Parts per Million of Propanol and Arsine as Responsible for the Poisoning of the Propylene Polymerization Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of the Pre-Catalyst
2.3. Polymerization Procedure for Propylene
2.4. Computational Details
Interaction of Propanol and Arsine with the Active Site of Ti (Inhibitor–Ti Interaction)
2.5. Determination of the Fraction Soluble in Xylene of PP
2.6. Melt Flow Index (MFI)
3. Results
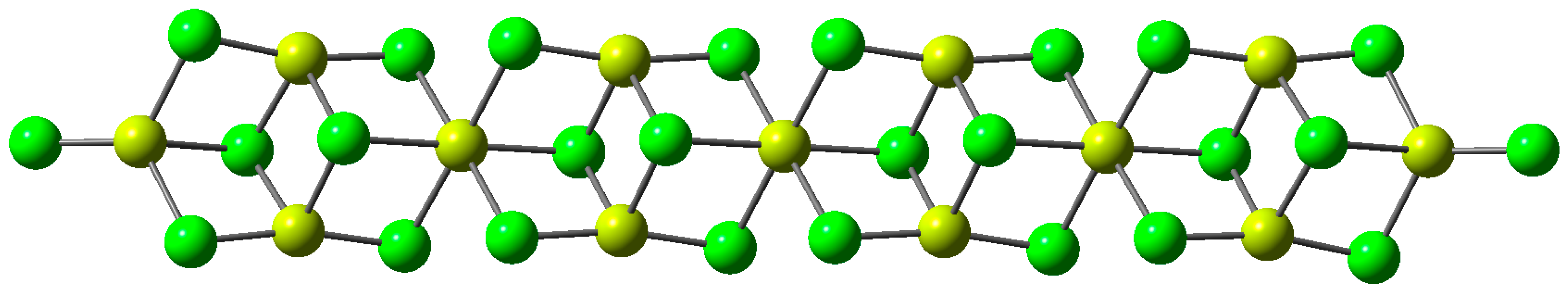
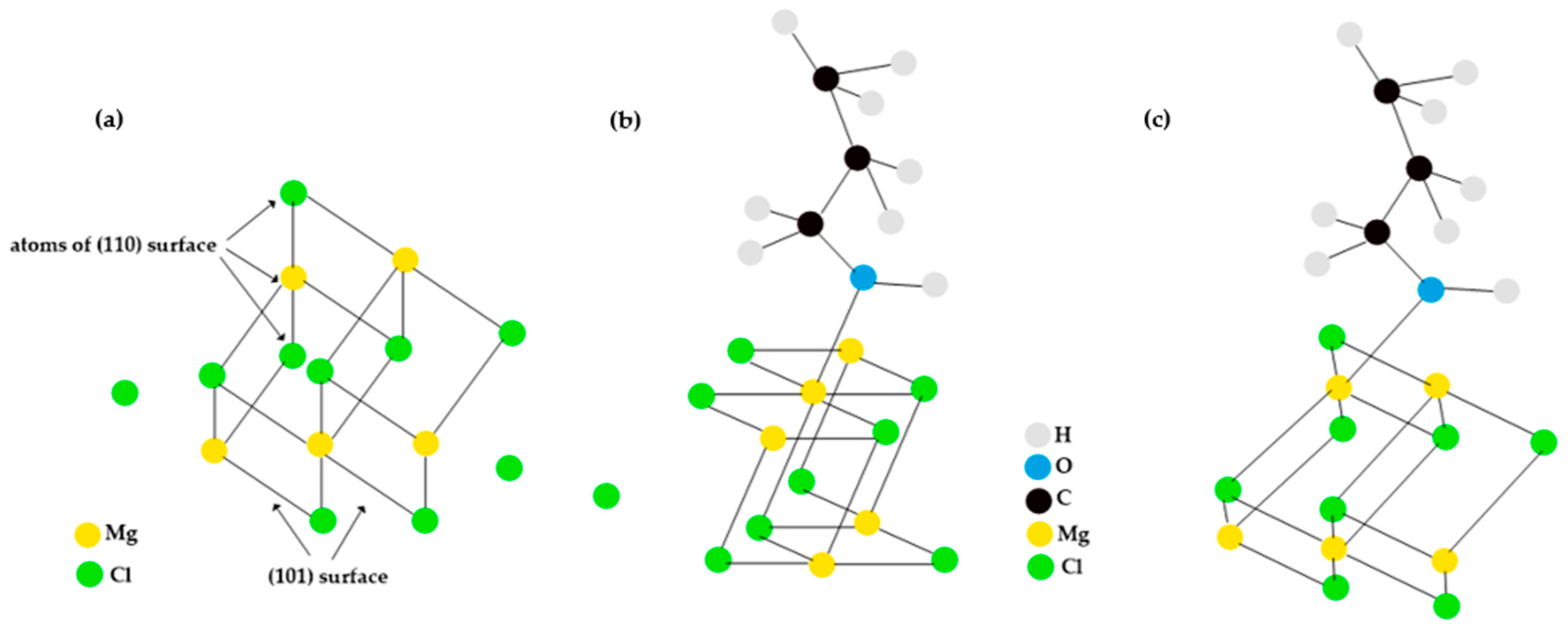
3.1. Interaction of Propanol with the Ziegler–Natta Catalyst
3.2. Adsorption of Propanol Molecules on TiCl4-MgCl2
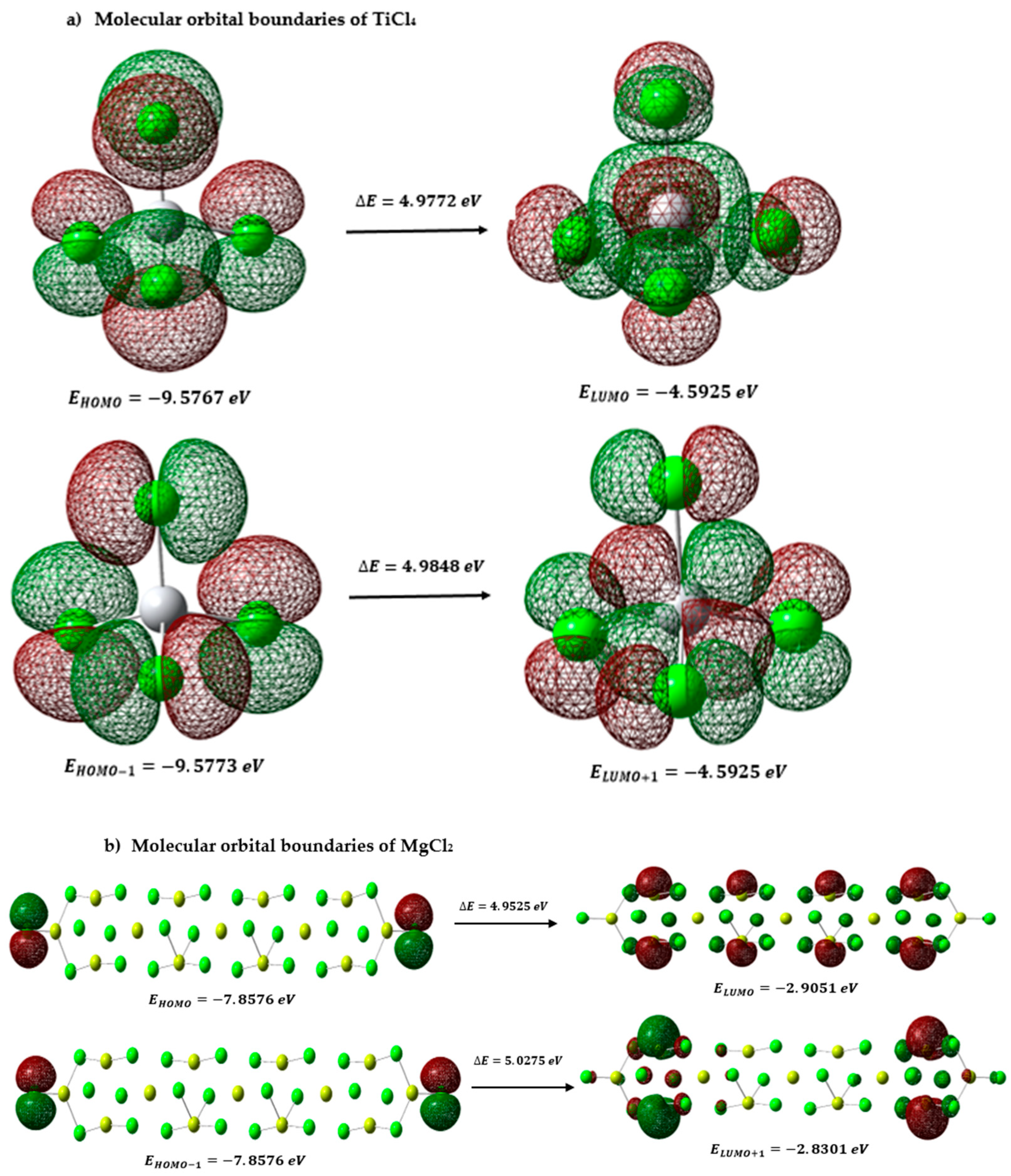
3.2.1. Frontier Molecular Orbitals and Global Descriptors of Propanol, Arsine, TiCl4, and MgCl2
