Influence of Molecular Weight on Thermal and Mechanical Properties of Carbon-Fiber-Reinforced Plastics Based on Thermoplastic Partially Crystalline Polyimide
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Polymers
2.3. Preparation of Carbon-Fiber-Reinforced Plastics Based on Polyimide R-BAPB with Different Molecular Weights
2.4. Measurements
3. Results and Discussion
3.1. Polymer Synthesis
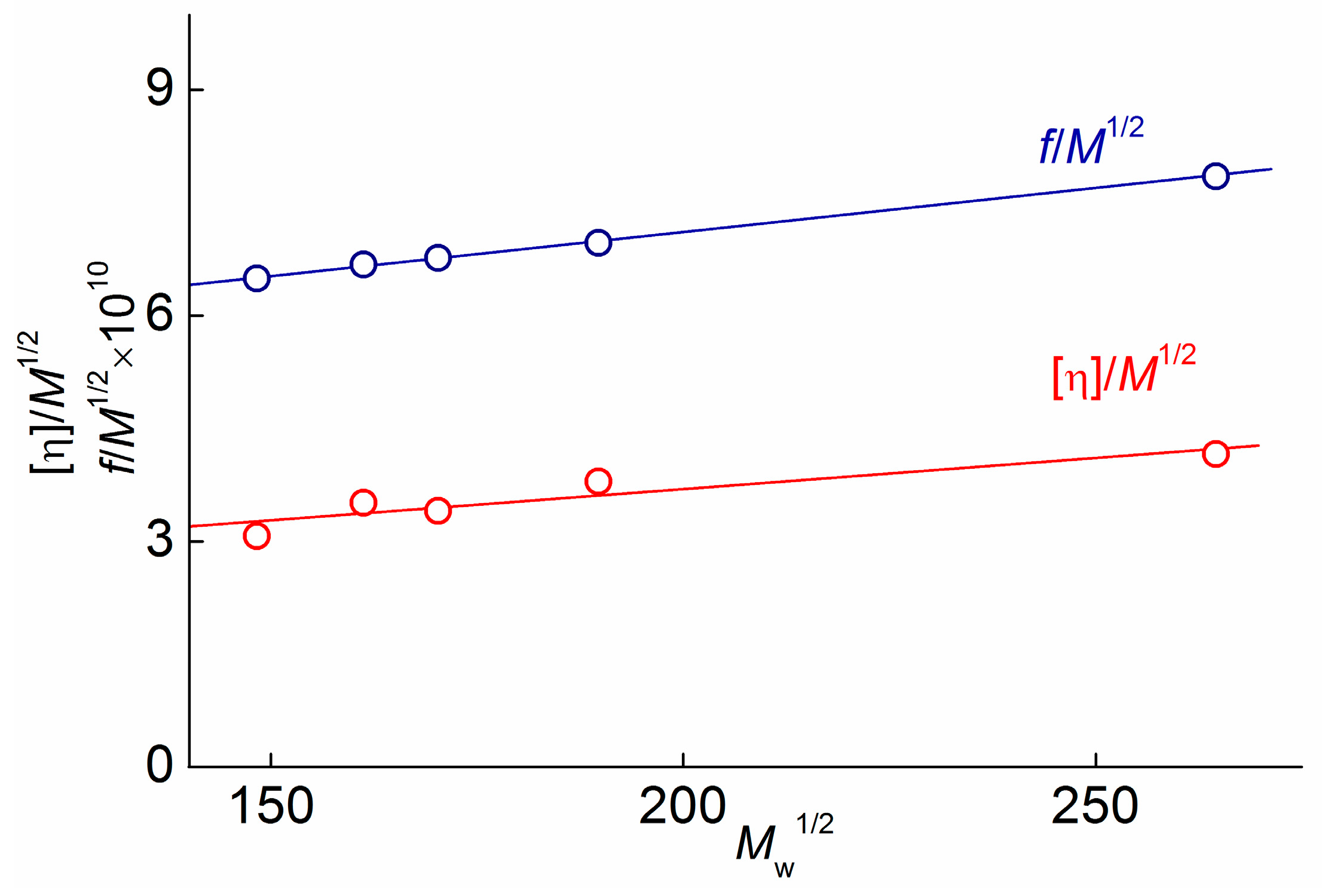
3.2. Structure Conformational and Molecular Hydrodynamic Characteristics
3.3. Equilibrium Rigidity of PAAs
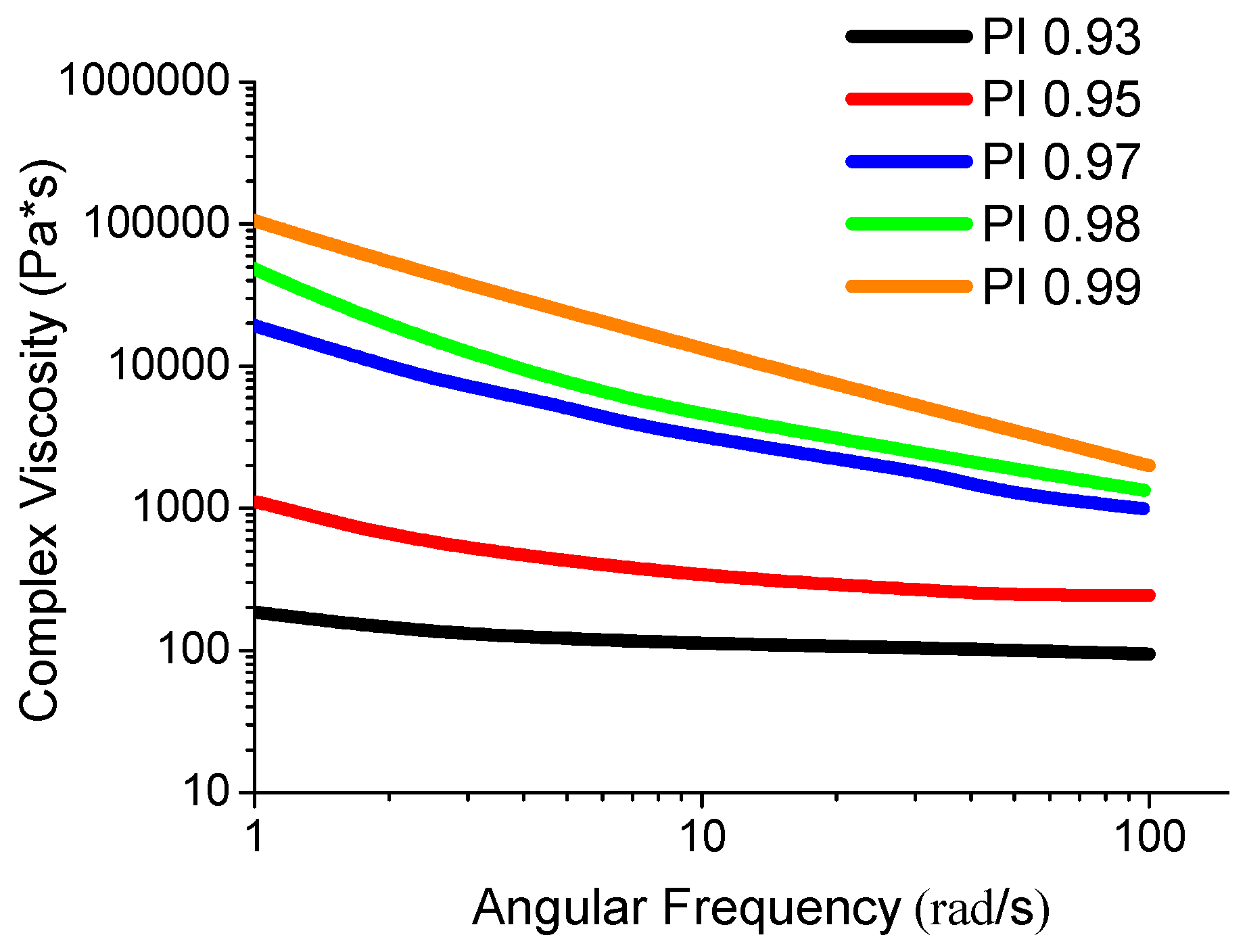
3.4. Investigation of the Viscosity of the Polyimide Melt
3.5. Investigation of Thermal Properties of CFRP
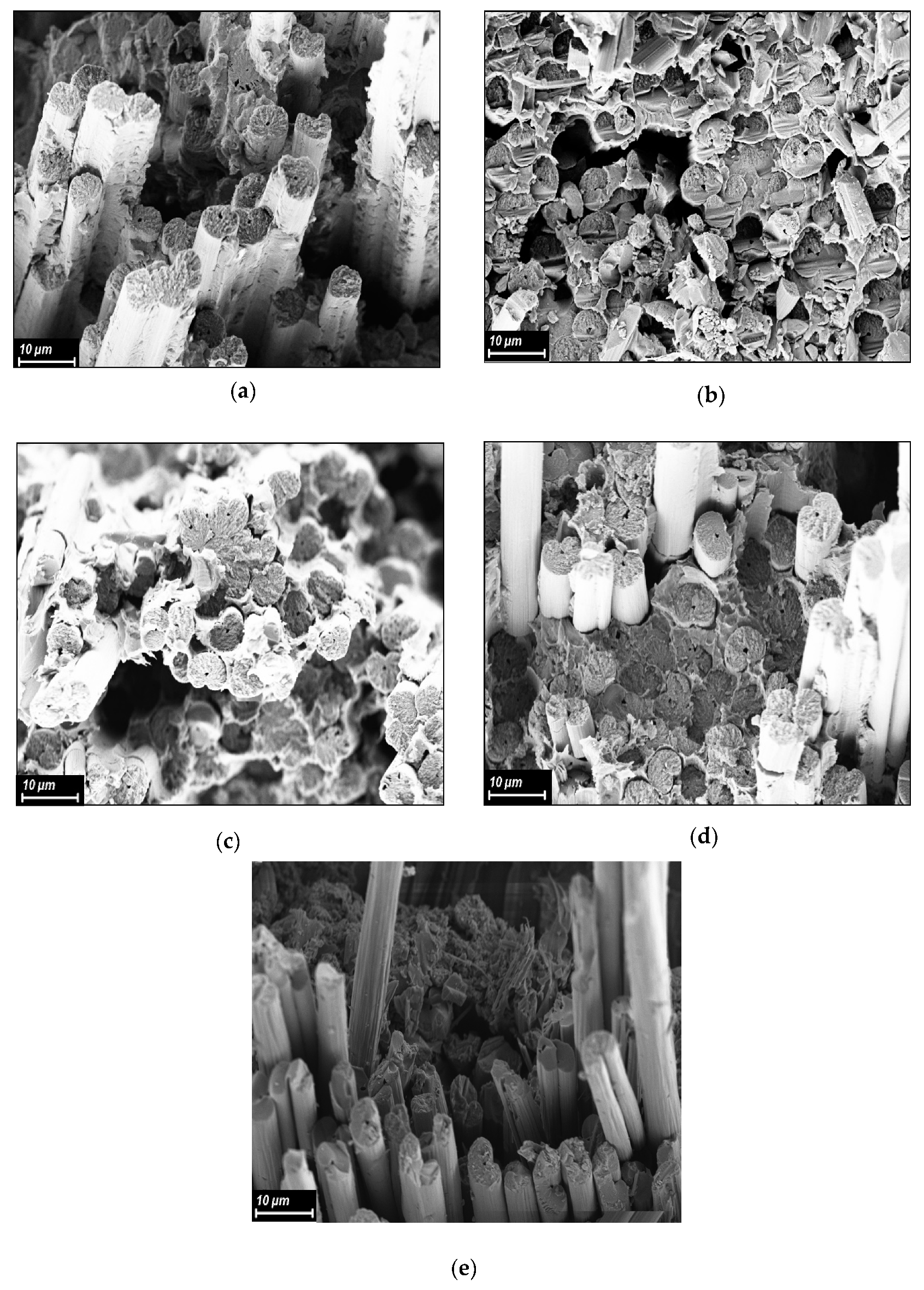
3.6. Investigation of Structure of CFRP
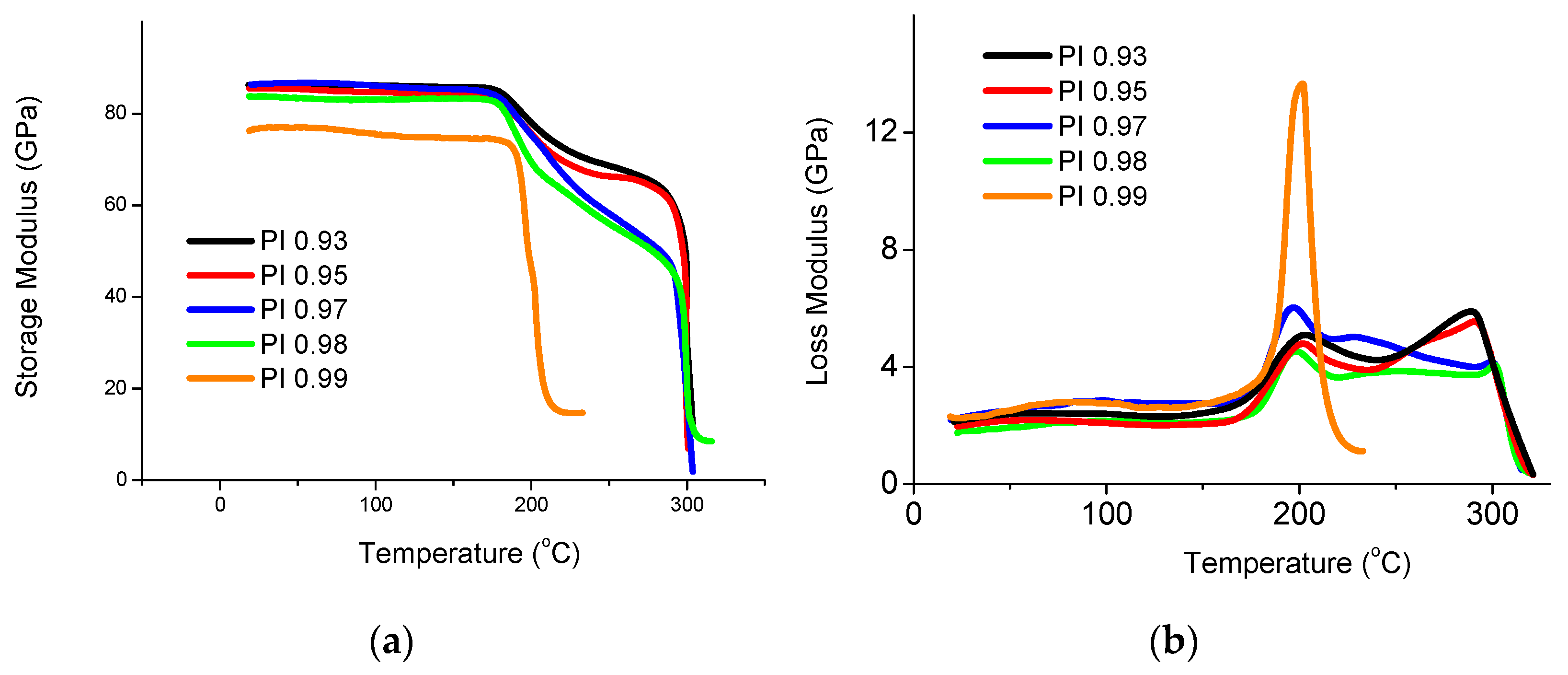
3.7. Investigation of Thermomechanical Properties of CFRP
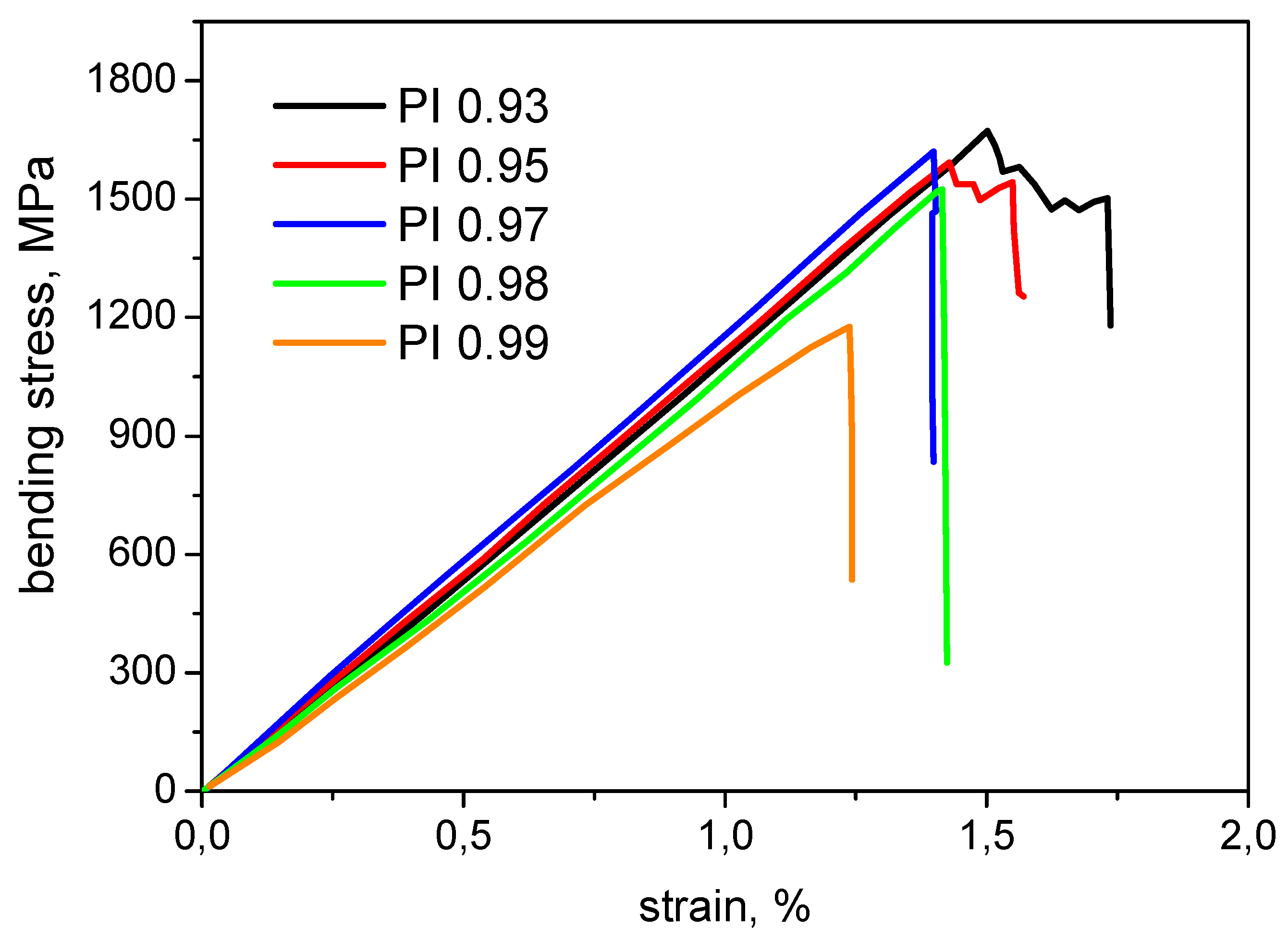
3.8. Investigation of Mechanical Properties of CFRP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, F.-L.; Lee, S.-Y.; Park, S.-J.; Info, A. Polymer matrices for carbon fiber-reinforced polymer composites. Carbon Lett. 2013, 14, 76–88. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.S.; Jin, F.L.; Rhee, K.Y.; Hui, D.; Park, S.J. Recent advances in carbon-fiber-reinforced thermoplastic composites: A review. Compos. Part B Eng. 2018, 142, 241–250. [Google Scholar] [CrossRef]
- Alshammari, B.A.; Alsuhybani, M.S.; Almushaikeh, A.M.; Alotaibi, B.M.; Alenad, A.M.; Alqahtani, N.B.; Alharbi, A.G. Comprehensive Review of the Properties and Modifications of Carbon Fiber-Reinforced Thermoplastic Composites. Polymers 2021, 13, 2474. [Google Scholar] [CrossRef] [PubMed]
