A Regression Approach to Model Refractive Index Measurements of Novel 3D Printable Photocurable Resins for Micro-Optofluidic Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- (i).
- NOA88—it is a low-viscosity (250 cps) UV-curing adhesive with a refractive index equal to 1.56 at 589 nm, requiring 5 J/cm2 of energy to fully cure a 25–50 micron bond, and having an absorption range ranging between 315 and 395 nm. According to the provided SDS (safety data sheet), it is a proprietary urethane-related resin-based formulation composed of a mixture of mercapto esters (the content of 50–65%) and triallyl isocyanuarte (the content of 30–55%);
- (ii).
- NOA160—it is a high-viscosity (2200 cps) UV-curing adhesive with a refractive index equal to 1.60 at 589 nm, requiring 6 J/cm2 of energy to fully cure a 25–50-micron bond. In accordance with the provided SDS (safety data sheet), it is a proprietary urethane-related resin-based formulation composed of a mixture of pentaerythritol tetra 3 (the content of 30–50%) and acrylic monomer (the content of 30–60%);
- (iii).
- NOA1665—it is a UV-curing adhesive characterized by a viscosity of 800–1000 cps, with a refractive index equal to 1.665 at 589 nm, which requires 6 J/cm2 of energy to fully cure a 25–50-micron bond. In line with the information provided by the SDS (safety data sheet), it is a proprietary urethane-related resin-based formulation composed of a mixture of metal oxide (the content of 30–60%) and acrylated resin (the content of 35–45%);
- (iv).
- NOA170—it is a very high-viscosity (4500–5500 cps) UV-curing adhesive characterized by a high refractive index, i.e., equal to 1.70 at 589 nm, that needs 6 J/cm2 of energy to fully cure a 25–50-micron bond. Based on the supplied SDS (safety data sheet), it is a proprietary acrylated-related resin-based formulation composed of a mixture of acrylated resin (content of 40–60%) and acrylic monomer (content of 10–30%).
Specimens Preparation
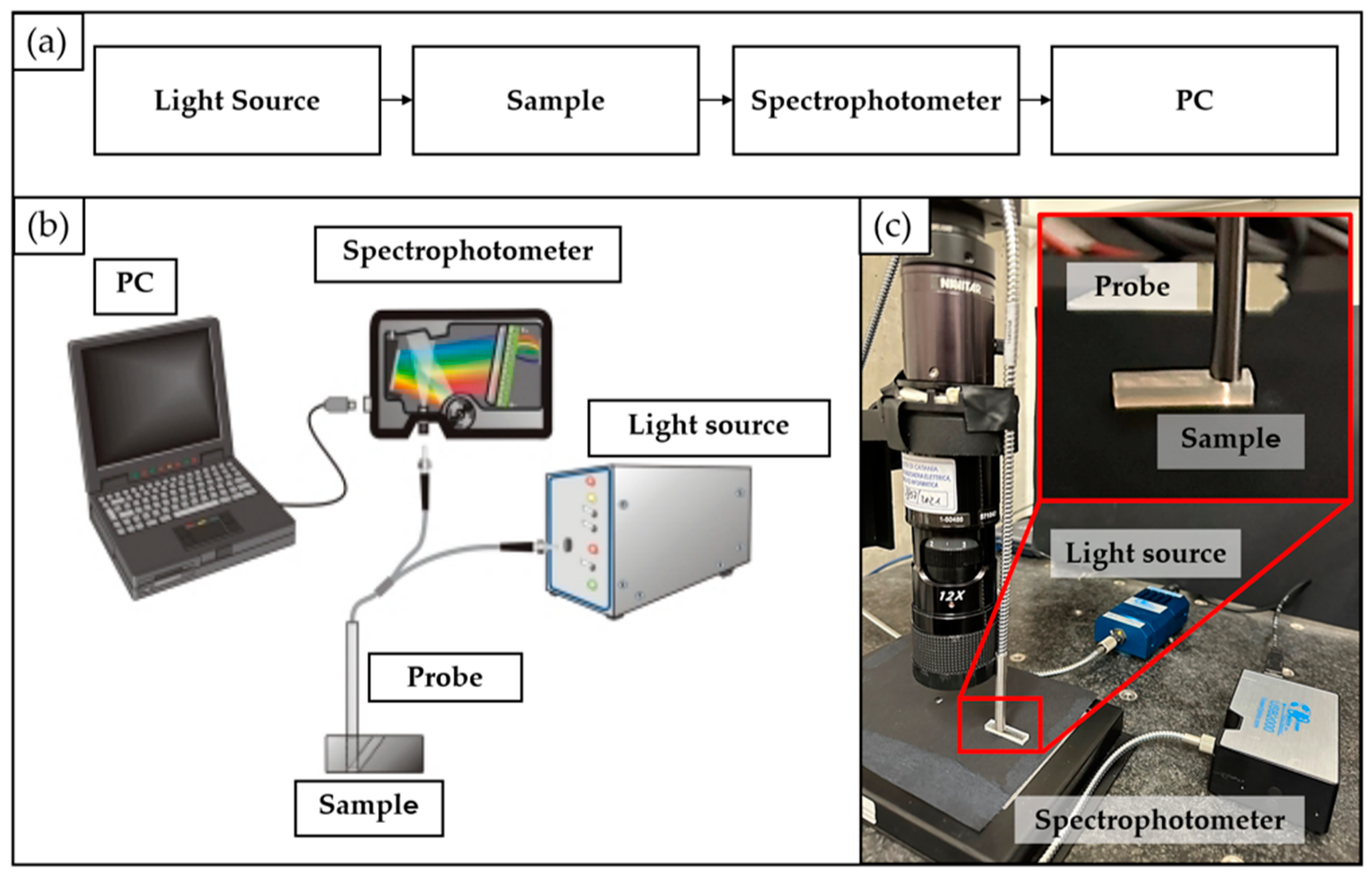
2.2. Transmission Measurements: Experimental Setup
2.3. Regression Model for Refractive Index Estimation
2.4. Application: Micro-Optofluidic Device for Slug-Flow Detection
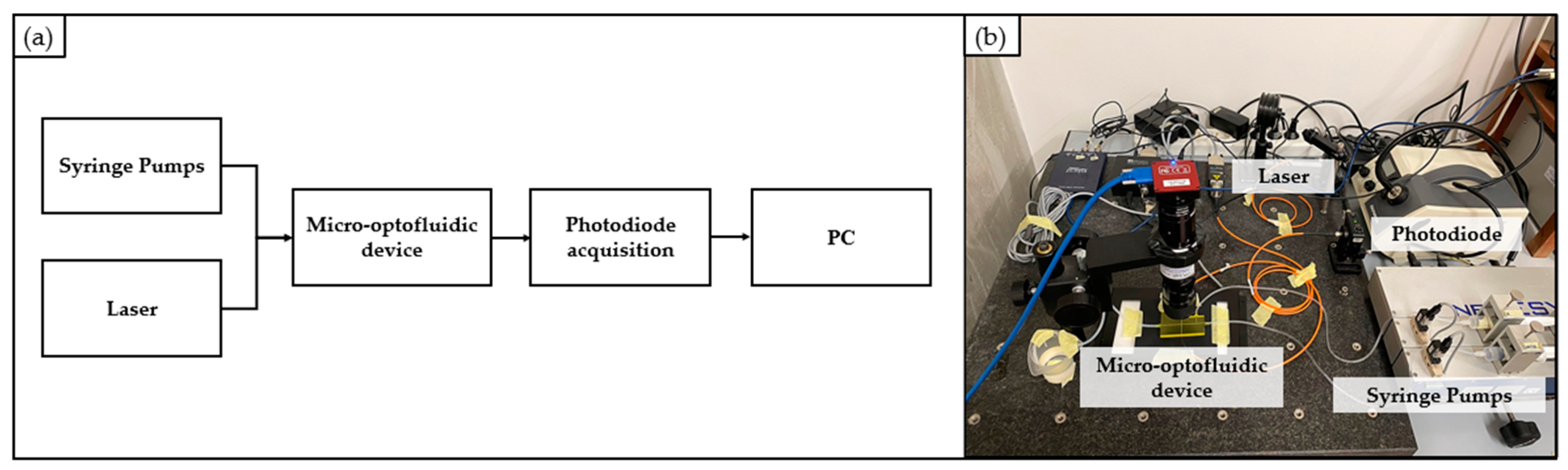
2.4.1. Optical Detection: Experimental Setup and Signals Processing
3. Results and Discussion
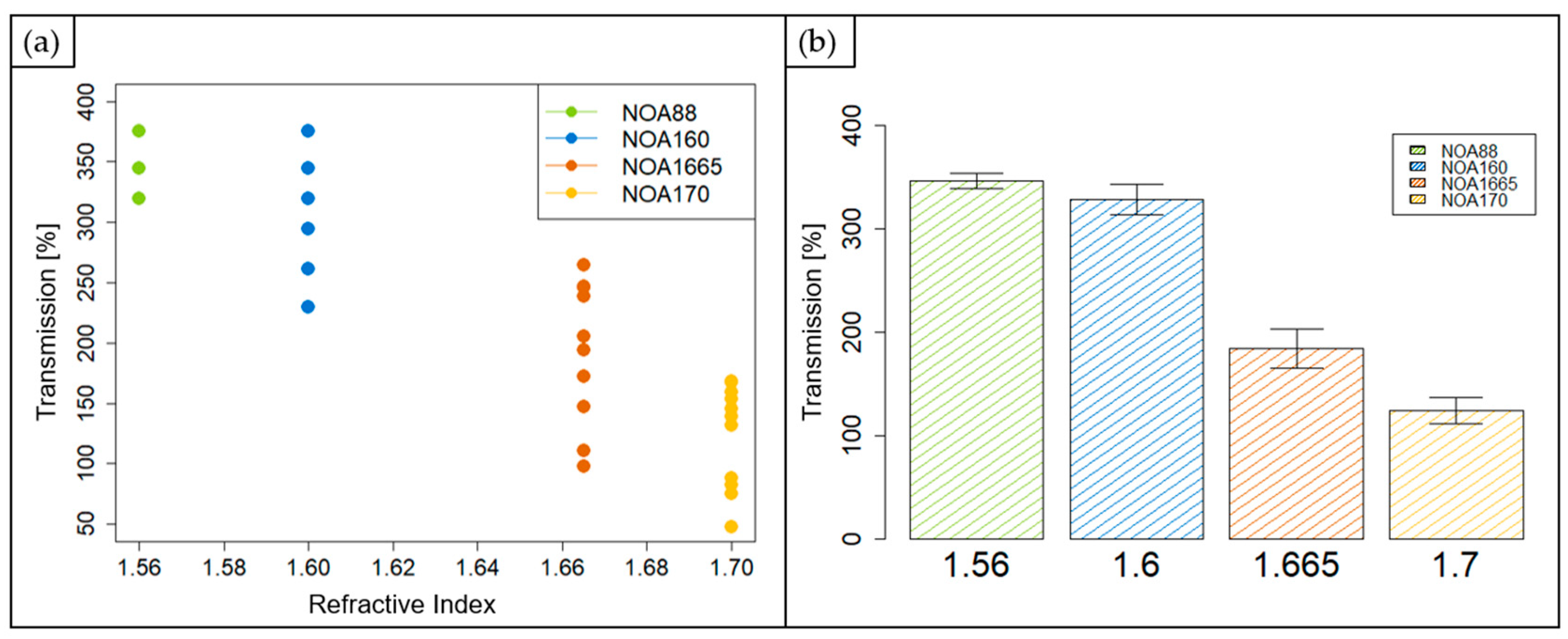
3.1. Transmission Measurements Results
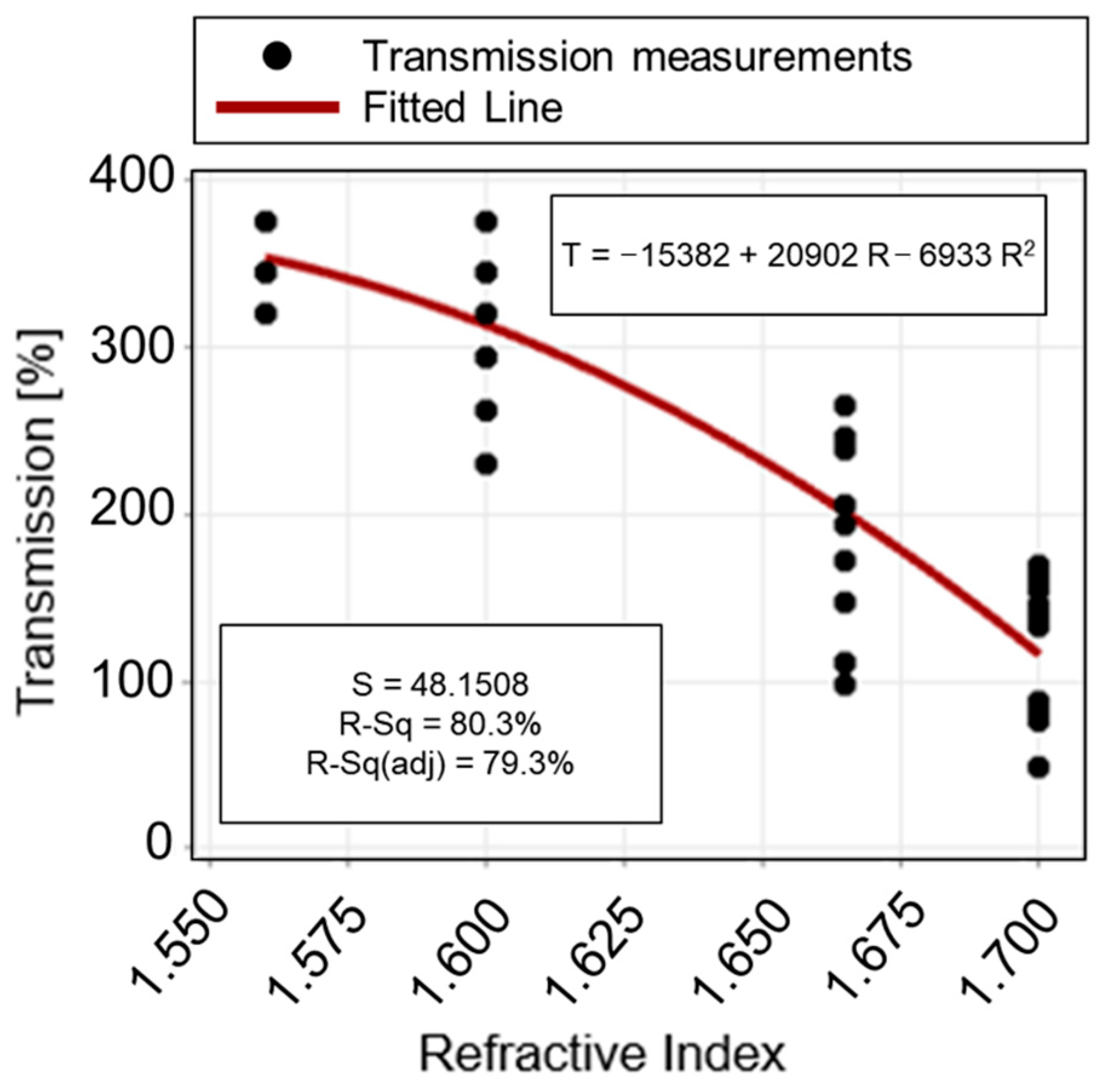
3.2. Quadratic Polynomial Regression Model for Refractive Index Value Determination
