Mechanical and Biological Characterization of PMMA/Al2O3 Composites for Dental Implant Abutments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of the PMMA/Al2O3 Composites
2.3. Morphological Analysis
2.4. WAXD Analysis
2.5. Mechanical Properties
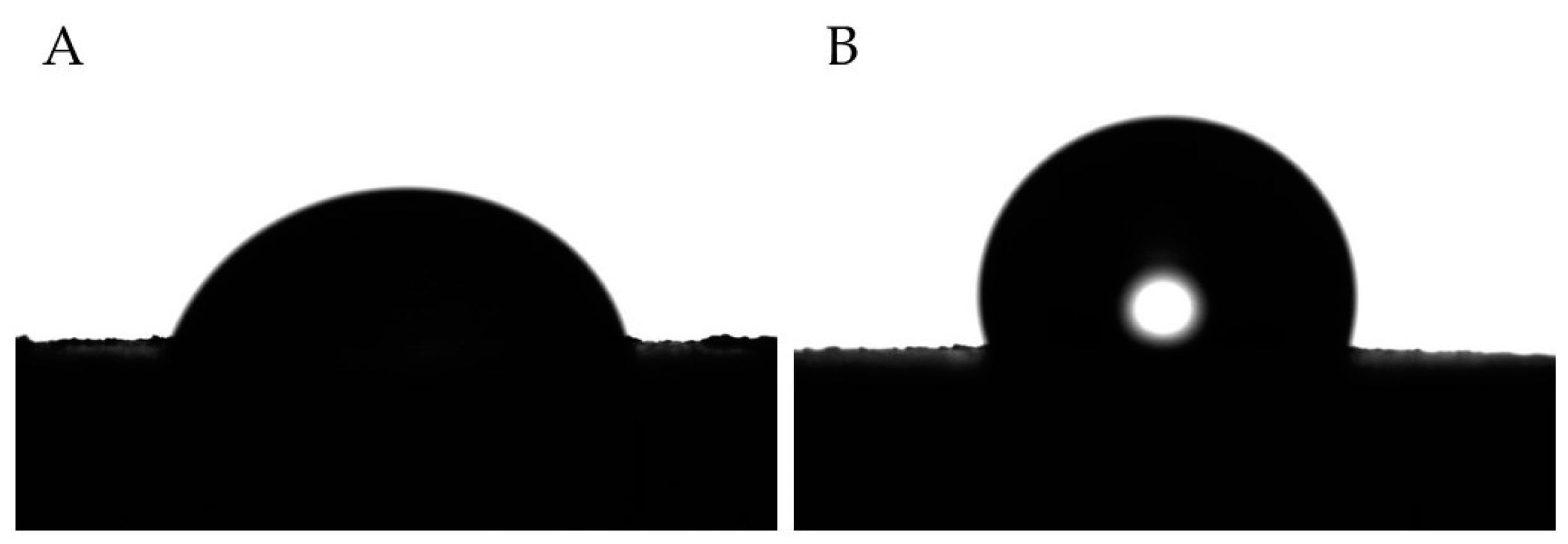
2.6. Surface Roughness and Wettability
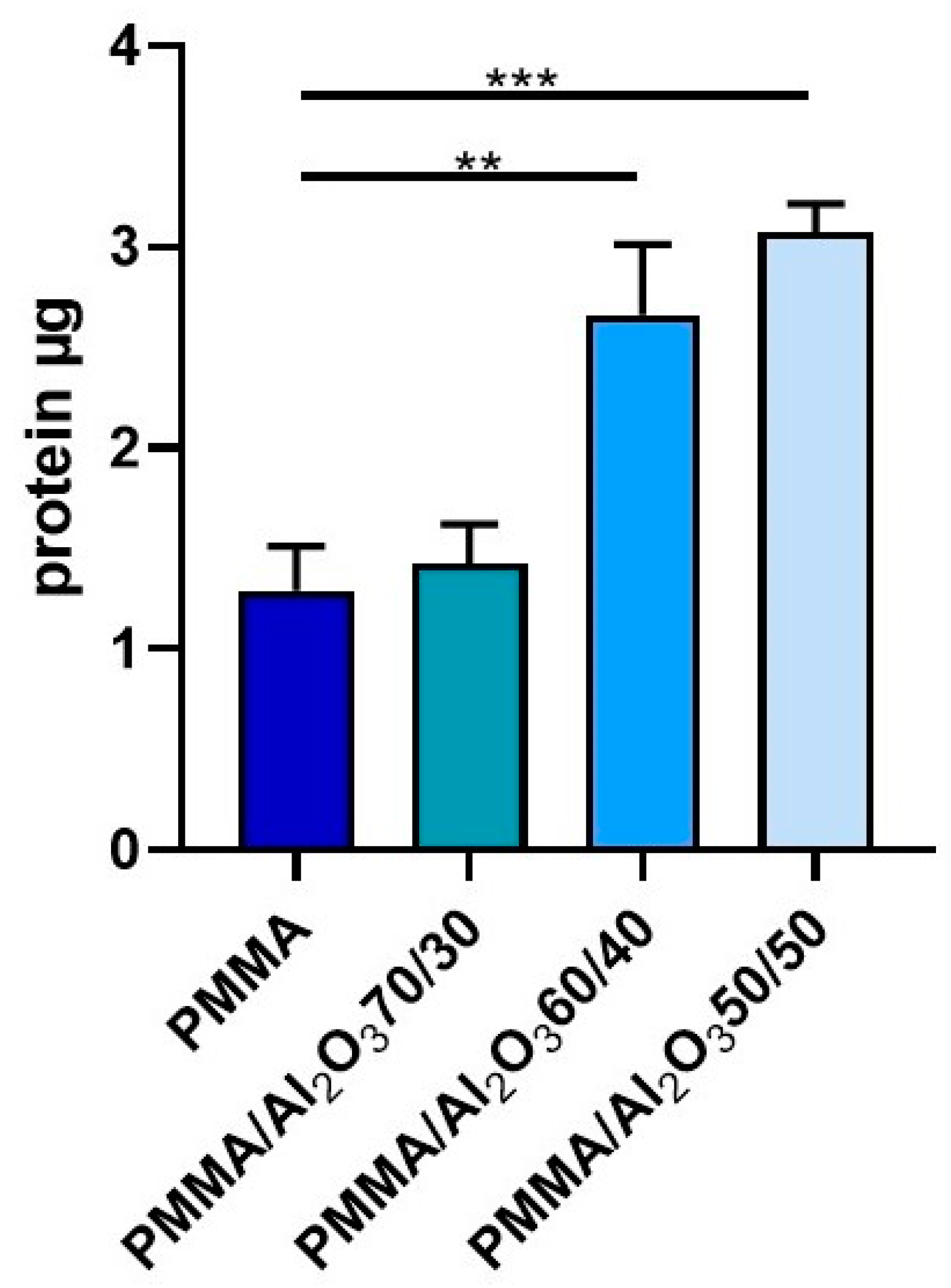
2.7. Protein Adsorption Assay
2.8. Cell Culture
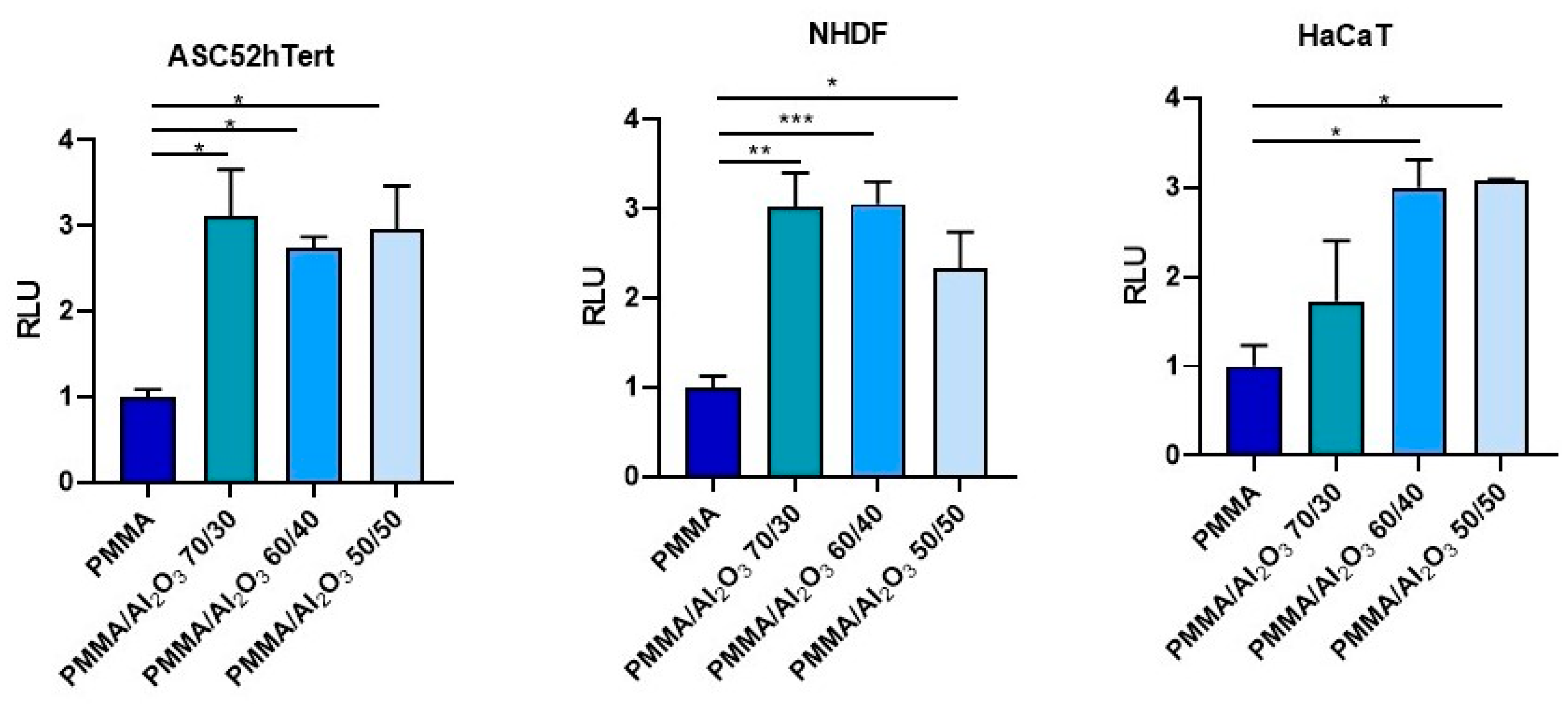
2.9. Cell Adhesion and Viability Assays
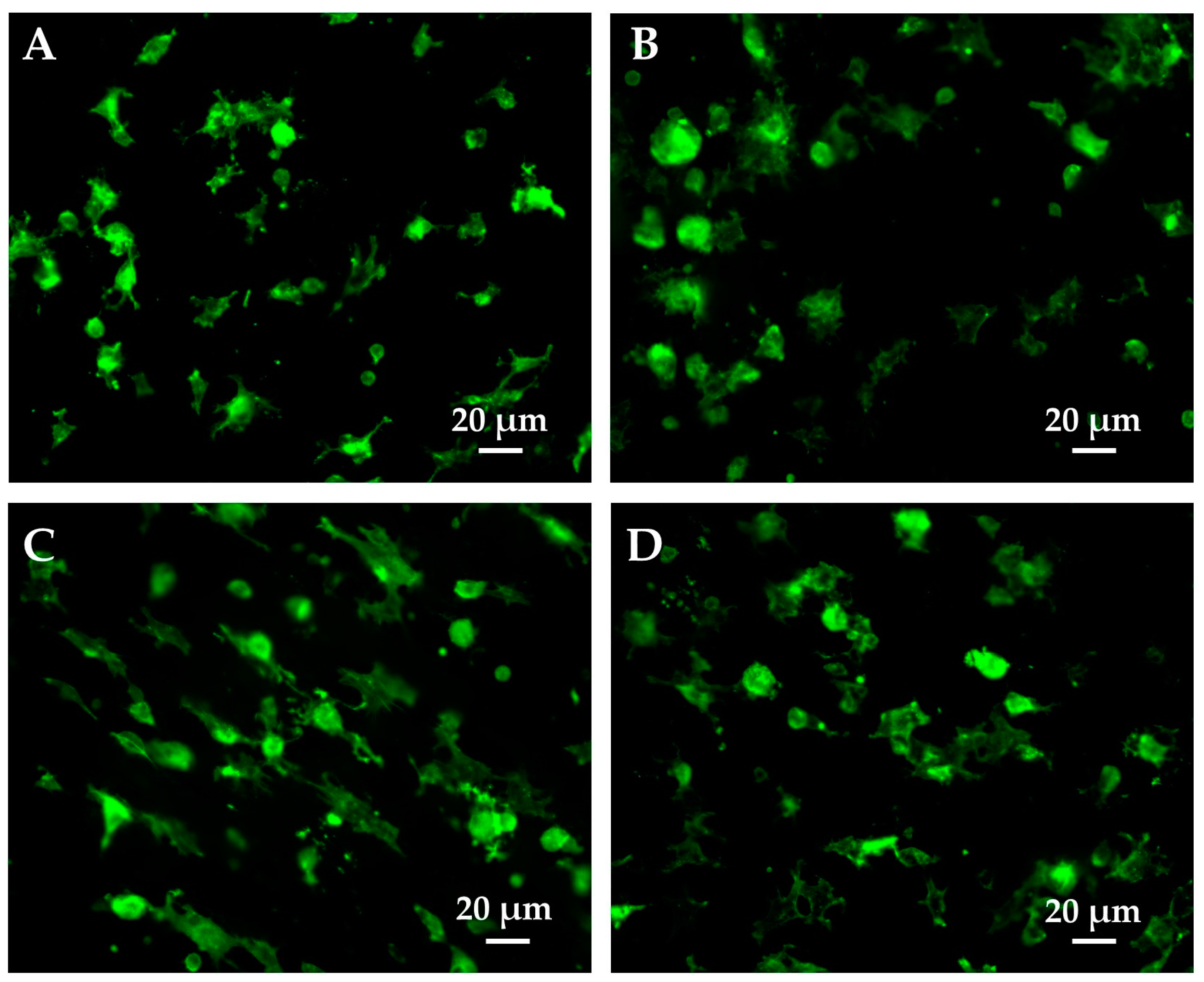
2.10. Cell Morphology and Orientation Analysis
2.11. Real-Time qRT-PCR
2.12. Statistical Analysis
3. Results
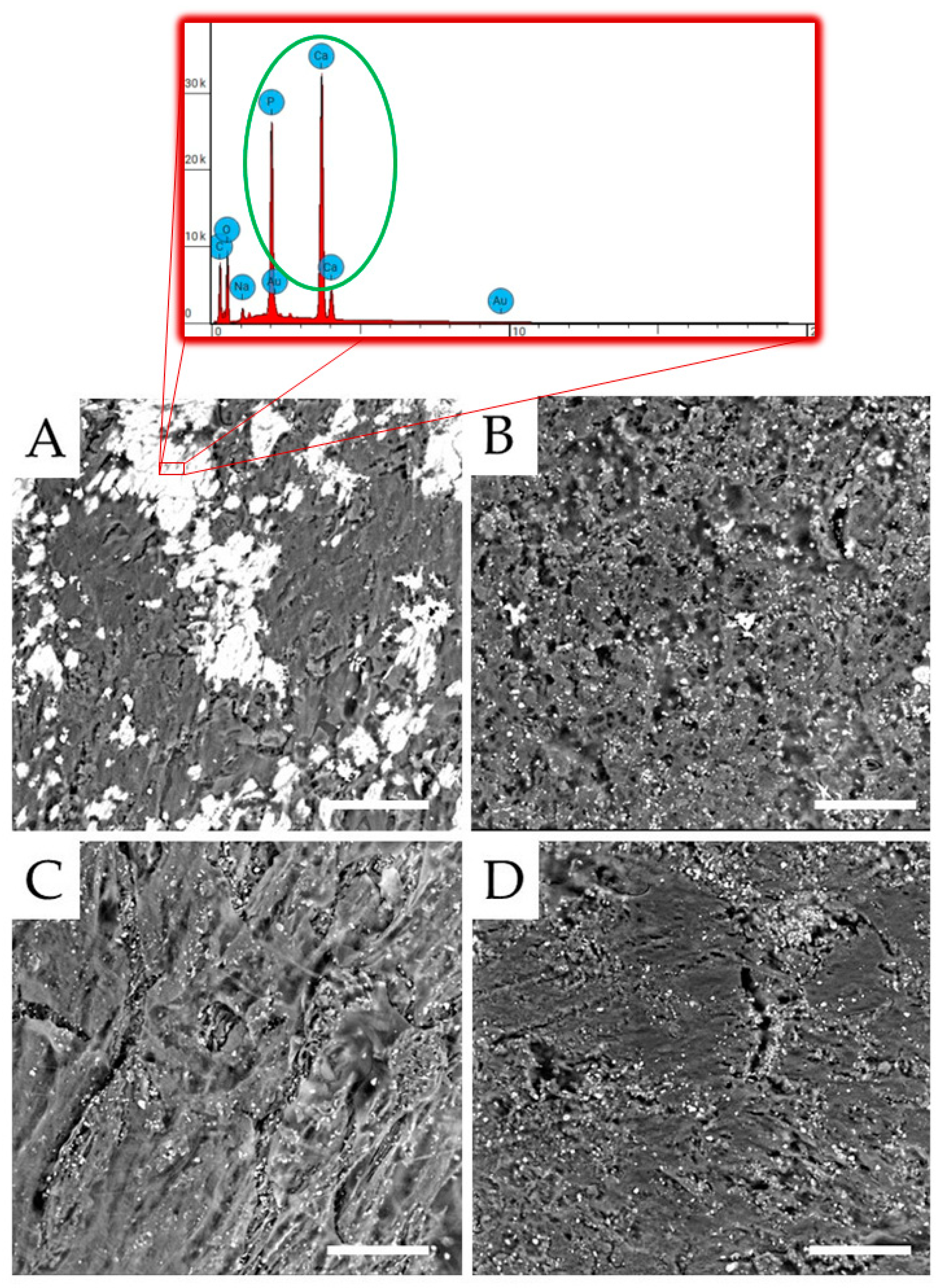
3.1. Morphological Analysis
3.2. Structural Analysis
3.3. Mechanical Properties
3.4. Surface Properties (Wettability and Roughness)
3.5. Protein Adsorption
3.6. Cell Adhesion and Viability
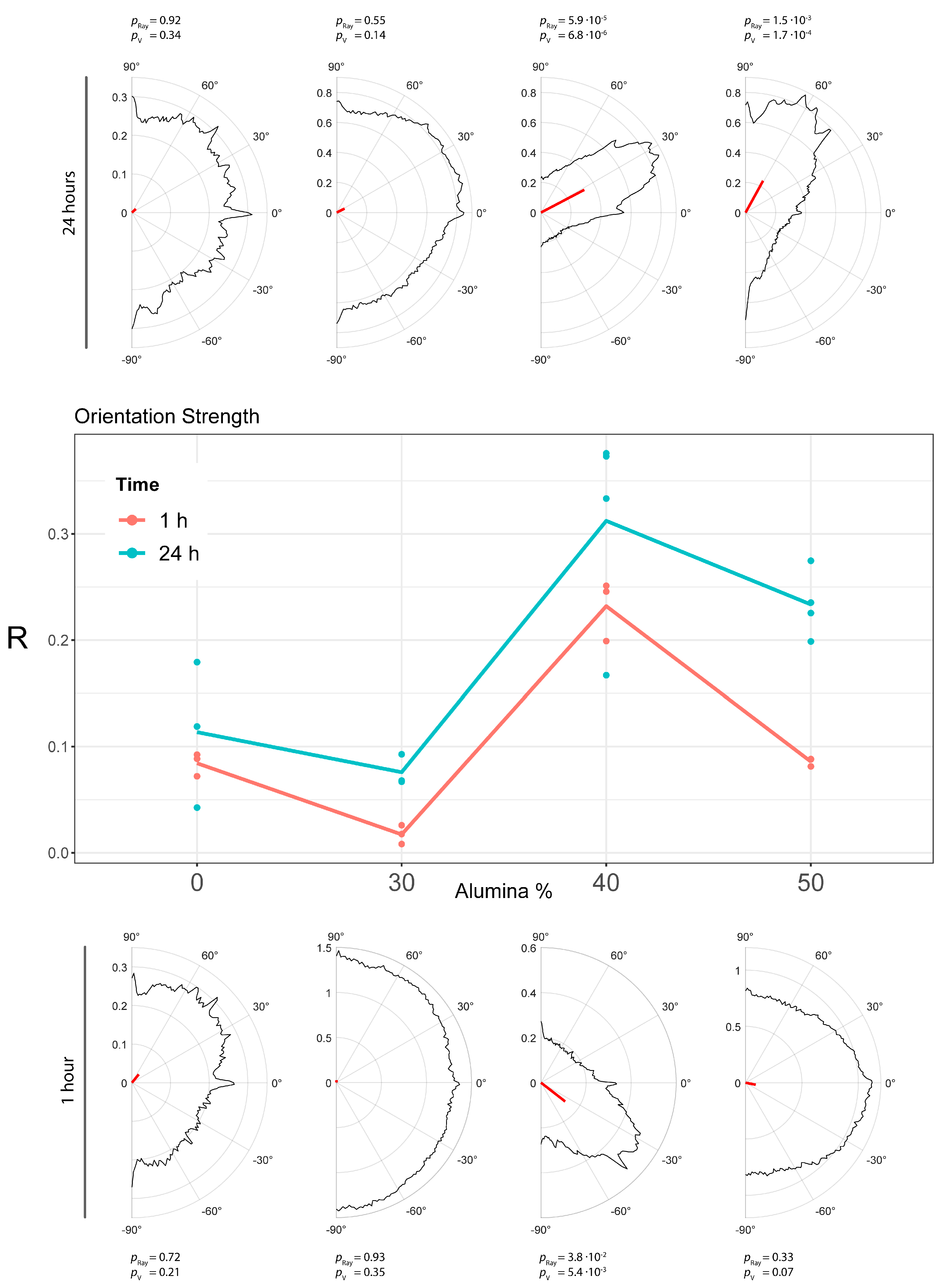
3.7. Cell morphology and Orientation Analysis
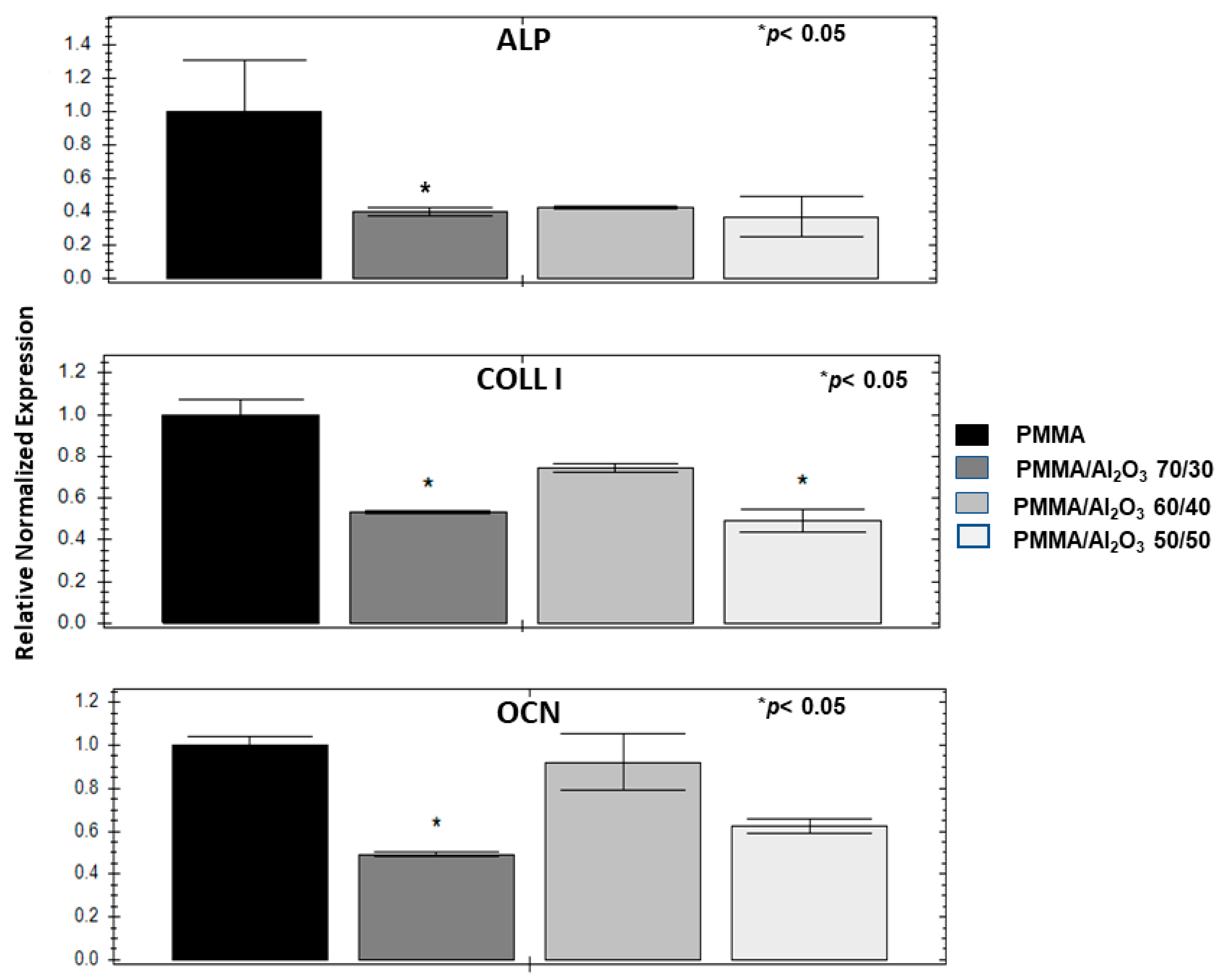
3.8. Alumina Filler Decreases Osteogenic Genes and Mineralization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wennerberg, A.; Albrektsson, T. On implant surfaces: A review of current knowledge and opinions. Int. J. Oral. Maxillofac. Implants 2010, 25, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Duraccio, D.; Mussano, F.; Faga, M.G. Biomaterials for dental implants: Current and future trends. J. Mater. Sci. 2015, 50, 4779–4812. [Google Scholar] [CrossRef]
