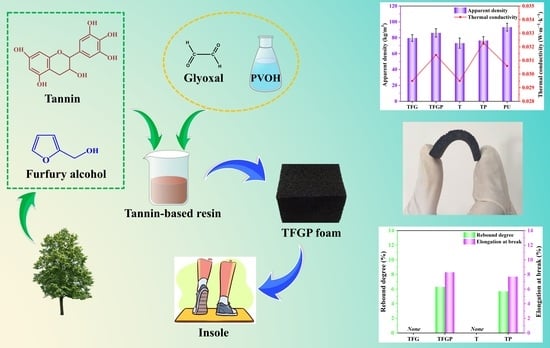
Preparation and Characterization of Biomass Tannin-Based Flexible Foam Insoles for Athletes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
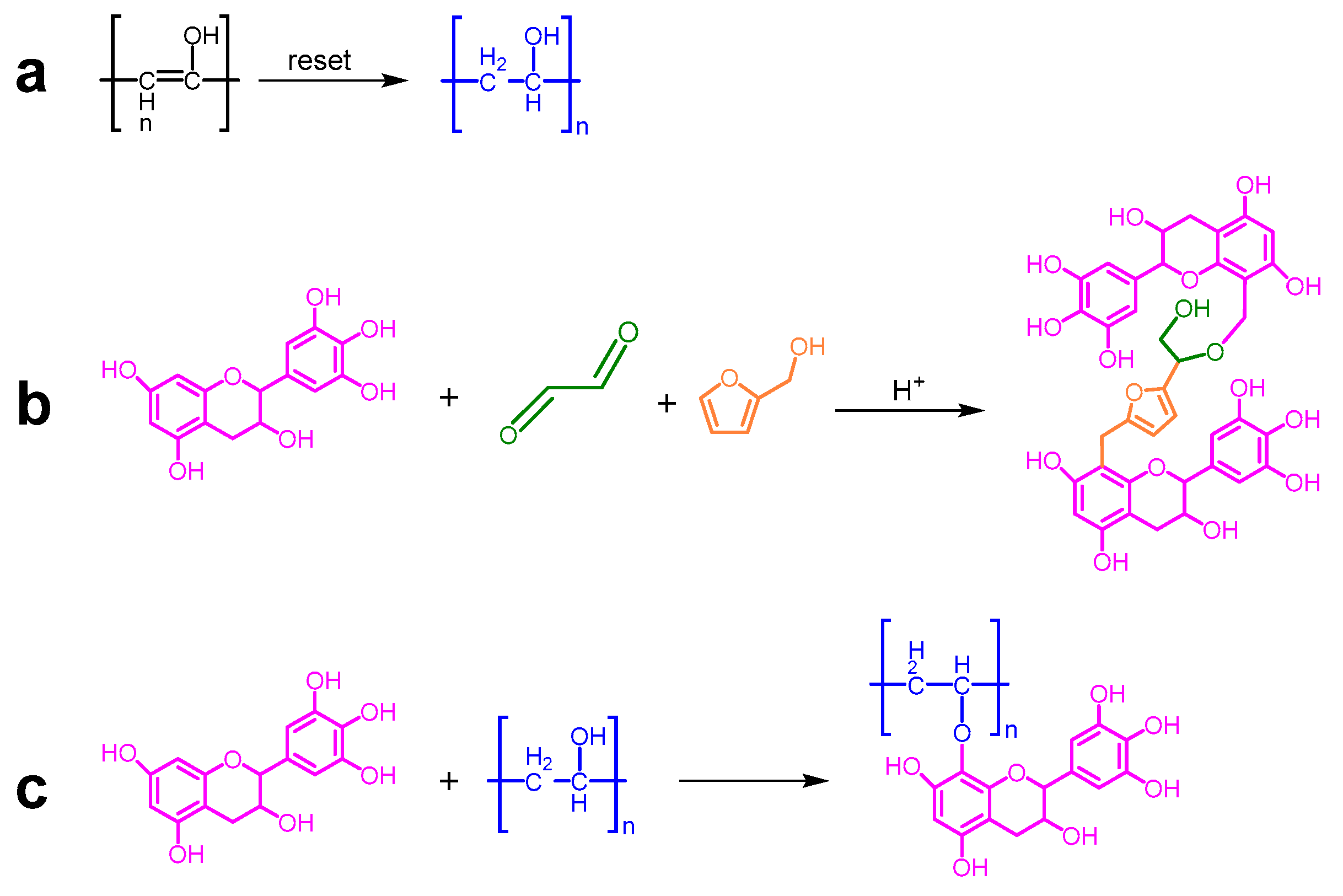
2.2. Preparation of Various Flexible