- Vieille, B.; Taleb, L. About the influence of temperature and matrix ductility on the behavior of carbon woven-ply PPS or epoxy laminates: Notched and unnotched laminates. Compos. Sci. Technol. 2011, 71, 998–1007. [Google Scholar] [CrossRef] [Green Version]
- Gabrion, X.; Placet, V.; Trivaudey, F.; Boubakar, L. About the thermomechanical behaviour of a carbon fibre reinforced high-temperature thermoplastic composite. Compos. Part B Eng. 2016, 95, 386–394. [Google Scholar] [CrossRef] [Green Version]
- Chukov, D.; Nematulloev, S.; Zadorozhnyy, M.; Tcherdyntsev, V.; Stepashkin, A.; Zherebtsov, D. Structure, Mechanical and Thermal Properties of Polyphenylene Sulfide and Polysulfone Impregnated Carbon Fiber Composites. Polymers 2019, 11, 684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.L.; Kim, J.K. Cooling rate influences in carbon fibre/PEEK composites. Part II: Interlaminar fracture toughness. Compos. -Part A Appl. Sci. Manuf. 2001, 32, 763–774. [Google Scholar] [CrossRef]
- Bashford, D. Polyphenylene Oxides (PPO). In Thermoplastics; Springer: Dordrecht, The Netherlands, 1997; pp. 433–440. [Google Scholar]
- Lu, Y.H.; Zhan, M.S.; Zheng, W.H. Preparation and properties of T300 carbon fiber-reinforced thermoplastic polyimide composites. J. Appl. Polym. Sci. 2006, 102, 646–654. [Google Scholar] [CrossRef]
- Zhang, R.; Fallon, J.J.; Joseph, R.M.; Thomas, J.A.; Hassan, M.S.; Choudhury, S.R.; Gilmer, E.L.; Kubota, M.; Deitzel, J.M.; Riffle, J.S.; et al. Preparation of Submicrometer High-Performance Poly(ether imide) Particles for Fabricating Carbon Fiber Reinforced Polymer Composites. Ind. Eng. Chem. Res. 2018, 57, 15346–15356. [Google Scholar] [CrossRef]