3.3. MoF Devices Optical Signals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scott, S.M.; Ali, Z. Fabrication Methods for Microfluidic Devices: An Overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Le, J.; Chen, Y.; Cai, Y.; Hong, Z.; Chai, Y. Recent Advances in Microfluidic Devices for Bacteria and Fungus Research. TrAC Trends Anal. Chem. 2019, 112, 175–195. [Google Scholar] [CrossRef]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.; Schueller, O.J.; Whitesides, G.M. Fabrication of Microfluidic Systems in Poly(Dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Fiorini, G.S.; Chiu, D.T. Disposable Microfluidic Devices: Fabrication, Function, and Application. Biotechniques 2005, 38, 429–446. [Google Scholar] [CrossRef] [Green Version]
- Whitesides, G.M. The Origins and the Future of Microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Convery, N.; Gadegaard, N. 30 Years of Microfluidics. Micro Nano Eng. 2019, 2, 76–91. [Google Scholar] [CrossRef]
- Gale, B.K.; Jafek, A.R.; Lambert, C.J.; Goenner, B.L.; Moghimifam, H.; Nze, U.C.; Kamarapu, S.K. A Review of Current Methods in Microfluidic Device Fabrication and Future Commercialization Prospects. Inventions 2018, 3, 60. [Google Scholar] [CrossRef] [Green Version]
- Minzioni, P.; Osellame, R.; Sada, C.; Zhao, S.; Omenetto, F.G.; Gylfason, K.B.; Haraldsson, T.; Zhang, Y.; Ozcan, A.; Wax, A.; et al. Roadmap for Optofluidics. J. Opt. 2017, 19, 093003. [Google Scholar] [CrossRef] [Green Version]
- Cairone, F.; Davi, S.; Stella, G.; Guarino, F.; Recca, G.; Cicala, G.; Bucolo, M. 3D-Printed Micro-Optofluidic Device for Chemical Fluids and Cells Detection. Biomed. Microdevices 2020, 22, 37. [Google Scholar] [CrossRef]
- Saitta, L.; Celano, G.; Cicala, G.; Fragalà, M.E.; Stella, G.; Barcellona, M.; Tosto, C.; Bucolo, M. Projection Micro-Stereolithography versus Master–Slave Approach to Manufacture a Micro-Optofluidic Device for Slug Flow Detection. Int. J. Adv. Manuf. Technol. 2022, 120, 4443–4460. [Google Scholar] [CrossRef]
- Stella, G.; Barcellona, M.; Saitta, L.; Tosto, C.; Cicala, G.; Gulino, A.; Bucolo, M.; Fragalà, M.E. 3D Printing Manufacturing of Polydimethyl-Siloxane/Zinc Oxide Micro-Optofluidic Device for Two-Phase Flows Control. Polymers 2022, 14, 2113. [Google Scholar] [CrossRef] [PubMed]
