidant activity, and applications. Front. Plant. Sci. 2021, 12, 792303. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S.; Pasukamonset, P.; Chusak, C. Clitoria ternatea beverages and antioxidant usage. In Pathology; Academic Press: Cambridge, MA, USA, 2020; pp. 189–196. [Google Scholar]
- Campbell, S.M.; Pearson, B.; Marble, S.C. Butterfly Pea (Clitoria ternatea) Flower. Extract (BPFE) and Its Use as a pH-Dependent Natural Colorant; University of Florida: Gainesville, FL, USA, 2019. [Google Scholar]
- Azima, A.S.; Noriham, A.; Manshoor, N. Phenolics, antioxidants and color properties of aqueous pigmented plant extracts: Ardisia colorata var. elliptica, Clitoria ternatea, Garcinia mangostana and Syzygium cumini. J. Funct. Foods 2017, 38, 232–241. [Google Scholar] [CrossRef]
- Balbinot-Alfaro, E.; Craveiro, D.V.; Lima, K.O.; Costa, H.L.G.; Lopes, D.R.; Prentice, C. Intelligent packaging with pH indicator potential. Food Eng. Rev. 2019, 11, 235–244. [Google Scholar] [CrossRef]
- Wiyantoko, B. Butterfly pea (Clitoria ternatea L.) extract as indicator of acid-base titration. Indones. J. Chem. 2020, 3, 22–32. [Google Scholar] [CrossRef]
- Basílio, N.; Pina, F. Chemistry and photochemistry of anthocyanins and related compounds: A thermodynamic and kinetic approach. Molecules 2016, 21, 1502. [Google Scholar] [CrossRef]
- Saptarini, N.M.; Suryasaputra, D.; Nurmalia, H. Application of Butterfly Pea (Clitoria ternatea Linn) extract as an indicator of acid-base titration. J. Chem. Pharm. Res. 2015, 7, 275–280. [Google Scholar]
- Sarwar, S.; Rahman, M.R.; Nahar, K.; Rahman, M.A. Analgesic and neuropharmacological activities of methanolic leaf extract of Clitoria ternatea Linn. J. Pharmacogn. Phytochem. 2014, 2, 110–114. [Google Scholar]
- Husin, N.; Rahim, M.Z.A.; Zulkhairi, M.; Azizan, M.; Mohd Noor, M.A.; Rashedi, M.; Ismail-Fitry, M.R.; Hassan, N. Real-time monitoring of food freshness using delphinidin-based visual. Malaysian J. Anal. Sci. 2020, 24, 558–569. [Google Scholar]
- Tena, N.; Asuero, A.G. Up-to-date analysis of the extraction methods for anthocyanins: Principles of the techniques, optimization, technical progress, and industrial application. Antioxidants 2022, 11, 286. [Google Scholar] [CrossRef]
- Ijod, G.; Musa, F.N.; Anwar, F.; Suleiman, N.; Adzahan, N.M.; Azman, E.M. Thermal and nonthermal pretreatment methods for the extraction of anthocyanins: A review. J. Food Process. Preserv. 2022, 46, e17255. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops—A review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef]
- Chemat, F.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation, and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Chong, F.C.; Gwee, X.F. Ultrasonic extraction of anthocyanin from Clitoria ternatea flowers using response surface methodology. Nat. Prod. Res. 2015, 29, 1485–1487. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Panja, P. Green extraction methods of food polyphenols from vegetable materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Sandhya, M.; Ramasamy, D.; Sudhakar, K.; Kadirgama, K.; Harun, W.S.W. Ultrasonication an intensifying tool for preparation of stable nanofluids and study the time influence on distinct properties of graphene nanofluids—A systematic overview. Ultrason. Sonochem. 2021, 73, 105479. [Google Scholar] [CrossRef]
- Nour, A.H.; Oluwaseun, A.R.; Nour, A.H.; Omer, M.S.; Ahmad, N. Microwave-assisted extraction of bioactive compounds. In Theory and Practice; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–31. [Google Scholar]
- Uzel, R.A. Microwave-assisted green extraction technology for sustainable food processing. In Emerging Microwave Technologies in Industrial, Agricultural, Medical and Food Processing; IntechOpen: London, UK, 2018; pp. 159–178. [Google Scholar]
- Alupului, A.; Calinescu, I.; Lavric, V. Microwave extraction of active principles from medicinal plants. UPB Sci. Bull. B Chem. Mater. Sci. 2012, 74, 129–142. [Google Scholar]
- Gamage, G.C.V.; Choo, W.S. Hot water extraction, ultrasound, microwave, and pectinase-assisted extraction of anthocyanins from blue pea flower. Food Chem. Adv. 2023, 2, 100209. [Google Scholar] [CrossRef]
- Marsin, A.M.; Jusoh, Y.M.M.; Abang, D.N.; Zaidel, Z.H.; Yusof, A.H.M.; Muhamad, I.I. Microwave-assisted encapsulation of blue pea flower (Clitoria ternatea) colourant: Maltodextrin concentration, power, and time. Chem. Eng. 2020, 78, 199–204. [Google Scholar] [CrossRef]
- Farzaneh, V.; Carvalho, I.S. Modelling of microwave assisted extraction (MAE) of anthocyanins (TMA). J. Appl. Res. Med. Aromat. Plants 2017, 6, 92–100. [Google Scholar] [CrossRef]