- Ivanov, D.A. Semicrystalline Polymers. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Mulhouse, France, 2012; Volume 1, pp. 227–258. ISBN 9780080878621. [Google Scholar]
- Bessonov, M.I.; Koton, M.M.; Kudryavtsev, V.V.; Laius, L.A. Polyimides–Thermally Stable Polymers, 1st ed.; Springer: New York, NY, USA, 1987; ISBN 9781461576365. [Google Scholar]
- Andrews, E.H. Morphology and Mechanical Properties in Semicrystalline Polymers. Pure Appl. Chem. 1974, 39, 179–194. [Google Scholar] [CrossRef] [Green Version]
- Vaganov, G.; Didenko, A.; Ivan’Kova, E.; Popova, E.; Yudin, V.; Elokhovskii, V.; Lasota, I. Development of new polyimide powder for selective laser sintering. J. Mater. Res. 2019, 34, 2895–2902. [Google Scholar] [CrossRef]
- Vaganov, G.; Ivan’kova, E.; Didenko, A.; Popova, E.; Elokhovskiy, V.; Kasatkin, I.; Yudin, V. High-performance crystallized composite carbon nanoparticles/polyimide fibers. J. Appl. Polym. Sci. 2022, 139, e52748. [Google Scholar] [CrossRef]
- Yudin, V.; Svetlichnyi, V.; Gubanova, N.; Didenko, A.; Popova, E.; Sukhanova, T.; Grigoriev, A.; Kostereva, T.; Arbel, I.; Marom, G. Influence of crystallinity of R-BAPB-type polyimide matrix on thermal and mechanical properties of carbon-fiber-reinforced composites. In Polyimides and Other High Temperature Polymers; CRC Press: Boca Raton, FL, USA, 2005; pp. 299–316. [Google Scholar]
- Ma, Y.; Bi, Y.; Zhang, X.; Wang, D.; Dang, G.; Zhou, H.; Chen, C. Effects of molecular weight on the dynamic mechanical properties and interfacial properties of carbon fiber fabric-reinforced polyetherketone cardo composites. High Perform. Polym. 2016, 28, 1210–1217. [Google Scholar] [CrossRef]