- Dawson, H.; Elias, J.; Etienne, P.; Calas-Etienne, S. The Rise of the Om-Loc: Opto-Microfluidic Enabled Lab-on-Chip. Micromachines 2021, 12, 1467. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.J.; Fluri, K.; Seiler, K.; Fan, Z.; Effenhauser, C.S.; Manz, A. Micromachining a Miniaturized Capillary Electrophoresis-Based Chemical Analysis System on a Chip. Science 1993, 261, 895–897. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.C.; Hergenroeder, R.; Koutny, L.B.; Ramsey, J.M. Open Channel Electrochromatography on a Microchip. Anal. Chem. 2002, 66, 2369–2373. [Google Scholar] [CrossRef]
- Jacobson, S.C.; Hergenroeder, R.; Koutny, L.B.; Ramsey, J.M. Fused Quartz Substrates for Microchip Electrophoresis. Anal. Chem. 2002, 67, 2059–2063. [Google Scholar] [CrossRef]
- Matzke, C.M.; Kottenstette, R.J.; Casalnuovo, S.A.; Frye-Mason, G.C.; Hudson, M.L.; Sasaki, D.Y.; Manginell, R.P.; Wong, C.C. Microfabricated Silicon Gas Chromatographic Microchannels: Fabrication and Performance. In Proceedings of the Micromachining and Microfabrication Process Technology IV, Santa Clara, CA, USA, 20–24 September 1998; Volume 3511, pp. 262–268. [Google Scholar] [CrossRef] [Green Version]
- Moore, A.W.; Jacobson, S.C.; Ramsey, J.M. Microchip Separations of Neutral Species via Micellar Electrokinetic Capillary Chromatography. Anal. Chem. 1995, 67, 4184–4189. [Google Scholar] [CrossRef]
- Sochol, R.D.; Sweet, E.; Glick, C.C.; Wu, S.Y.; Yang, C.; Restaino, M.; Lin, L. 3D Printed Microfluidics and Microelectronics. Microelectron. Eng. 2018, 189, 52–68. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Urrios, A.; Kang, S.; Folch, A. The Upcoming 3D-Printing Revolution in Microfluidics. Lab Chip 2016, 16, 1720–1742. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, N.P.; Cabot, J.M.; Smejkal, P.; Guijt, R.M.; Paull, B.; Breadmore, M.C. Comparing Microfluidic Performance of Three-Dimensional (3D) Printing Platforms. Anal. Chem 2017, 89, 3858–3866. [Google Scholar] [CrossRef]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D Printed Microfluidic Devices: Enablers and Barriers. Lab Chip 2016, 16, 1993–2013. [Google Scholar] [CrossRef] [Green Version]
- Au, A.K.; Lee, W.; Folch, A. Mail-Order Microfluidics: Evaluation of Stereolithography for the Production of Microfluidic Devices. Lab Chip 2014, 14, 1294–1301. [Google Scholar] [CrossRef] [Green Version]
- Stella, G.; Saitta, L.; Ongaro, A.E.; Cicala, G.; Kersaudy-Kerhoas, M.; Bucolo, M. Advanced Technologies in the Fabrication of a Micro-Optical Light Splitter. Micro 2023, 3, 338–352. [Google Scholar] [CrossRef]
- Luo, Y.; Canning, J.; Zhang, J.; Peng, G.D. Toward Optical Fibre Fabrication Using 3D Printing Technology. Opt. Fiber Technol. 2020, 58, 102299. [Google Scholar] [CrossRef]