Tannin–Furan Resin-Based Foams
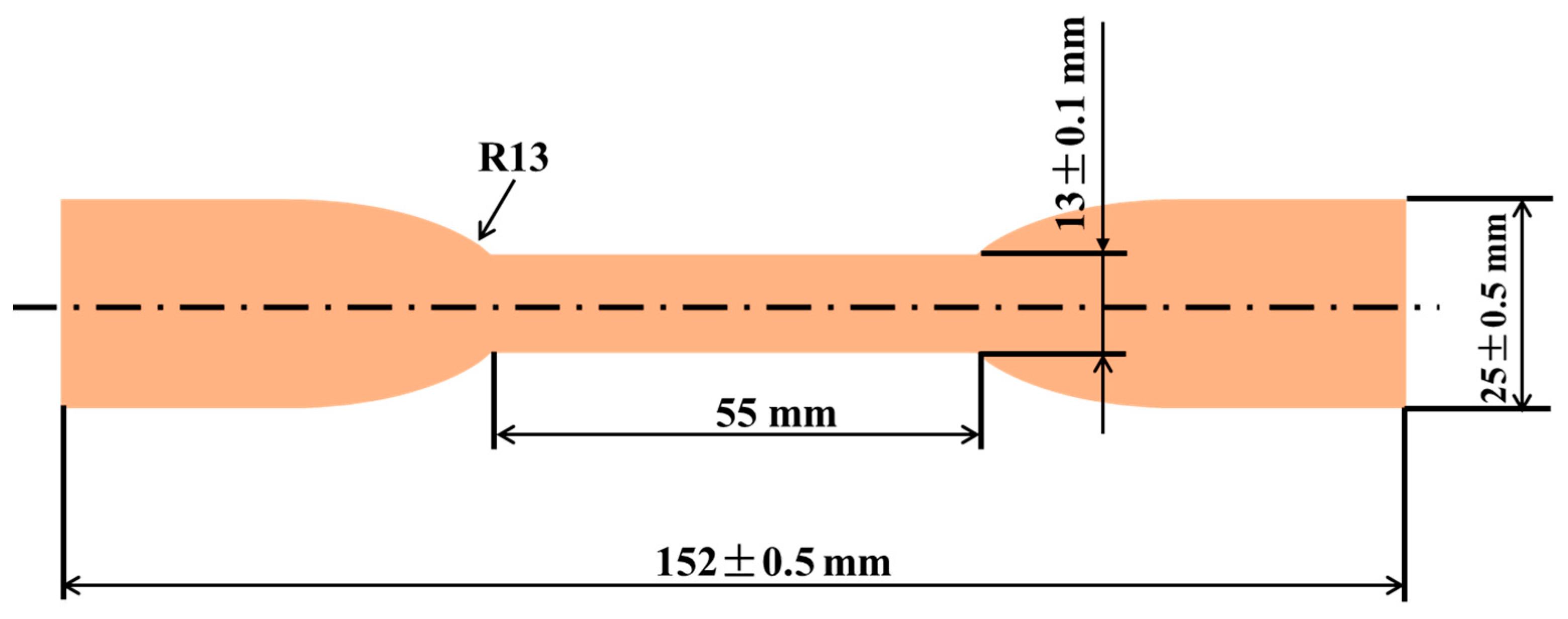
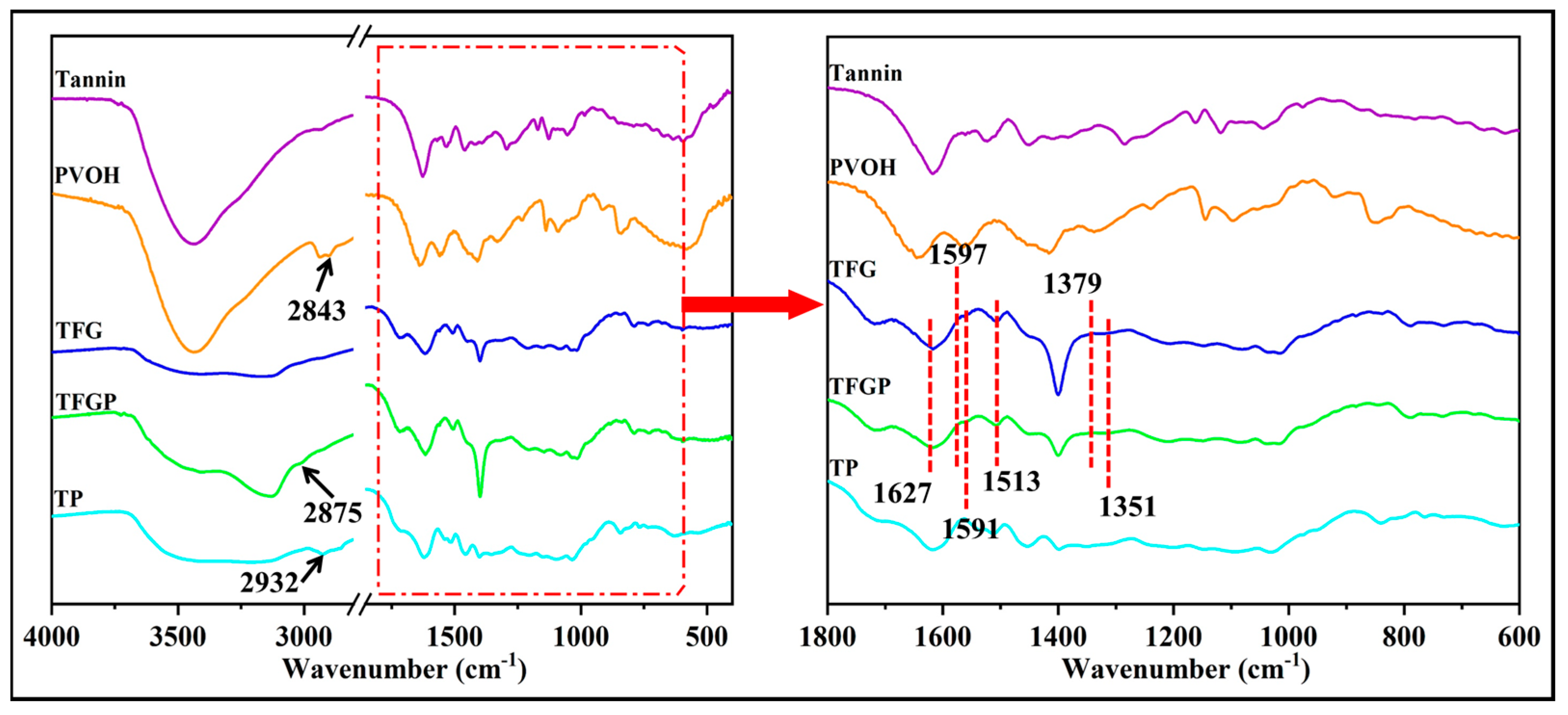
2.3. Characterizations