- Sharma, M.; Bijwe, J. Influence of molecular weight on performance properties of polyethersulphone and its composites with carbon fabric. Wear 2012, 274–275, 388–394. [Google Scholar] [CrossRef]
- Tanaka, K.; Koriyama, H.; Isshiki, S.; Katayama, T.; Shinohara, M. Effect Of The Molecular Weight of Polycarbonate on the Impact Resistance of Continuous CarbonFiber Reinforced Polycarbonate Composites. WIT Trans. Built Environ. 2014, 137, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Friedrich, K.; Cutolo, D.; Savadori, A. Manufacture of CF/PEEK composites from powder/sheath fibre preforms. Compos. Manuf. 1994, 5, 41–50. [Google Scholar] [CrossRef]
- Goud, V.; Alagirusamy, R.; Das, A.; Kalyanasundaram, D. Dry Electrostatic Spray Coated Towpregs for Thermoplastic Composites. Fibers Polym. 2018, 19, 364–374. [Google Scholar] [CrossRef]
- Vaidya, U.K.; Chawla, K.K. Processing of fibre reinforced thermoplastic composites. Int. Mater. Rev. 2008, 53, 185–218. [Google Scholar] [CrossRef]
- Vaganov, G.; Yudin, V.; Vuorinen, J.; Molchanov, E. Influence of multiwalled carbon nanotubes on the processing behavior of epoxy powder compositions and on the mechanical properties of their fiber reinforced composites. Polym. Compos. 2016, 37, 2377–2383. [Google Scholar] [CrossRef]
- Chu, B. Light Scattering Studies of Polymer Solutions and Melts. Polym. J. 1985, 17, 225–238. [Google Scholar] [CrossRef] [Green Version]
- Schärtl, W. Light Scattering from Polymer Solutions and Nanoparticle Dispersions, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Kratochvíl, P. Classical Light Scattering from Polymer Solutions; Elsevier: Amsterdam, The Netherlands, 1987; ISBN 0444428909. [Google Scholar]