- Toledano-Serrabona, J.; Camps-Font, O.; Pompéu de Moraes, D.; Corte-Rodríguez, M.; Montes-Bayón, M.; Valmaseda-Castellón, E.; Gay-Escoda, C.; Sánchez-Garcés, M.A. Ion release and local effects of titanium metal particles from dental implants: An experimental study in rats. J. Periodontol. 2023, 94, 119–129. [Google Scholar] [CrossRef]
- Kheder, W.; Al Kawas, S.; Khalaf, K.; Samsudin, A.R. Impact of tribocorrosion and titanium particles release on dental implant complications—A narrative review. Jpn. Dent. Sci. Rev. 2021, 57, 182–189. [Google Scholar] [CrossRef]
- Lozano, P.; Peña, M.; Herrero-Climent, M.; Rios-Santos, J.V.; Rios-Carrasco, B.; Brizuela, A.; Gil, J. Corrosion Behavior of Titanium Dental Implants with Implantoplasty. Materials 2022, 15, 1563. [Google Scholar] [CrossRef]
- Fage, S.W.; Muris, J.; Jakobsen, S.S.; Thyssen, J.P. Titanium: A review on exposure, release, penetration, allergy, epidemiology, and clinical reactivity. Contact. Dermat. 2016, 74, 323–345. [Google Scholar] [CrossRef] [Green Version]
- Nistor, L.; Grădinaru, M.; Rîcă, R.; Mărășescu, P.; Stan, M.; Manolea, H.; Ionescu, A.; Moraru, I. Zirconia Use in Dentistry Manufacturing and Properties. Curr. Health Sci. J. 2019, 45, 28–35. [Google Scholar] [CrossRef]