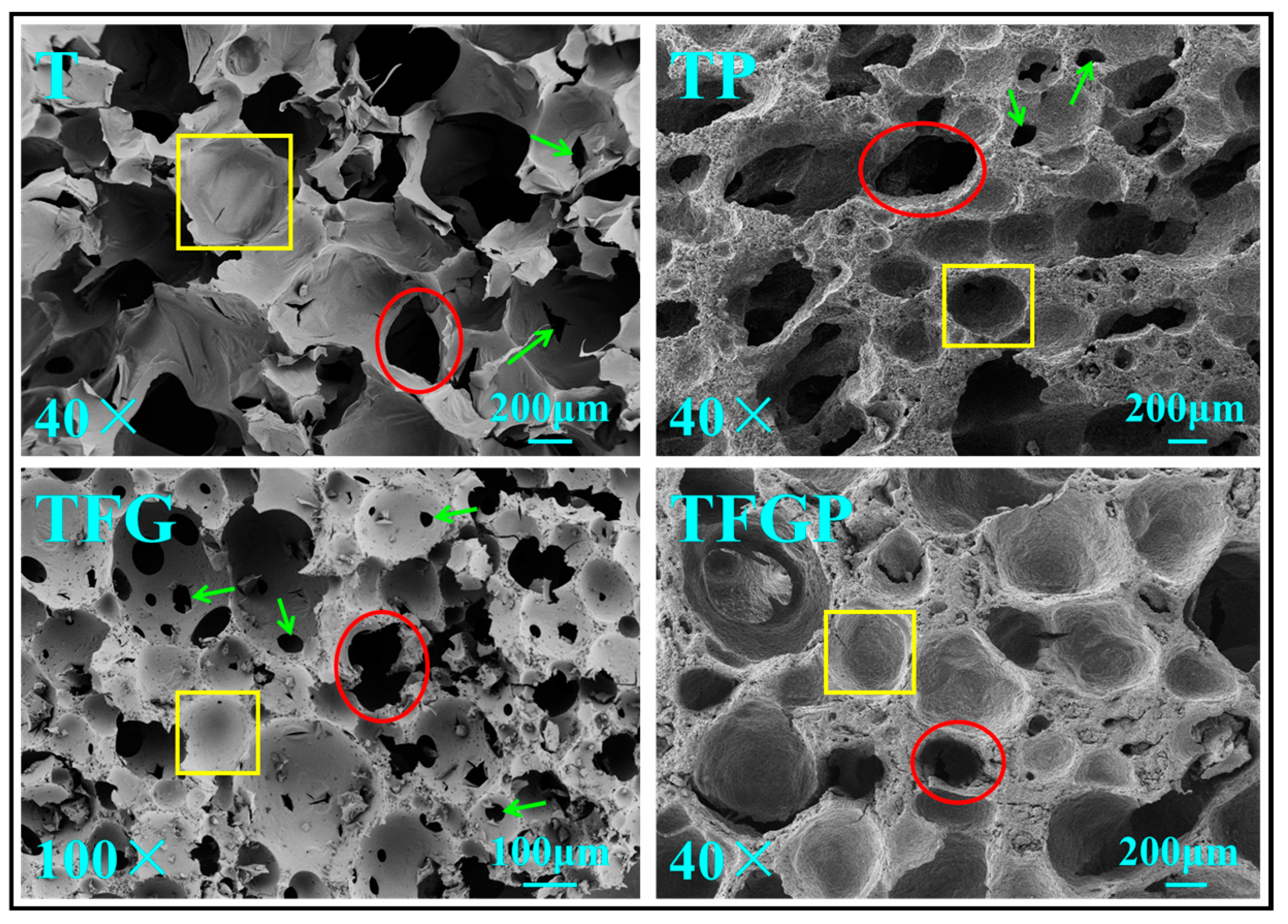
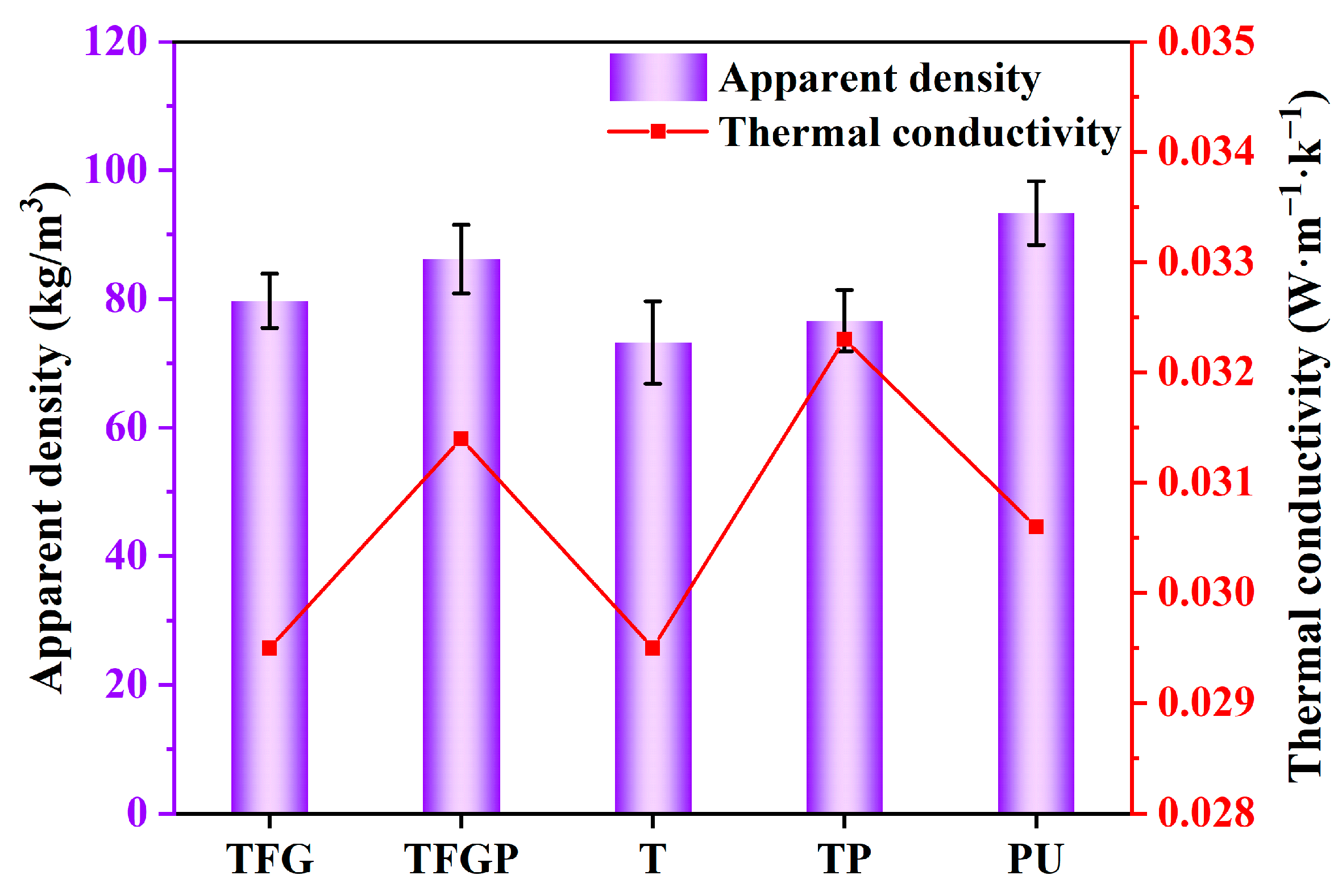
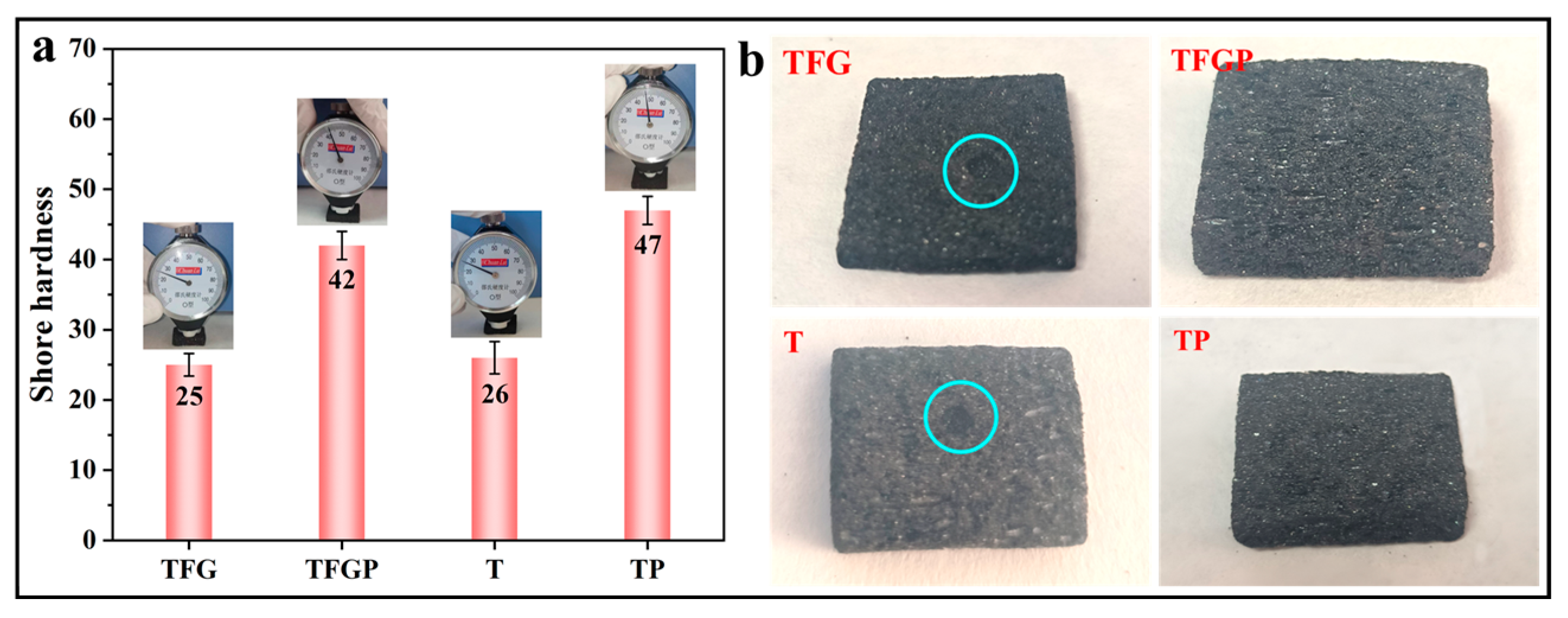