- Tsvetkov, V.N. Rigid-Chain Polymers: Hydrodynamic and Optical Properties in Solution: T︠S︡vetkov, V.N. (Viktor Nikolaevich): Free Download, Borrow, and Streaming: Internet Archive, 1st ed.; Plenum Press: New York, NY, USA, 1989. [Google Scholar]
- Tsvetkov, V.N.; Lavrenko, P.N.; Bushin, S.V. Hydrodynamic invariant of polymer molecules. J. Polym. Sci. Part A 1984, 22, 3447–3480. [Google Scholar] [CrossRef]
- Tsvetkov, V.N.; Lavrenko, P.N.; Bushin, S. V A Hydrodynamic Invariant of Polymeric Molecules. Russ. Chem. Rev. 1982, 51, 975–993. [Google Scholar] [CrossRef]
- Simonova, M.; Kamorin, D.; Sadikov, A.; Filippov, A.; Kazantsev, O. The Influence of Synthesis Method on Characteristics of Buffer and Organic Solutions of Thermo-and pH-Responsive Poly(N-[3-(diethylamino)propyl]methacrylamide)s. Polymers 2022, 14, 282. [Google Scholar] [CrossRef] [PubMed]
- Simonova, M.; Kamorin, D.; Kazantsev, O.; Nepomnyashaya, M.; Filippov, A. Conformation, self-organization and thermoresponsibility of polymethacrylate molecular brushes with oligo(Ethylene glycol)-block-oligo(propylene glycol) side chains. Polymers 2021, 13, 2715. [Google Scholar] [CrossRef]
- Filippov, A.P.; Zamyshlyaeva, O.G.; Tarabukina, E.B.; Simonova, M.A.; Kozlov, A.V.; Semchikov, Y.D. Structural and conformational properties of hyperbranched copolymers based on perfluorinated germanium hydrides1. Polym. Sci.-Ser. A 2012, 54, 319–329. [Google Scholar] [CrossRef]
- Simonova, M.A.; Tarasova, E.V.; Dudkina, M.M.; Tenkovtsev, A.V.; Filippov, A.P. Synthesis and hydrodynamic and conformation properties of star-shaped polystyrene with calix[8]arene core. Int. J. Polym. Anal. Charact. 2019, 24, 87–95. [Google Scholar] [CrossRef]