- Lo Giudice, R.; Sindoni, A.; Tribst, J.P.M.; Dal Piva, A.M.d.O.; Lo Giudice, G.; Bellezza, U.; Lo Giudice, G.; Famà, F. Evaluation of Zirconia and High Performance Polymer Abutment Surface Roughness and Stress Concentration for Implant- Supported Fixed Dental Prostheses. Coatings 2022, 12, 238. [Google Scholar] [CrossRef]
- Bottino, M.A.; de Oliveira, F.R.; Sabino, C.F.; Dinato, J.C.; Silva-Concílio, L.R.; Tribst, J.P.M. Survival Rate and Deformation of External Hexagon Implants with One-Piece Zirconia Crowns. Metals 2021, 11, 1068. [Google Scholar] [CrossRef]
- Chevalier, J.; Cales, B.; Drouin, J.M. Low-temperature aging of Y-TZP ceramics. J. Am. Ceram. Soc. 1999, 82, 2150. [Google Scholar] [CrossRef]
- Paratelli, A.; Perrone, G.; Ortega, R.; Gómez-Polo, M. Polyetheretherketone in implant prosthodontics: A scoping review. Int. J. Prosthodont. 2020, 33, 671–679. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Kuo, M.; Huang, J.; Chen, M. On the PEEK composites reinforced by surface-modified nano-silica. Mater. Sci. Eng. A 2007, 458, 158–169. [Google Scholar] [CrossRef] [Green Version]
- Sidhom, M.; Zaghloul, H.; Mosleh, I.E.-S.; Eldwakhly, E. Effect of Different CAD/CAM Milling and 3D Printing Digital Fabrication Techniques on the Accuracy of PMMA Working Models and Vertical Marginal Fit of PMMA Provisional Dental Prosthesis: An In Vitro Study. Polymers 2022, 14, 1285. [Google Scholar] [CrossRef]