- Whitesides, G.M. Controlling Flows in Microchannels with Patterned Surface Charge and Topography. Acc. Chem. Res. 2003, 36, 597–604. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft Lithography. Angew. Chem. Int. Ed. 1998, 37, 550–575. [Google Scholar] [CrossRef]
- Fleck, E.; Sunshine, A.; Denatale, E.; Keck, C.; McCann, A.; Potkay, J. Advancing 3d-Printed Microfluidics: Characterization of a Gas-Permeable, High-Resolution Pdms Resin for Stereolithography. Micromachines 2021, 12, 1266. [Google Scholar] [CrossRef]
- Zhu, Y.; Ramadani, E.; Egap, E. Thiol Ligand Capped Quantum Dot as an Efficient and Oxygen Tolerance Photoinitiator for Aqueous Phase Radical Polymerization and 3D Printing under Visible Light. Polym. Chem. 2021, 12, 5106–5116. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, J.; Corrigan, N.; Boyer, C. Controlling Mechanical Properties of 3D Printed Polymer Composites through Photoinduced Reversible Addition-Fragmentation Chain Transfer (RAFT) Polymerization. Polym. Chem. 2022, 13, 44–57. [Google Scholar] [CrossRef]
- Chao, T.-C.; Ros, A. Microfluidic Single-Cell Analysis of Intracellular Compounds. J. R. Soc. Interface 2008, 5 (Suppl. S2), S139–S150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, E.; Sott, K.; Lundqvist, F.; Sveningsson, M.; Scrimgeour, J.; Hanstorp, D.; Goksör, M.; Granéli, A. A Microfluidic Device for Reversible Environmental Changes around Single Cells Using Optical Tweezers for Cell Selection and Positioning. Lab Chip 2010, 10, 617–625. [Google Scholar] [CrossRef]
- Zheng, B.; Roach, L.S.; Ismagilov, R.F. Screening of Protein Crystallization Conditions on a Microfluidic Chip Using Nanoliter-Size Droplets. J. Am. Chem. Soc. 2003, 125, 11170–11171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, D.T.; Jeon, N.L.; Huang, S.; Kane, R.S.; Wargo, C.J.; Choi, I.S.; Ingber, D.E.; Whitesides, G.M. Patterned Deposition of Cells and Proteins onto Surfaces by Using Three-Dimensional Microfluidic Systems. Proc. Natl. Acad. Sci. USA 2000, 97, 2408–2413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, D.L.; Manginell, R.P.; Samara, M.A.; Kim, B.-I.; Bunker, B.C. Programmed Adsorption and Release of Proteins in a Microfluidic Device. Science 2003, 301, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Paunović, N.; Bao, Y.; Brian Coulter, F.; Masania, K.; Karoline Geks, A.; Klein, K.; Rafsanjani, A.; Cadalbert, J.; Kronen, P.W.; Kleger, N.; et al. Digital Light 3D Printing of Customized Bioresorbable Airway Stents with Elastomeric Properties. Sci. Adv. 2021, 7, eabe9499. [Google Scholar] [CrossRef] [PubMed]