- Stockmayer, W.H.; Fixman, M. On the estimation of unperturbed dimensions from intrinsic viscositiesxcin. J. Polym. Sci. Part C Polym. Symp. 1963, 1, 137–141. [Google Scholar] [CrossRef]
- Cowie, J.M.G.; Bywater, S. The use of frictional coefficients to evaluate unperturbed dimensions in dilute polymer solutions. Polymer 1965, 6, 197–204. [Google Scholar] [CrossRef]
- Radu-Dan, R.; Damaceanu, M.; Bruma, M.; Ronova, I.A. Effect of Conformational Parameters on Thermal Properties of Some Poly(oxadiazole-naphthylimide)s. Eur. Polym. J. 2011, 20, 29–40. [Google Scholar]
- Cho, H.; Kim, Y.C.; Kim, S.; Chung, I. Persistence length calculation from light scattering and intrinsic viscosity of dilute semiflexible polyimide solutions with different degree of imidization. Korea-Aust. Rheol. J. 2000, 12, 69–76. [Google Scholar]
- Ronova, I.A.; Dubronna, L.V.; Kovalevskii, A.Y.; Hamciuc, C.; Bruma, M. The effect of side substituents on rotation hindrance in polyheteroarylenes. Russ. Chem. Bull. 1998, 47, 1248–1256. [Google Scholar] [CrossRef]
- Tarabukina, E.; Amirova, A.; Belyaeva, E.; Krasova, A.; Simonova, M.; Filippov, A.; Meleshko, T.; Ilgach, D.; Bogorad, N.; Yakimansky, A. Conformational Characteristics of Polyimide Initiator for the Synthesis of Poly(Methylmethacrylate) Grafted Block-Copolymers. J. Macromol. Sci. Part B 2013, 52, 1545–1557. [Google Scholar] [CrossRef]
- Birshtein, T.M.; Goriunov, A.M. The theoretical analysis of the elasticity of polyimides and polyaminoacids. Polym. Sci. Ser. A 1979, 21, I990. [Google Scholar]