3.2.2. Local Descriptors for Propanol, Arsine, TiCl4, and MgCl2
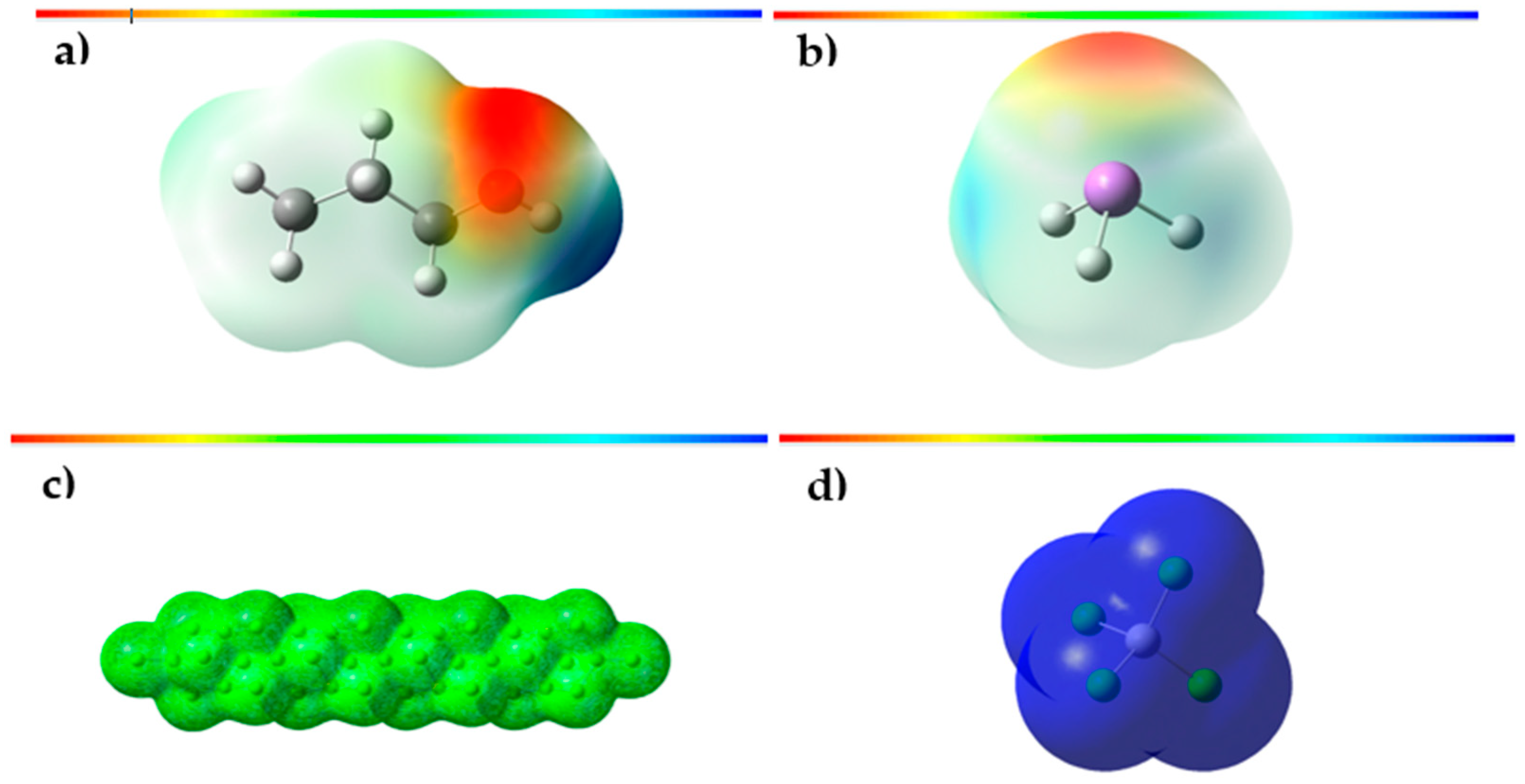
3.3. Molecular Electrostatic Potential
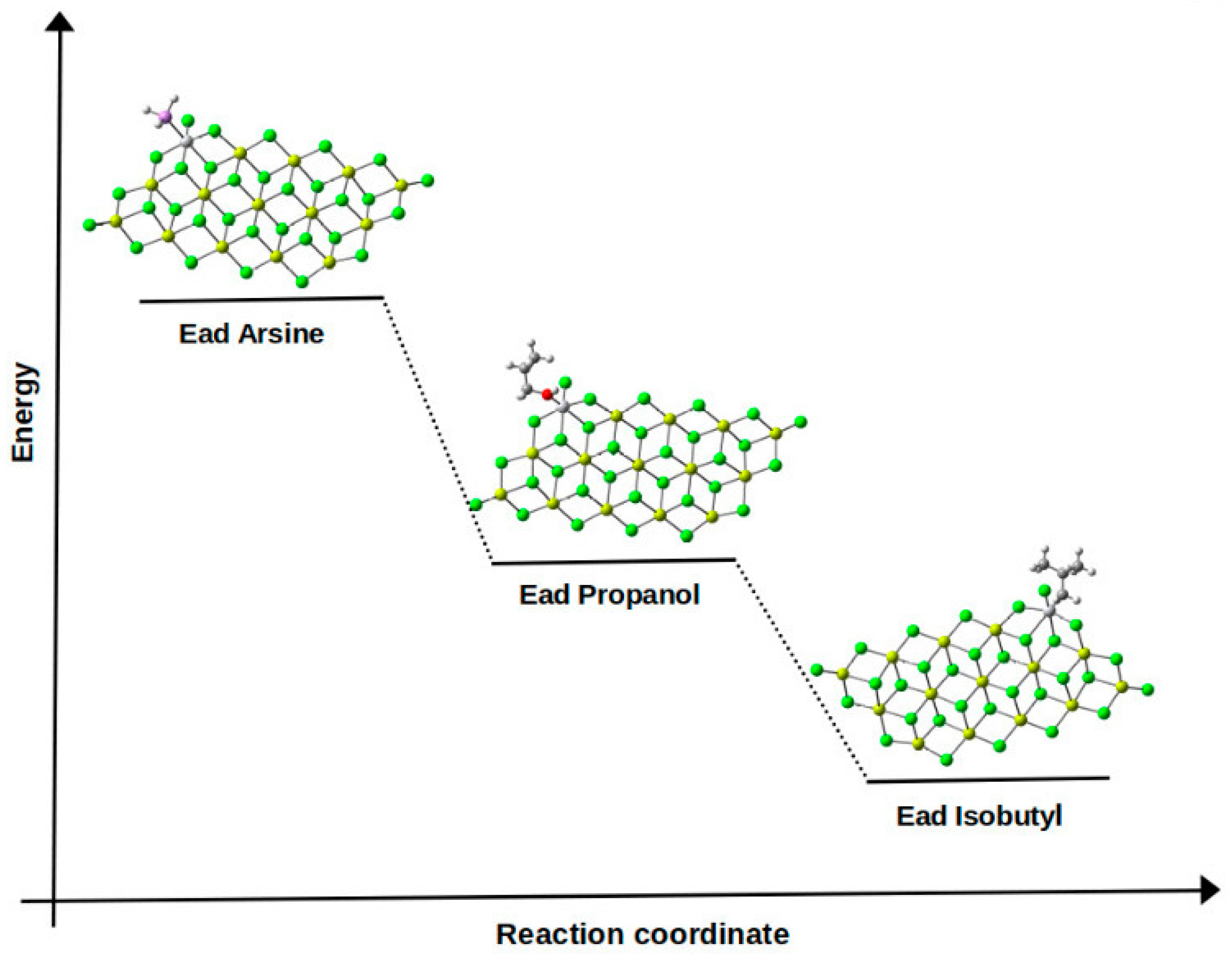
3.4. Proposed Mechanisms of the Inhibition Process of Propanol and Arsine to the Ziegler–Natta Catalyst
Interaction of Methanol, Arsine, and Isobutyl with the Ti-Active Center (Inhibitor–Ti Interaction)