- Maity, N.; Mansour, N.; Chakraborty, P.; Bychenko, D.; Gazit, E.; Cohn, D. A Personalized Multifunctional 3D Printed Shape Memory-Displaying, Drug Releasing Tracheal Stent. Adv. Funct. Mater. 2021, 31, 2108436. [Google Scholar] [CrossRef]
- Borandeh, S.; van Bochove, B.; Teotia, A.; Seppälä, J. Polymeric Drug Delivery Systems by Additive Manufacturing. Adv. Drug Deliv. Rev. 2021, 173, 349–373. [Google Scholar] [CrossRef]
- Robles-Martinez, P.; Xu, X.; Trenfield, S.J.; Awad, A.; Goyanes, A.; Telford, R.; Basit, A.W.; Gaisford, S. 3D Printing of a Multi-Layered Polypill Containing Six Drugs Using a Novel Stereolithographic Method. Pharmaceutics 2019, 11, 274. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, N.; Parra-Cabrera, C.; Kim, Y.T.; Kuo, A.P.; Folch, A. Desktop-Stereolithography 3D-Printing of a Poly(Dimethylsiloxane)-Based Material with Sylgard-184 Properties. Adv. Mater. 2018, 30, e1800001. [Google Scholar] [CrossRef]
- Au, A.K.; Huynh, W.; Horowitz, L.F.; Folch, A. Mikrofluidik Aus Dem 3D-Drucker. Angew. Chem. 2016, 128, 3926–3946. [Google Scholar] [CrossRef]
- Bao, Y. Recent Trends in Advanced Photoinitiators for Vat Photopolymerization 3D Printing. Macromol. Rapid Commun. 2022, 43, 2200202. [Google Scholar] [CrossRef]
- Yu, C.; Schimelman, J.; Wang, P.; Miller, K.L.; Ma, X.; You, S.; Guan, J.; Sun, B.; Zhu, W.; Chen, S. Photopolymerizable Biomaterials and Light-Based 3D Printing Strategies for Biomedical Applications. Chem. Rev. 2020, 120, 10695–10743. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, R.; Fabbri, P.; Leoni, E.; Mazzanti, F.; Akbari, R.; Antonini, C. Additive Manufacturing by Digital Light Processing: A Review. Prog. Addit. Manuf. 2022, 8, 331–351. [Google Scholar] [CrossRef]
- Zhang, Z.-C.; Li, P.-L.; Chu, F.-T.; Shen, G. Influence of the Three-Dimensional Printing Technique and Printing Layer Thickness on Model Accuracy. J. Orofac. Orthop. 2019, 80, 194–204. [Google Scholar] [CrossRef]
- Martín-Montal, J.; Pernas-Sánchez, J.; Varas, D. Experimental Characterization Framework for SLA Additive Manufacturing Materials. Polymers 2021, 13, 1147. [Google Scholar] [CrossRef]