- Lyulin, S.V.; Larin, S.V.; Gurtovenko, A.A.; Lukasheva, N.V.; Yudin, V.E.; Svetlichnyi, V.M.; Lyulin, A.V. Effect of the SO2 group in the diamine fragment of polyimides on their structural, thermophysical, and mechanical properties. Polym. Sci.-Ser. A 2012, 54, 631–643. [Google Scholar] [CrossRef]
- Van Krevelen, D.W.; Te Nijenhuis, K. Rheological Properties of Polymer Melts. In Properties of Polymers; Elsevier: Amsterdam, The Netherlands, 2009; pp. 525–597. ISBN 978-0-08-054819-7. [Google Scholar]
- Botelho, E.C.; Figiel, Ł.; Rezende, M.C.; Lauke, B. Mechanical behavior of carbon fiber reinforced polyamide composites. Compos. Sci. Technol. 2003, 63, 1843–1855. [Google Scholar] [CrossRef]
- Durai Prabhakaran, R.T.; Pillai, S.; Charca, S.; Oshkovr, S.A.; Knudsen, H.; Andersen, T.L.; Ilsted Bech, J.; Thomsen, O.T.; Lilholt, H. Mechanical characterization and fractography of glass fiber/polyamide (PA6) composites. Polym. Compos. 2015, 36, 834–853. [Google Scholar] [CrossRef]


| Molar Ratio of R/BAPB | Name PAA | Name PI |
|---|---|---|
| 0.93:1 | PAA 0.93 | PI 0.93 |
| 0.95:1 | PAA 0.95 | PI 0.95 |
| 0.97:1 | PAA 0.97 | PI 0.97 |
| 0.98:1 | PAA 0.98 | PI 0.98 |
| 0.99:1 | PAA 0.99 | PI 0.99 |
| Polymers | Mw × 10–3, g·mol−1 | Rh-D, nm | f∙10−7 g·c−1 | A2∙10−4, cm3·mole·g−2 | [η], cm3·g−1 | N | L, nm |
|---|---|---|---|---|---|---|---|
| PAA 0.93 | 22 ± 2 | 5.1 ± 0.4 | 0.963 | 32 | 45.6 ± 3 | 30 | 97 |
| PAA 0.95 | 26 ± 3 | 5.7 ± 0.3 | 1.080 | 39 | 56.6 ± 2 | 35 | 114 |
| PAA 0.97 | 29 ± 3 | 6.1 ± 0.2 | 1.150 | 22 | 58.0 ± 2 | 40 | 130 |
| PAA 0.98 | 36 ± 4 | 7.0 ± 0.2 | 1.320 | 19 | 72.0 ± 3 | 49 | 159 |
| PAA 0.99 | 70 ± 5 | 11.0 ± 0.3 | 2.080 | 17 | 110.0 ± 7 | 95 | 310 |
| Sample of CFRP | Tg, °C | Tm, °C | ∆Hm, J/g | χ, % | τ5, °C |
|---|---|---|---|---|---|
| PI 0.93 | 198 | 320 | 42.3 | 47.0 | 572 |