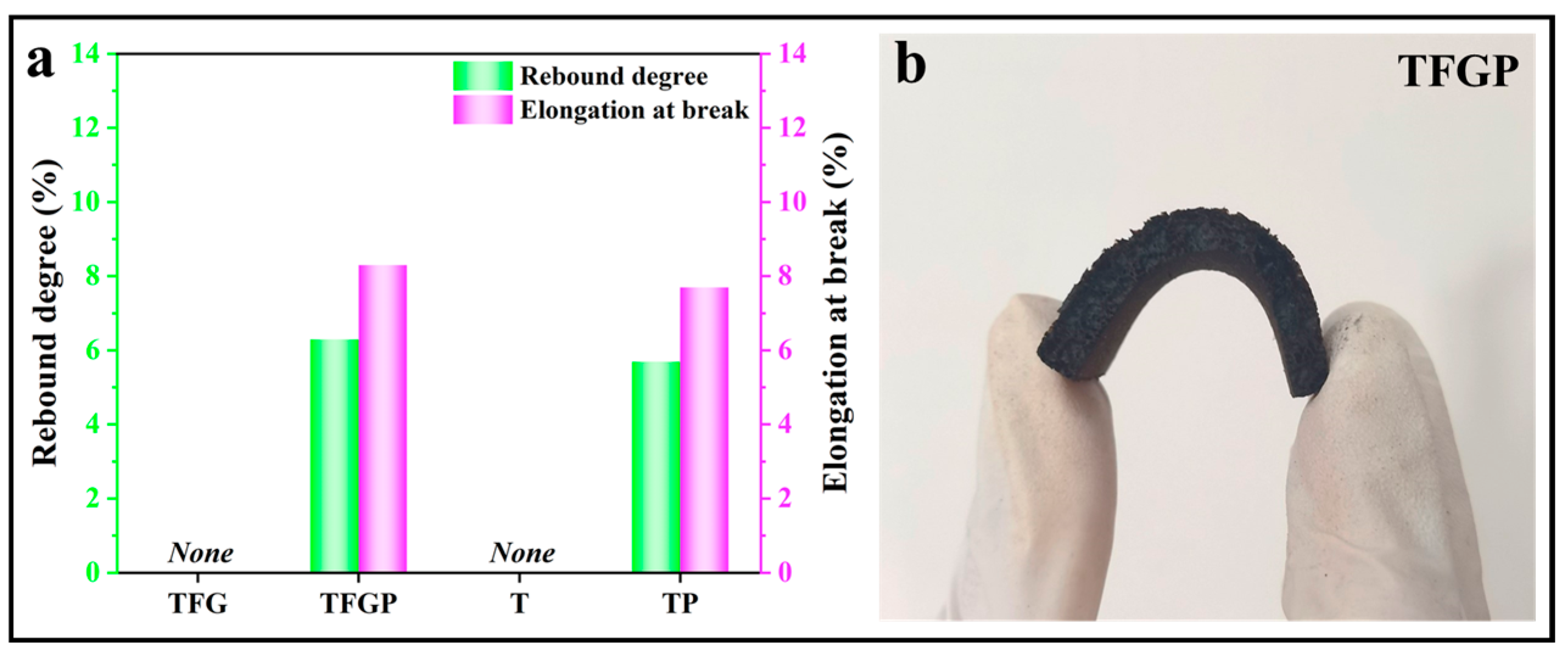
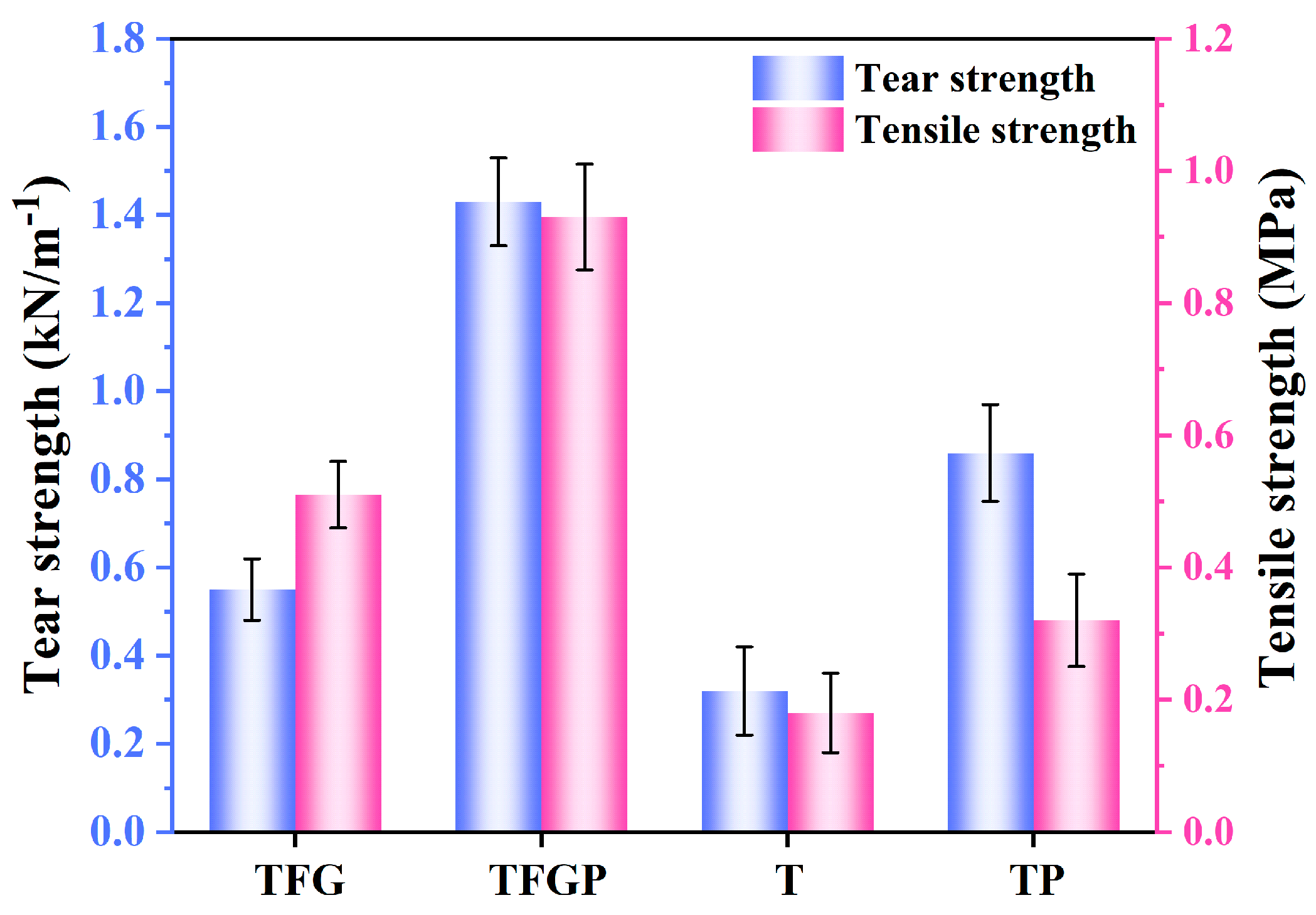
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: www.worldfootwear.com/news/european-footwear-production-drops-in-the-last-4-decades/8723.html (accessed on 1 July 2023).