- Ge, Q.; Li, Z.; Wang, Z.; Kowsari, K.; Zhang, W.; He, X.; Zhou, J.; Fang, N.X. Projection Micro Stereolithography Based 3D Printing and Its Applications. Int. J. Extrem. Manuf. 2020, 2, 022004. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Ye, F.; Ma, X.; Zhao, W.; Wang, H. Dependence of Macro-Bending Loss on Bending Configuration of Multimode Optical Fibers Studied by Ray-Tracing Simulation. Opt. Rev. 2020, 27, 290–295. [Google Scholar] [CrossRef]
- SCAD College of Engineering and Technology; Institute of Electrical and Electronics Engineers. Comprehensive and Analytical Review on Optical Fiber Refractive Index Sensor; IEEE Xplore: Piscataway, NJ, USA, 2020; ISBN 9781728155180. [Google Scholar]
- Cennamo, N.; Saitta, L.; Tosto, C.; Arcadio, F.; Zeni, L.; Fragalá, M.E.; Cicala, G. Microstructured Surface Plasmon Resonance Sensor Based on Inkjet 3d Printing Using Photocurable Resins with Tailored Refractive Index. Polymers 2021, 13, 2518. [Google Scholar] [CrossRef]
- Saitta, L.; Arcadio, F.; Celano, G.; Cennamo, N.; Zeni, L.; Tosto, C.; Cicala, G. Design and Manufacturing of a Surface Plasmon Resonance Sensor Based on Inkjet 3D Printing for Simultaneous Measurements of Refractive Index and Temperature. Int. J. Adv. Manuf. Technol. 2023, 124, 2261–2278. [Google Scholar] [CrossRef]
- Khan, R.; Gul, B.; Khan, S.; Nisar, H.; Ahmad, I. Refractive Index of Biological Tissues: Review, Measurement Techniques, and Applications. Photodiagn. Photodyn. Ther. 2021, 33, 102192. [Google Scholar] [CrossRef]
- Sequeira, F.; Cennamo, N.; Rudnitskaya, A.; Nogueira, R.; Zeni, L.; Bilro, L. D-Shaped POF Sensors for Refractive Index Sensing—The Importance of Surface Roughness. Sensors 2019, 19, 2476. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Jeong, T.Y.; Jung, S.; Yee, K.J. Refractive Index Dispersion of Hexagonal Boron Nitride in the Visible and Near-Infrared. Phys. Status Solidi B Basic Res. 2019, 256, 1800417. [Google Scholar] [CrossRef]
- Liu, M.; Plum, E.; Li, H.; Li, S.; Xu, Q.; Zhang, X.; Zhang, C.; Zou, C.; Jin, B.; Han, J.; et al. Temperature-Controlled Optical Activity and Negative Refractive Index. Adv. Funct. Mater. 2021, 31, 2010249. [Google Scholar] [CrossRef]
- Alsaad, A.M.; Al-Bataineh, Q.M.; Ahmad, A.A.; Albataineh, Z.; Telfah, A. Optical Band Gap and Refractive Index Dispersion Parameters of Boron-Doped ZnO Thin Films: A Novel Derived Mathematical Model from the Experimental Transmission Spectra. Optik 2020, 211, 164641. [Google Scholar] [CrossRef]
- Niskanen, I.; Lauri, J.; Yokota, M.; Heikkila, R.; Hashimoto, T.; Fabritius, T. Determination of the Refractive Index of Particles through the Immersion Solid Matching Method. IEEE Trans. Instrum. Meas. 2021, 70, 1–5. [Google Scholar] [CrossRef]