- Alauddin, M.S.; Baharuddin, A.S.; Mohd Ghazali, M.I. The Modern and Digital Transformation of Oral Health Care: A Mini Review. Healthcare 2021, 9, 118. [Google Scholar] [CrossRef]
- Arinc, H. Effects of Prosthetic Material and Framework Design on Stress Distribution in Dental Implants and Peripheral Bone: A Three-Dimensional Finite Element Analysis. Med. Sci. Monit. 2018, 24, 4279–4287. [Google Scholar] [CrossRef]
- Reinedahl, D.; Johansson, P.; Galli, S.; Kjellin, P.; Albrektsson, T.; Wennerberg, A. Review of PEEK implants and biomechanical and immunological responses to a zirconium phosphate nano-coated PEEK, a blasted PEEK, and a turned titanium implant surface. Am. J. Dent. 2022, 35, 152–160. [Google Scholar] [PubMed]
- Al Wadei, M.H.D.; Sayed, M.E.; Jain, S.; Aggarwal, A.; Alqarni, H.; Gupta, S.G.; Alqahtani, S.M.; Alahmari, N.M.; Alshehri, A.H.; Jain, M.; et al. Marginal Adaptation and Internal Fit of 3D-Printed Provisional Crowns and Fixed Dental Prosthesis Resins Compared to CAD/CAM-Milled and Conventional Provisional Resins: A Systematic Review and Meta-Analysis. Coatings 2022, 12, 1777. [Google Scholar] [CrossRef]
- Luo, C.; Liu, Y.; Peng, B.; Chen, M.; Liu, Z.; Li, Z.; Kuang, H.; Gong, B.; Li, Z.; Sun, H. PEEK for Oral Applications: Recent Advances in Mechanical and Adhesive Properties. Polymers 2023, 15, 386. [Google Scholar] [CrossRef]