- Bernardini, J.; Anguillesi, I.; Coltelli, M.B.; Cinelli, P.; Lazzeri, A. Optimizing the lignin based synthesis of flexible polyurethane foams employing reactive liquefying agents. Polym. Int. 2015, 64, 1235–1244. [Google Scholar] [CrossRef]
- Kohan, L.; Martins, C.R.; Duarte, L.O.; Pinheiro, L.; Baruque-Ramos, J. Panorama of natural fibers applied in Brazilian footwear: Materials and market. SN Appl. Sci. 2019, 1, 895. [Google Scholar] [CrossRef]
- Morita, J.; Ando, Y.; Komatsu, S.; Matsumura, K.; Okazaki, T.; Asano, Y.; Nakatani, M.; Tanaka, H. Mechanical Properties and Reliability of Parametrically Designed Architected Materials Using Urethane Elastomers. Polymers 2021, 13, 842. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Yu, P.; Yang, X.; Sun, Z.; Zhang, Y.; Wu, Y.; Jiang, C. Ultrasensitive and wide-range reduced graphene oxide/natural rubber foam sensors for multifunctional self-powered wireless wearable applications. Compos. Sci. Technol. 2022, 226, 109560. [Google Scholar] [CrossRef]
- Conti, T.M.; Catto, A.L.; Amico, S.C. Composite for insole shoe assembly based on polyvinyl acetate and polyester fabric waste from the footwear industry. Polym. Compos. 2022, 43, 7360–7371. [Google Scholar] [CrossRef]
- Li, N.; Yick, K.; Yu, A. Novel weft-knitted spacer structure with silicone tube and foam inlays for cushioning insoles. J. Ind. Text. 2022, 51, 6463S–6483S. [Google Scholar] [CrossRef]
- Aristri, M.A.; Lubis, M.A.R.; Yadav, S.M.; Antov, P.; Papadopoulos, A.N.; Pizzi, A.; Iswanto, A.H. Recent developments in lignin-and tannin-based non-isocyanate polyurethane resins for wood adhesives—A review. Appl. Sci. 2021, 11, 4242. [Google Scholar] [CrossRef]
- Chen, M.S.; Luo, J.; Shi, R.Q.; Zhang, J.Z.; Gao, Q.; Li, J. Improved adhesion performance of soy protein-based adhesives with a larch tannin-based resin. Polymers 2017, 9, 408. [Google Scholar] [CrossRef]
- Ghahri, S.; Chen, X.; Pizzi, A.; Hajihassani, R.; Papadopoulos, A.N. Natural tannins as new cross-linking materials for soy-based adhesives. Polymers 2021, 13, 595. [Google Scholar] [CrossRef]
- Esmaeili, N.; Salimi, A.; Zohuriaan-Mehr, M.J.; Vafayan, M.; Meyer, W. Bio-based thermosetting epoxy foam: Tannic acid valorization toward dye-decontaminating and thermo-protecting applications. J. Hazard. Mater. 2018, 357, 30–39. [Google Scholar] [CrossRef]
- Mitrus, M.; Moscicki, L. Extrusion-cooking of starch protective loose-fill foams. Chem. Eng. Res. Des. 2014, 92, 778–783. [Google Scholar] [CrossRef]
- Khundamri, N.; Aouf, C.; Fulcrand, H.; Dubreucq, E.; Tanrattanakul, V. Bio-based flexible epoxy foam synthesized from epoxidized soybean oil and epoxidized mangosteen tannin. Ind. Crops Prod. 2019, 128, 556–565. [Google Scholar] [CrossRef]
- Akrami, M.; Ghasemi, I.; Azizi, H.; Karrabi, M.; Seyedabadi, M. A new approach in compatibilization of the poly(lactic acid)/thermoplastic starch (PLA/TPS) blends. Carbohydr. Polym. 2016, 144, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Lagel, M.C.; Pizzi, A.; Basso, M.C.; Abdalla, S. Development and characterization of abrasive grinding wheels with a tanninfuranic resins matrix. Ind. Crops Prod. 2015, 65, 343–348. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, Y.; Essawy, H.; Feng, S.; Li, X.; Liao, J.; Zhou, X.; Zhang, J.; Xie, S. Formaldehyde Free Renewable Thermosetting Foam Based on Biomass Tannin with a Lignin Additive. J. Renew. Mater. 2022, 10, 3009–3024. [Google Scholar] [CrossRef]
- Chen, X.; Guigo, N.; Pizzi, A.; Sbirrazzuoli, N.; Li, B.; Fredon, E.; Gerardin, C. Ambient Temperature Self-Blowing Tannin-Humins Biofoams. Polymers 2020, 12, 2732. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Nicollin, A.; Pizzi, A.; Zhou, X.; Sauget, A.; Delmotte, L. Natural tannin–furanic thermosetting moulding plastics. RSC Adv. 2013, 3, 17732–17740. [Google Scholar] [CrossRef]
- Reyer, A.; Tondi, G.; Berger, R.J.F.; Petutschnigg, A.; Musso, M. Raman spectroscopic investigation of tannin-furanic rigid foams. Vib. Spectrosc. 2016, 84, 58–66. [Google Scholar] [CrossRef]
- Varila, T.; Romar, H.; Luukkonen, T.; Hilli, T.; Lassi, U. Characterization of lignin enforced tannin/furanic foams. Heliyon 2020, 6, e03228. [Google Scholar] [CrossRef]