- Tompkins, H.G.; Jovanovich, H.B.; San, B.; New, D.; London, Y.; Toronto, S.T. A User’s Guide to Ellipsometry; Courier Corporation: North Chelmsford, MA, USA, 2006. [Google Scholar]
- Sharda, T.; Soga, T.; Jimbo, T. Optical Properties of Nanocrystalline Diamond Films by Prism Coupling Technique. J. Appl. Phys. 2003, 93, 101–105. [Google Scholar] [CrossRef]
- Kersten, R. A New Method for Measuring Refractive Index and Thickness of Liquid and Deposited Solid Thin Films. Opt. Commun. 1975, 13, 327–329. [Google Scholar] [CrossRef]
- Sultanova, N.G.; Kasarova, S.N.; Nikolov, I.D. Characterization of Optical Properties of Optical Polymers. Opt. Quantum Electron. 2013, 45, 221–232. [Google Scholar] [CrossRef]
- Nussbaumer, R.J.; Halter, M.; Tervoort, T.; Caseri, W.R.; Smith, P. A Simple Method for the Determination of Refractive Indices of (Rough) Transparent Solids. J. Mater. Sci. 2005, 40, 575–582. [Google Scholar] [CrossRef]
- Ojena, S.M.; De Forest, P.R. Precise Refractive Index Determination by the Immersion Method, Using Phase Contrast Microscopy and the Mettler Hot Stage. J. Forensic Sci. Soc. 1972, 12, 315–329. [Google Scholar] [CrossRef]
- Stoiber, R.E.; Morse, A.S. The Immersion Method. In Crystal Identification with the Polarizing Microscope; Springer: Boston, MA, USA, 1994; pp. 49–75. [Google Scholar]
- Schembri, F.; Bucolo, M. Periodic Input Flows Tuning Nonlinear Two-Phase Dynamics in a Snake Microchannel. Microfluid. Nanofluidics 2011, 11, 189–197. [Google Scholar] [CrossRef]
- Hassani, A.; Skorobogatiy, M. Design Criteria for Microstructured-Optical-Fiber-Based Surface-Plasmon-Resonance Sensors. J. Opt. Soc. Am. B 2007, 24, 1423–1429. [Google Scholar] [CrossRef]
- Debnath, N.; Live, L.S.; Poudineh, M. A Microfluidic Plasma Separation Device Combined with a Surface Plasmon Resonance Biosensor for Biomarker Detection in Whole Blood. Lab Chip 2023, 23, 572–579. [Google Scholar] [CrossRef] [PubMed]





| NOA Commercial Name | Refractive Index Value | UV-Curing Energy |
|---|---|---|
| NOA88 | 1.56 @ 589 nm | 5 J/cm2 |
| NOA160 | 1.60 @ 589 nm | 6 J/cm2 |
| NOA1665 | 1.665 @ 589 nm | 6 J/cm2 |
| NOA170 | 1.70 @ 589 nm | 6 J/cm2 |
| MoF Device | Material | Ra [nm] | RMS [nm] | Peak to Peak [nm] |
|---|---|---|---|---|
| Device 1 | PDMS | 1.097 | 0.763 | 35.158 |
| Device 2 | HTL resin | 47.056 | 37.324 | 259.121 |
| Factor | Symbol | Type | Unit | Levels (a = 4) | |||