- Khandaker, M.; Vaughan, M.B.; Morris, T.L.; White, J.J.; Meng, Z. Effect of additive particles on mechanical, thermal, and cell functioning properties of poly (methyl methacrylate) cement. Int. J. Nanomed. 2014, 9, 2699–2712. [Google Scholar] [CrossRef] [Green Version]
- Chaijareenont, P.; Takahashi, H.; Nishiyama, N.; Arksornnukit, M. Effect of different amounts of 3-methacryloxypropyltrimethoxysilane on the flexural properties and wear resistance of alumina reinforced PMMA. Dent. Mater. J. 2012, 31, 623–628. [Google Scholar] [CrossRef] [Green Version]
- Pawar, R.O.; Narote, P.S.; Gawai, K.T.; Amte, M.P.; Singh, S.; Sonkesriya, S. Comparative Analysis of Biofilm Formation on Materials Used for the Fabrication of Implant Supported Prostheses. J. Pharm. Bioallied. Sci. 2022, 14 (Suppl. S1), S812–S815. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. PMMA-Based Nanocomposites for Odontology Applications: A State-of-the-Art. Int. J. Mol. Sci. 2022, 23, 10288. [Google Scholar] [CrossRef]
- Siegel, R.W.; Chang, S.K.; Ash, B.J.; Stone, J.; Ajayan, P.M.; Doremus, R.W.; Schadler, L.S. Mechanical behavior of polymer and ceramic matrix nanocomposites. Scr. Mater. 2001, 44, 2061–2064. [Google Scholar] [CrossRef]