- Romero-díez, R.; Matos, M.; Rodrigues, L.; Bronze, M.R.; Rodríguez-rojo, S. Microwave and ultrasound pre-treatments to enhance anthocyanins extraction from different wine lees. Food Chem. 2019, 272, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Izirwan, I.; Munusamy, T.D.; Hamidi, N.H.; Sulaiman, S.Z. Optimization of microwave-assisted extraction of anthocyanin from Clitoria ternatea flowers. Int. J. Mech. Eng. Robot. Res. 2020, 9, 1246–1252. [Google Scholar] [CrossRef]
- Pereira de Abreu, D.A.; Cruz, J.M.; Paseiro Losada, P. Active and intelligent packaging for the food industry. Food Rev. Int. 2012, 28, 146–187. [Google Scholar] [CrossRef]
- Realini, C.E.; Marcos, B. Active and intelligent packaging systems for a modern society. Meat Sci. 2014, 98, 404–419. [Google Scholar] [CrossRef]
- Heising, J.K.; Dekker, M.; Bartels, P.V.; Van Boekel, M.A.J.S. Monitoring the quality of perishable foods: Opportunities for intelligent packaging. Crit. Rev. Food Sci. Nutr. 2014, 54, 645–654. [Google Scholar] [CrossRef]
- Fang, Z.; Zhao, Y.; Warner, R.D.; Johnson, S.K. Active and intelligent packaging in meat industry. Trends Food Sci. Technol. 2017, 61, 60–71. [Google Scholar] [CrossRef]
- Vanderroost, M.; Ragaert, P.; Devlieghere, F.; De Meulenaer, B. Intelligent food packaging: The next generation. Trends Food Sci. Technol. 2014, 39, 47–62. [Google Scholar] [CrossRef]
- Müller, P.; Schmid, M. Intelligent packaging in the food sector: A brief overview. Foods 2019, 8, 16. [Google Scholar] [CrossRef]
- Kuswandi, B. Freshness sensors for food packaging. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Jarray, A.; Gerbaud, V.; Hemati, M. Polymer-plasticizer compatibility during coating formulation: A multi-scale investigation. Prog. Org. Coat. 2016, 101, 195–206. [Google Scholar] [CrossRef]
- Horan, T.J. Method for Determining Deleterious Bacterial Growth in Packaged Food Utilizing Hydrophilic Polymers. U.S. Patent 6,149,952, 21 November 2000. [Google Scholar]
- Ledakowicz, S.; Paździor, K. Recent achievements in dyes removal focused on advanced oxidation processes integrated with biological methods. Molecules 2021, 26, 870. [Google Scholar] [CrossRef]
- Grzebieniarz, W.; Tkaczewska, J.; Juszczak, L.; Kawecka, A.; Krzyściak, P.; Nowak, N.; Guzik, P.; Kasprzak, M.; Janik, M.; Jamróz, E. The influence of aqueous butterfly pea (Clitoria ternatea) flower extract on active and intelligent properties of furcellaran Double-Layered films-in vitro and in vivo research. Food Chem. 2023, 413, 135612. [Google Scholar] [CrossRef]
- Du, Y.; Sun, J.; Wang, L.; Wu, C.; Gong, J.; Lin, L.; Pang, J. Development of antimicrobial packaging materials by incorporation of gallic acid into Ca2+ crosslinking konjac glucomannan/gellan gum films. Int. J. Biol. Macromol. 2019, 137, 1076–1085. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Guzik, P.; Duda, I. The verification of intelligent properties of furcellaran films with plant extracts on the stored fresh Atlantic mackerel during storage at 2 °C. Food Hydrocoll. 2019, 97, 105211. [Google Scholar] [CrossRef]
- Boziaris, I.S.; Stamatiou, A.P.; Nychas, G.J.E. Microbiological aspects and shelf life of processed seafood products. J. Sci. Food Agric. 2013, 93, 1184–1190. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Wang, Z.; Cai, S.; Zhu, B.; Dong, X. Recent advances in fishy odour in aquatic fish products, from formation to control. Int. J. Food Sci. Technol. 2021, 56, 4959–4969. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasanah, N.N.; Mohamad Azman, E.; Rozzamri, A.; Zainal Abedin, N.H.; Ismail-Fitry, M.R. A Systematic Review of Butterfly Pea Flower (Clitoria ternatea L.): Extraction and Application as a Food Freshness pH-Indicator for Polymer-Based Intelligent Packaging. Polymers 2023, 15, 2541. https://doi.org/10.3390/polym15112541
Hasanah NN, Mohamad Azman E, Rozzamri A, Zainal Abedin NH, Ismail-Fitry MR. A Systematic Review of Butterfly Pea Flower (Clitoria ternatea L.): Extraction and Application as a Food Freshness pH-Indicator for Polymer-Based Intelligent Packaging. Polymers. 2023; 15(11):2541. https://doi.org/10.3390/polym15112541
Chicago/Turabian StyleHasanah, Nur Nabilah, Ezzat Mohamad Azman, Ashari Rozzamri, Nur Hanani Zainal Abedin, and Mohammad Rashedi Ismail-Fitry. 2023. "A Systematic Review of Butterfly Pea Flower (Clitoria ternatea L.): Extraction and Application as a Food Freshness pH-Indicator for Polymer-Based Intelligent Packaging" Polymers 15, no. 11: 2541. https://doi.org/10.3390/polym15112541