| PI 0.95 | 200 | 321 | 40.9 | 45.4 | 570 |
| PI 0.97 | 204 | 322 | 37.2 | 41.3 | 570 |
| PI 0.98 | 206 | 324 | 36.0 | 40.0 | 573 |
| PI 0.99 | 210 | 326 | 1.0 | 1.1 | 589 |
| Sample of CFRP | G1c J/m2 | σb, MPa | E′, GPa |
|---|---|---|---|
| PI 0.93 | 350 ± 25 | 1590 ± 177 | 114 ± 7 |
| PI 0.95 | 370 ± 10 | 1580 ± 81 | 118 ± 10 |
| PI 0.97 | 720 ± 81 | 1560 ± 81 | 119 ± 10 |
| PI 0.98 | 830 ± 13 | 1510 ± 145 | 111 ± 6 |
| PI 0.99 | 1808 ± 65 | 1146 ± 102 | 101 ± 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaganov, G.; Simonova, M.; Romasheva, M.; Didenko, A.; Popova, E.; Ivan’kova, E.; Kamalov, A.; Elokhovskiy, V.; Vaganov, V.; Filippov, A.; et al. Influence of Molecular Weight on Thermal and Mechanical Properties of Carbon-Fiber-Reinforced Plastics Based on Thermoplastic Partially Crystalline Polyimide. Polymers 2023, 15, 2922. https://doi.org/10.3390/polym15132922
Vaganov G, Simonova M, Romasheva M, Didenko A, Popova E, Ivan’kova E, Kamalov A, Elokhovskiy V, Vaganov V, Filippov A, et al. Influence of Molecular Weight on Thermal and Mechanical Properties of Carbon-Fiber-Reinforced Plastics Based on Thermoplastic Partially Crystalline Polyimide. Polymers. 2023; 15(13):2922. https://doi.org/10.3390/polym15132922
Chicago/Turabian StyleVaganov, Gleb, Maria Simonova, Margarita Romasheva, Andrey Didenko, Elena Popova, Elena Ivan’kova, Almaz Kamalov, Vladimir Elokhovskiy, Vyacheslav Vaganov, Alexander Filippov, and et al. 2023. "Influence of Molecular Weight on Thermal and Mechanical Properties of Carbon-Fiber-Reinforced Plastics Based on Thermoplastic Partially Crystalline Polyimide" Polymers 15, no. 13: 2922. https://doi.org/10.3390/polym15132922