- Mallakpour, S.; Khadem, E. Recent Development in the Synthesis of Polymer Nanocomposites Based on Nano-Alumina. Prog. Polym. Sci. 2014, 51, 74–93. [Google Scholar] [CrossRef]
- Derazkola, H.; Simchi, A. Effects of alumina nanoparticles on the microstructure, strength and wear resistance of poly(methyl methacrylate)-based nanocomposites prepared by friction stir processing. J. Mech. Behav. Biomed. Mater. 2018, 79, 246–253. [Google Scholar] [CrossRef]
- de Sá, J.; Vieira, F.; Aroso, C.M.; Cardoso, M.; Mendes, J.M.; Silva, A.S. The Influence of Saliva pH on the Fracture Resistance of Three Complete Denture Base Acrylic Resins. Int. J. Dent. 2020, 2020, 8941876. [Google Scholar] [CrossRef]
- Montoya, C.; Kurylec, J.; Baraniya, D.; Tripathi, A.; Puri, S.; Orrego, S. Antifungal Effect of Piezoelectric Charges on PMMA Dentures. ACS Biomater. Sci. Eng. 2021, 7, 4838–4846. [Google Scholar] [CrossRef]
- McKinney, R.V., Jr.; Steflik, D.E.; Koth, D.L. Evidence for a junctional epithelial attachment to ceramic dental implants. A transmission electron microscopic study. J. Periodontol. 1985, 56, 579–591. [Google Scholar] [CrossRef]
- ASTM D2240; Standard Test Method for Rubber Property—Durometer Hardness. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D790-03; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM International: West Conshohocken, PA, USA, 2017.