|---|---|---|---|---|---|---|---|
| Material | A | Categorical | [-] | NOA88 | NOA160 | NOA1665 | NOA170 |
| Source | DF | SS | MS | F | p | |
|---|---|---|---|---|---|---|
| Regression | Linear | 1 | 379,065 | - | 154.76 | 0.000 |
| Quadratic | 1 | 7815 | - | 3.37 | 0.074 | |
| Error | 41 | 95,059 | 2319 | |||
| Total | 43 | 481,939 | ||||
| S | 48.1508 | |||||
| R2 | 80.28% | |||||
| R2-adj | 79.31% |
| Resin | Replication (n) | T [%] |
|---|---|---|
| BIO | 1 | 151.564 |
| BIO | 2 | 150.701 |
| BIO | 3 | 151.564 |
| HTL | 1 | 104.113 |
| HTL | 2 | 102.558 |
| HTL | 3 | 103.515 |
| Resin | Replication (n) | ||
|---|---|---|---|
| BIO | 1 | 1.3108 | 1.7040 |
| BIO | 2 | 1.3103 | 1.7046 |
| BIO | 3 | 1.3106 | 1.7043 |
| HTL | 1 | 1.3291 | 1.6858 |
| HTL | 2 | 1.3287 | 1.6861 |
| HTL | 3 | 1.3291 | 1.6858 |
| PDMS | BIO | HTL | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 [mW] | 3 [mW] | 5 [mW] | 1 [mW] | 3 [mW] | 5 [mW] | 1 [mW] | 3 [mW] | 5 [mW] | |
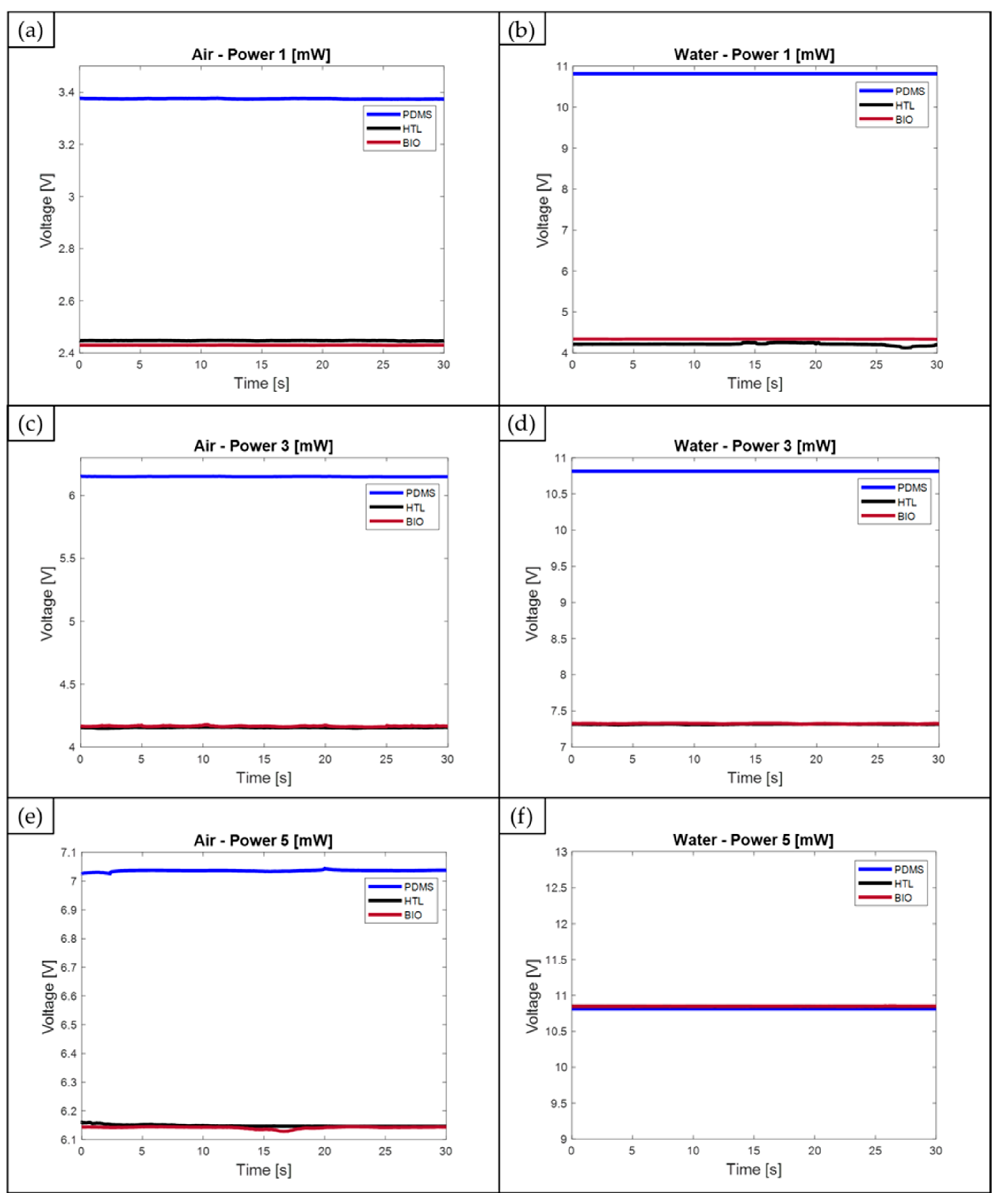
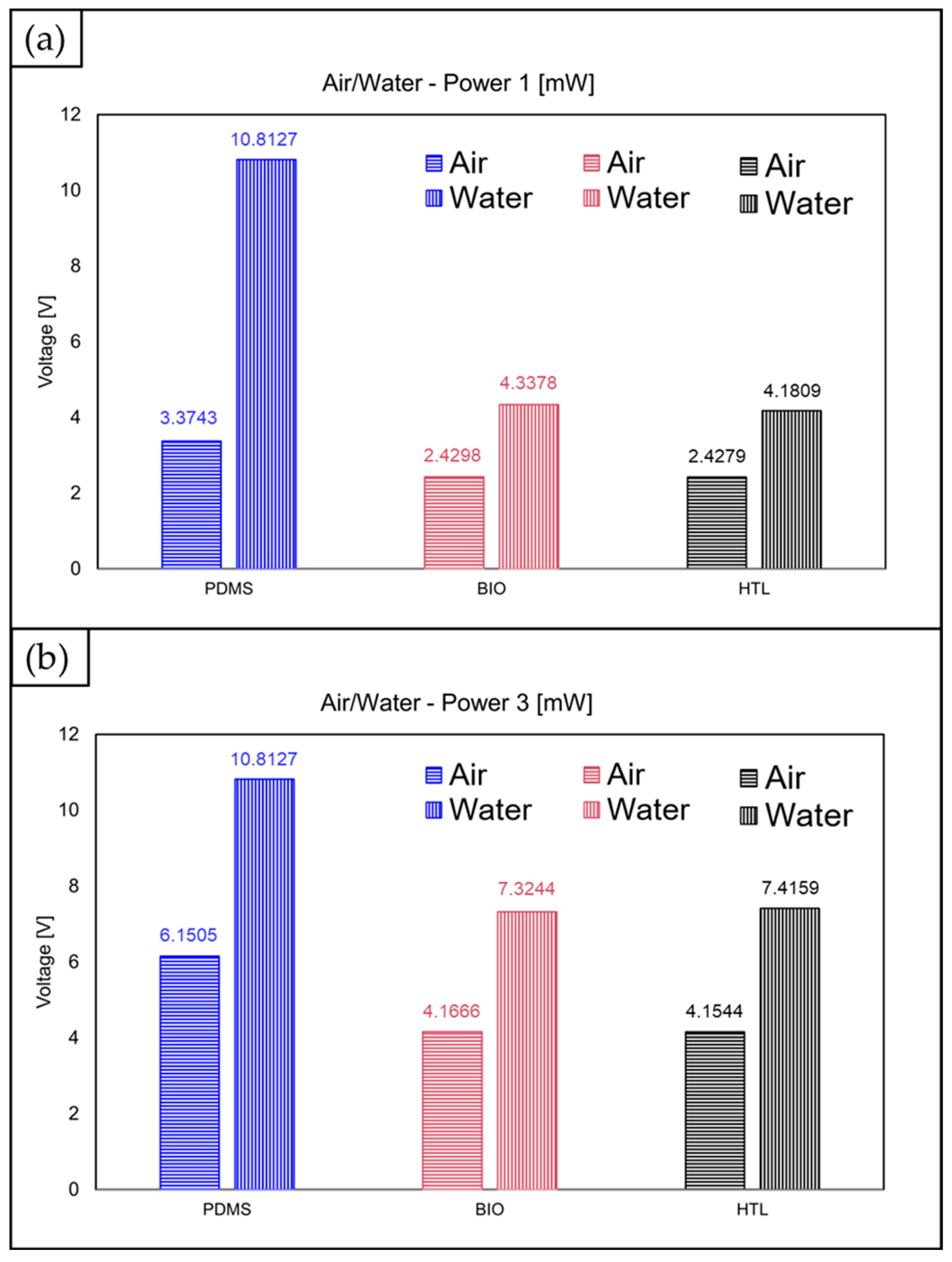
| Air | 3.3743 ± 1.30 × 10−3 | 6.1505 ± 1.10 × 10−3 | 7.0920 ± 7.91 × 10−2 | 2.4298 ± 1.90 × 10−4 | 4.1666 ± 3.30 × 10−3 | 6.0996 ± 7.02 × 10−2 | 2.4279 ± 5.30 × 10−2 | 4.1544 ± 2.10 × 10−3 | 6.1471 ± 2.5 × 10−3 |
| Water | 10.8127 ± 2.15 × 10−6 | 10.8127 ± 2.15 × 10−6 | 10.8127 ± 2.15 × 10−6 | 4.3378 ± 2.5 × 10−3 | 7.3244 ± 2.8 × 10−3 | 10.8494 ± 8.26 × 10−4 | 4.1809 ± 4.44 × 10−2 | 7.4159 ± 2.4 × 10−3 | 10.8487 ± 6.28 × 10−4 |
| Range | 7.4 | 3.7 | 3.8 | 1.9 | 3.2 | 4.7 | 1.8 | 3.3 | 4.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saitta, L.; Cutuli, E.; Celano, G.; Tosto, C.; Stella, G.; Cicala, G.; Bucolo, M. A Regression Approach to Model Refractive Index Measurements of Novel 3D Printable Photocurable Resins for Micro-Optofluidic Applications. Polymers 2023, 15, 2690. https://doi.org/10.3390/polym15122690
Saitta L, Cutuli E, Celano G, Tosto C, Stella G, Cicala G, Bucolo M. A Regression Approach to Model Refractive Index Measurements of Novel 3D Printable Photocurable Resins for Micro-Optofluidic Applications. Polymers. 2023; 15(12):2690. https://doi.org/10.3390/polym15122690
Chicago/Turabian StyleSaitta, Lorena, Emanuela Cutuli, Giovanni Celano, Claudio Tosto, Giovanna Stella, Gianluca Cicala, and Maide Bucolo. 2023. "A Regression Approach to Model Refractive Index Measurements of Novel 3D Printable Photocurable Resins for Micro-Optofluidic Applications" Polymers 15, no. 12: 2690. https://doi.org/10.3390/polym15122690