- Eckardt, J.; Sepperer, T.; Cesprini, E.; Šket, P.; Tondi, G. Comparing Condensed and Hydrolysable Tannins for Mechanical Foaming of Furanic Foams: Synthesis and Characterization. Molecules 2023, 28, 2799. [Google Scholar] [CrossRef]
- Mubarok, M.; Azadeh, E.; Akong, F.O.; Dumarçay, S.; Gérardin, P.; Charbonnier, C.G. Effect of Tannins Addition on Thermal Stability of Furfurylated Wood. Polymers 2023, 15, 2044. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wu, Z.; Lei, H.; Xi, X.; Li, T.; Du, G. The Reaction between Furfuryl Alcohol and Model Compound of Protein. Polymers 2017, 37, 711. [Google Scholar] [CrossRef] [PubMed]
- Lacoste, C.; Basso, M.; Pizzi, A.; Laborie, M.; Garcia, D.; Celzard, A. Bioresourced pine tannin/furanic foams with glyoxal and glutaraldehyde. Ind. Crops Prod. 2013, 45, 401–405. [Google Scholar] [CrossRef]
- Merle, J.; Trinsoutrot, P.; Bouhtoury, F.C. Optimization of the formulation for the synthesis of bio-based foams. Eur. Polym. J. 2016, 84, 577–588. [Google Scholar] [CrossRef]
- Zhou, W.; Zuo, J. Mechanical, thermal and electrical properties of epoxy modified with a reactive hydroxyl-terminated polystyrene-butadiene liquid rubber. J. Reinf. Plast. Compos. 2013, 32, 1359–1369. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, F.; Liu, Y.; Leng, J. Design and Synthesis of Shape Memory Phenol-Formaldehyde with Good Irradiation Resistance, Thermal, and Mechanical Properties. ACS Appl. Polym. Mater. 2022, 4, 5789–5799. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Guo, H. Research Progress of Polyvinyl Alcohol Water-Resistant Film Materials. Membranes 2022, 12, 347. [Google Scholar] [CrossRef]
- Li, Y.; Deng, J.; Zhang, J. Porous poly(vinyl formal) foam prepared using poly(vinyl alcohol) of low degree of polymerization. Polym. Int. 2018, 67, 1438–1444. [Google Scholar] [CrossRef]
- Apicella, A.; Barbato, A.; Garofalo, E.; Incarnato, L.; Scarfato, P. Effect of PVOH/PLA + Wax Coatings on Physical and Functional Properties of Biodegradable Food Packaging Films. Polymers 2022, 14, 935. [Google Scholar] [CrossRef]
- Wang, M.; Bai, J.; Shao, K.; Tang, W.; Zhao, X.; Lin, D.; Huang, S.; Chen, C.; Ding, Z.; Ye, J. Poly(vinyl alcohol) Hydrogels: The Old and New Functional Materials. Int. J. Polym. Sci. 2021. [Google Scholar] [CrossRef]
- Zhou, X.; Li, B.; Xu, Y.; Essawy, H.; Wu, Z.; Du, G. Tannin-furanic resin foam reinforced with cellulose nanofibers (CNF). Ind. Crops Prod. 2019, 134, 107–112. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, W.; Li, Y.; Tao, W. Experimental study of the thermal conductivity of polyurethane foams. Appl. Therm. Eng. 2017, 115, 528–538. [Google Scholar] [CrossRef]
- Baltich, J.; Maurer, C.; Nigg, B.M. Increased vertical impact forces and altered running mechanics with softer midsole shoes. PLoS ONE 2015, 10, e0125196. [Google Scholar] [CrossRef] [PubMed]
- Anggoro, P.W.; Bawono, B.; Jamari, J.; Tauviqirrahman, M.; Bayuseno, A.P. Advanced design and manufacturing of custom orthotics insoles based on hybrid Taguchi-response surface method. Heliyon 2021, 7, e06481. [Google Scholar] [CrossRef] [PubMed]


| Type | Tannin/g | Distilled Water/g | Furfuryl Alcohol/g | Glyoxal/g | Ether/g | Tween 80/g | Guar Gum/g | pTSA/g | PVOH/g |
|---|---|---|---|---|---|---|---|---|---|
| T | 20 | 10 | 0 | 0 | 0 | 4 | 2 | 0 | 0 |
| TP | 20 | 10 | 0 | 0 | 0 | 4 | 2 | 0 | 15 |
| TFG | 30 | 0 | 20 | 10 | 2 | 4 | 2 | 15 | 0 |
| TFGP | 30 | 0 | 20 | 10 | 2 | 4 | 2 | 15 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, Z.; Liu, B.; Essawy, H.; Huang, Z.; Tang, J.; Miao, Z.; Chen, F.; Zhang, J. Preparation and Characterization of Biomass Tannin-Based Flexible Foam Insoles for Athletes. Polymers 2023, 15, 3480. https://doi.org/10.3390/polym15163480
Zuo Z, Liu B, Essawy H, Huang Z, Tang J, Miao Z, Chen F, Zhang J. Preparation and Characterization of Biomass Tannin-Based Flexible Foam Insoles for Athletes. Polymers. 2023; 15(16):3480. https://doi.org/10.3390/polym15163480
Chicago/Turabian StyleZuo, Zhikai, Bowen Liu, Hisham Essawy, Zhigang Huang, Jun Tang, Zhe Miao, Fei Chen, and Jun Zhang. 2023. "Preparation and Characterization of Biomass Tannin-Based Flexible Foam Insoles for Athletes" Polymers 15, no. 16: 3480. https://doi.org/10.3390/polym15163480