- ISO 4288; Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Rules and Procedures for the Assessment of Surface Texture. ISO: Geneva, Switzerland, 1996.
- Ruffinatti, F.A.; Genova, T.; Mussano, F.; Munaron, L. MORPHEUS: An automated tool for unbiased and reproducible cell morphometry. J. Cell. Physiol. 2020, 235, 10110–10115. [Google Scholar] [CrossRef]
- D’Amelio, P.; Tamone, C.; Sassi, F.; D’Amico, L.; Roato, I.; Patanè, S.; Ravazzoli, M.; Veneziano, L.; Ferracini, R.; Pescarmona, G.P.; et al. Teriparatide increases the maturation of circulating osteoblast precursors. Osteoporos. Int. 2012, 23, 1245–1253. [Google Scholar] [CrossRef]
- Hanemann, T.; Szabó, D.V. Polymer-Nanoparticle Composites: From Synthesis to Modern Applications. Materials 2010, 3, 3468–3517. [Google Scholar] [CrossRef]
- Hanemann, T. Influence of particle properties on the viscosity of polymer–alumina composites. Ceram. Int. 2008, 34, 2099–2105. [Google Scholar] [CrossRef]
- Duraccio, D.; Arrigo, R.; Strongone, V.; Capra, P.P.; Malucelli, G. Rheological, mechanical, thermal and electrical properties of UHMWPE/CNC composites. Cellulose 2021, 28, 10953. [Google Scholar] [CrossRef]
- Lovell, R.; Windle, A.H. Determination of the local conformation of PMMA from wide-angle X-ray scattering. Polymer 1981, 22, 175. [Google Scholar] [CrossRef]
- Feret, F.; Roy, D.; Boulanger, C. Determination of alpha and beta alumina in ceramic alumina by X-ray diffraction. Spectrochim. Acta Part. B 2000, 55, 1051–1061. [Google Scholar] [CrossRef]
- Hada, T.; Kanazawa, M.; Iwaki, M.; Katheng, A.; Minakuchi, S. Comparison of Mechanical Properties of PMMA Disks for Digitally Designed Dentures. Polymers 2021, 13, 1745. [Google Scholar] [CrossRef]
- Duraccio, D.; Strongone, V.; Malucelli, G.; Auriemma, F.; De Rosa, C.; Mussano, F.D.; Genova, T.; Faga, M.G. The role of alumina-zirconia loading on the mechanical and biological properties of UHMWPE for biomedical applications. Compos. Part. B Eng. 2019, 164, 800–808. [Google Scholar] [CrossRef]
- Ma, Y.; Cao, X.; Feng, X.; Ma, Y.; Zou, H. Fabrication of super-hydrophobic film from PMMA with intrinsic water contact angle below 90°. Polymer 2007, 48, 7455–7460. [Google Scholar] [CrossRef]
- Alrahlah, A.; Khan, R.; Vohra, F.; Alqahtani, I.M.; Alruhaymi, A.A.; Haider, S.; Al-Odayni, A.-B.M.; Saeed, W.S.; Ananda Murthy, H.C.; Bautista, L. Influence of the physical inclusion of ZrO2/TiO2 nanoparticles on physical, mechanical, and morphological characteristics of PMMA based interim restorative material. BioMed Res. Int. 2022, 2022, 1743019. [Google Scholar] [CrossRef]
- Cascione, M.; De Matteis, V.; Pellegrino, P.; Albanese, G.; De Giorgi, M.L.; Paladini, F.; Corsalini, M.; Rinaldi, R. Improvement of PMMA Dental Matrix Performance by Addition of Titanium Dioxide Nanoparticles and Clay Nanotubes. Nanomaterials 2021, 11, 2027. [Google Scholar] [CrossRef]
- Wei, J.; Igarashi, T.; Okumori, N.; Igarashi, T.; Maetani, T.; Liu, B.; Yoshinari, M. Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts. Biomed. Mater. 2009, 4, 045002. [Google Scholar] [CrossRef]
- Silva-Bermudez, P.; Rodil, S.E. An overview of protein adsorption on metal oxide coatings for biomedical implants. Surf. Coat. Technol. 2013, 233, 147–158. [Google Scholar] [CrossRef]
- Mussano, F.; Genova, T.; Laurenti, M.; Zicola, E.; Munaron, L.; Rivolo, P.; Mandracci, P.; Carossa, S. Early Response of fibroblasts and epithelial cells to pink-shaded anodized dental implant abutments: An in vitro study. Int. J. Oral. Maxillofac. Implant. 2018, 33, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.D.; Di Gregorio, M.; He, P.; McCulloch, C.A. TRPV4 Mediates the Calcium Influx Required for Flightless-Non-Muscle Myosin Interaction and Collagen Remodeling. J. Cell Sci. 2017, 130, 2196–2208. [Google Scholar] [CrossRef] [Green Version]
- Chiang, C.C.; Hsieh, M.K.; Wang, C.Y.; Tuan, W.H.; Lai, P.L. Cytotoxicity and Cell Response of Preosteoblast in Calcium Sulfate-Augmented PMMA Bone Cement. Biomed. Mater. 2021, 16, 055014. [Google Scholar] [CrossRef] [PubMed]




| Hardness (Shore D) | Flexural Modulus (GPa) | Flexural Strength (MPa) | Elongation at Break (%) | Water Contact Angle (°) | Roughness (µm) | |
|---|---|---|---|---|---|---|
| PMMA | 91.8 ± 1.5 | 3.86 ± 0.80 | 104.2 ± 15.5 | 3.21 ± 0.63 | 77.2 ± 3.8 | 1.94 ± 0.33 |
| PMMA/Al2O3 70/30 | 96.8 ± 1.2 | 4.39 ± 0.26 | 80.5 ± 4.6 | 2.04 ± 0.12 | 99.7 ± 5.3 | 2.28 ± 0.35 |
| PMMA/Al2O3 60/40 | 96.8 ± 1.6 | 4.50 ± 0.13 | 72.1 ± 3.7 | 1.78 ± 0.06 | 103.6 ± 10.0 | 2.32 ± 0.39 |
| PMMA/Al2O3 50/50 | 97.0 ± 1.2 | 4.44 ± 0.13 | 65.4 ± 3.2 | 1.64 ± 0.08 | 105.8 ± 4.6 | 2.45 ± 0.44 |
| Row | Mean | R | S | Rayleigh_Pval | Vtest_Pval |
|---|---|---|---|---|---|
| PMMA_0A_1_1 | 35.67701600 | 0.072035935 | 0.927964065 | 0.474441214 | 0.110761126 |
| PMMA_0A_1_2 | 50.63379808 | 0.088506355 | 0.911493645 | 0.721981796 | 0.208632835 |
| PMMA_0A_1_3 | 52.86819961 | 0.092353823 | 0.907646177 | 0.701346336 | 0.198654567 |
| PMMA_0A_24_1 | 82.74359410 | 0.118870528 | 0.881129472 | 0.352655849 | 0.074071439 |
| PMMA_0A_24_2 | 42.39168138 | 0.042559193 | 0.957440807 | 0.921683113 | 0.342375407 |
| PMMA_0A_24_3 | 73.89992077 | 0.179232651 | 0.820767349 | 0.136471136 | 0.022975108 |
| PMMA_30A_1_1 | −61.48795269 | 0.025929487 | 0.974070513 | 0.965958569 | 0.395724331 |
| PMMA_30A_1_2 | −68.56930945 | 0.008236939 | 0.991763061 | 0.996173616 | 0.464959623 |
| PMMA_30A_1_3 | 86.88319288 | 0.017617649 | 0.982382351 | 0.927777572 | 0.349160372 |
| PMMA_30A_24_1 | 28.58235944 | 0.066870283 | 0.933129717 | 0.527206483 | 0.128637695 |
| PMMA_30A_24_2 | 26.84561799 | 0.067942743 | 0.932057257 | 0.548369580 | 0.136178979 |
| PMMA_30A_24_3 | 43.90423995 | 0.092727566 | 0.907272434 | 0.625224085 | 0.165392251 |
| PMMA_40A_1_1 | −1214876817 | 0.199050932 | 0.800949068 | 0.002521487 | 0.000282729 |
| PMMA_40A_1_2 | −26.82576711 | 0.245658642 | 0.754341358 | 0.021715219 | 0.002912159 |
| PMMA_40A_1_3 | −37.88083450 | 0.251121755 | 0.748878245 | 0.038089695 | 0.005407698 |
| PMMA_40A_24_1 | 72.53467140 | 0.375807448 | 0.624192552 | 7.21462 × 10−7 | 8.27644 × 10−8 |
| PMMA_40A_24_2 | 79.77644706 | 0.333230014 | 0.666769986 | 0.000127143 | 1.40318 × 10−5 |
| PMMA_40A_24_3 | 60.54074263 | 0.166938425 | 0.833061575 | 0.097362791 | 0.015490374 |
| PMMA_40A_24_4 | 27.69148361 | 0.372972822 | 0.627027178 | 5.90978 × 10−5 | 6.82014 × 10−6 |
| PMMA_50A_1_1 | −4.806702539 | 0.088296361 | 0.911703639 | 0.663567024 | 0.181657465 |
| PMMA_50A_1_2 | −17.28843119 | 0.087947123 | 0.912052877 | 0.647910275 | 0.174922305 |
| PMMA_50A_1_3 | −11.75231262 | 0.081283560 | 0.918716440 | 0.337785021 | 0.070193658 |
| PMMA_50A_24_1 | −61.55226548 | 0.235267368 | 0.764732632 | 0.005880955 | 0.000708117 |
| PMMA_50A_24_2 | 73.61116062 | 0.225430377 | 0.774569623 | 0.006611798 | 0.000799675 |
| PMMA_50A_24_3 | 73.74353041 | 0.198685652 | 0.801314348 | 0.008193347 | 0.000998012 |
| PMMA_50A_24_4 | 61.23645716 | 0.274637545 | 0.725362455 | 0.001507763 | 0.000171249 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roato, I.; Genova, T.; Duraccio, D.; Ruffinatti, F.A.; Zanin Venturini, D.; Di Maro, M.; Mosca Balma, A.; Pedraza, R.; Petrillo, S.; Chinigò, G.; et al. Mechanical and Biological Characterization of PMMA/Al2O3 Composites for Dental Implant Abutments. Polymers 2023, 15, 3186. https://doi.org/10.3390/polym15153186
Roato I, Genova T, Duraccio D, Ruffinatti FA, Zanin Venturini D, Di Maro M, Mosca Balma A, Pedraza R, Petrillo S, Chinigò G, et al. Mechanical and Biological Characterization of PMMA/Al2O3 Composites for Dental Implant Abutments. Polymers. 2023; 15(15):3186. https://doi.org/10.3390/polym15153186
Chicago/Turabian StyleRoato, Ilaria, Tullio Genova, Donatella Duraccio, Federico Alessandro Ruffinatti, Diletta Zanin Venturini, Mattia Di Maro, Alessandro Mosca Balma, Riccardo Pedraza, Sara Petrillo, Giorgia Chinigò, and et al. 2023. "Mechanical and Biological Characterization of PMMA/Al2O3 Composites for Dental Implant Abutments" Polymers 15, no. 15: 3186. https://doi.org/10.3390/polym15153186