3.5. Effects on the Melt Flow Index (MFI) and Mw of the PP
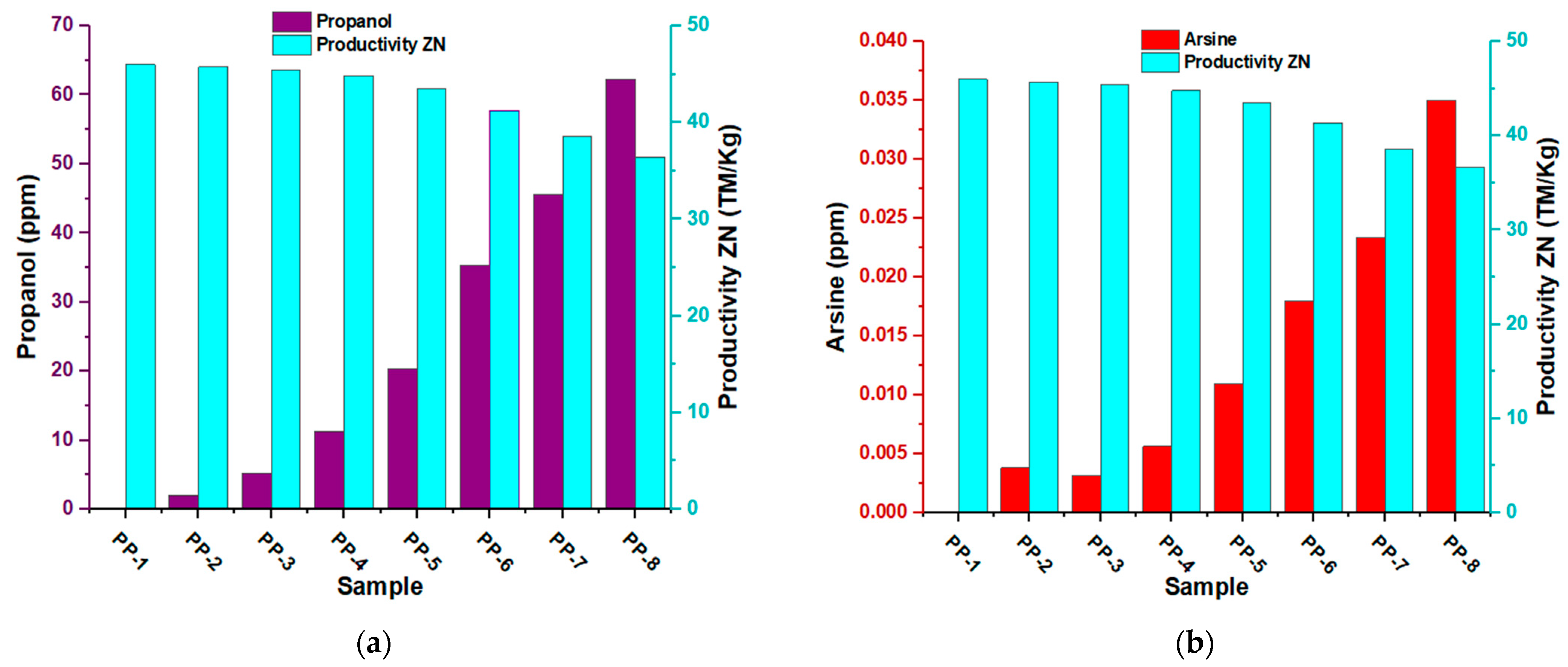
3.6. Effects of Inhibitors on Catalyst Productivity
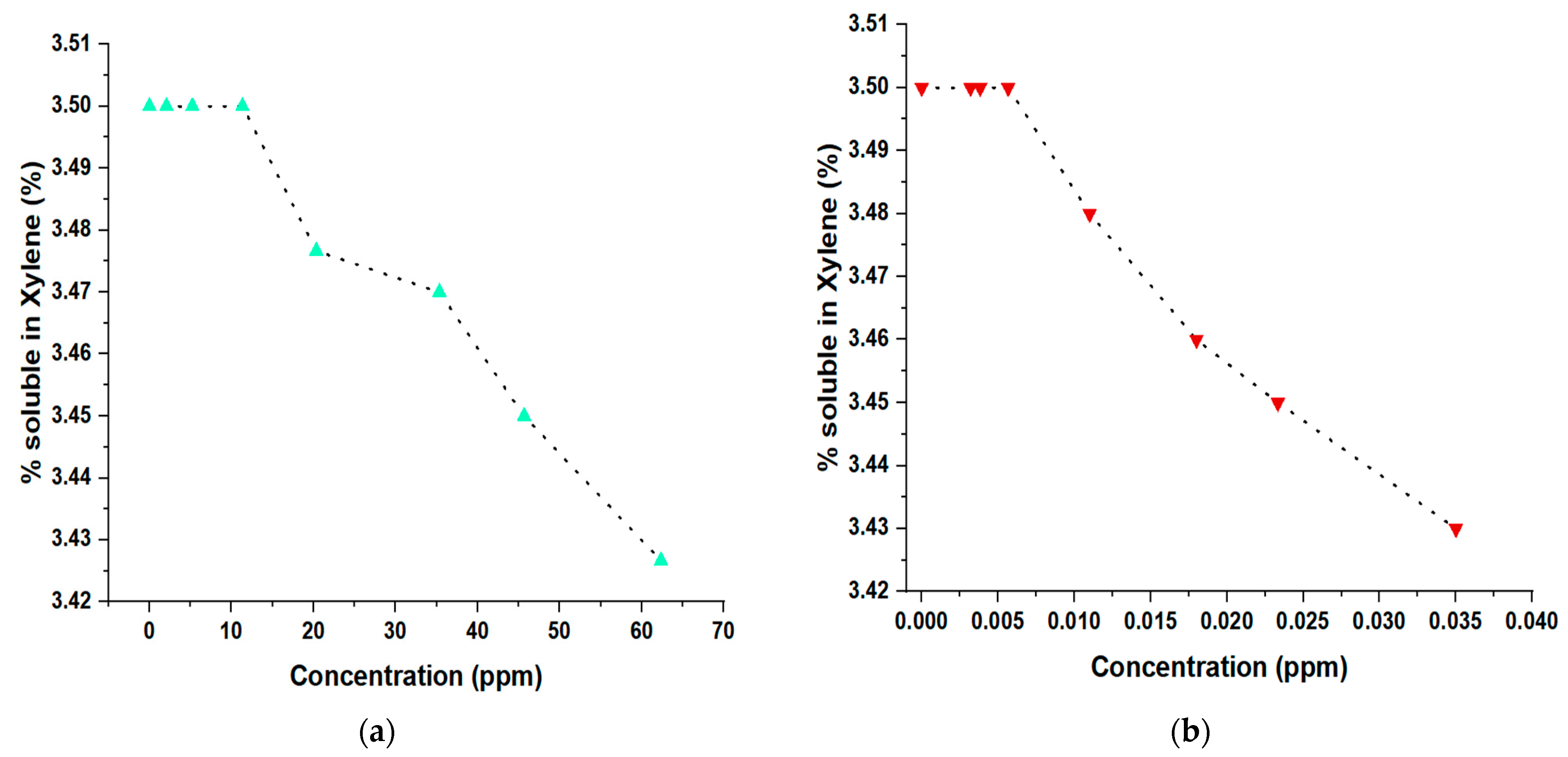
3.7. Determination of the Isotacticity of PP with Traces of Propanol and Arsine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shamiri, A.; Chakrabarti, M.H.; Jahan, S.; Hussain, M.A.; Kaminsky, W.; Aravind, P.V.; Yehye, W.A. The Influence of Ziegler-Natta and Metallocene Catalysts on Polyolefin Structure, Properties, and Processing Ability. Materials 2014, 7, 5069–5108. [Google Scholar] [CrossRef] [PubMed]
- Igin, K.J.; Rooney, J.J.; Stewart, C.D.; Green, M.L.; Mahtab, R. Mecanismo para la polimerización estereoespecífica de olefinas por catalizadores Ziegler—Natta. Revista de la Sociedad Química. Comun. Químicas 1978, 14, 604–606. [Google Scholar]
- Mulhaupt, R. Catálisis de polimerización catalítica y post polimerización cincuenta años después del descubrimiento de los catalizadores de Ziegler. Macromol. Chem. Phys. 2003, 204, 289–327. [Google Scholar]
- Tangjituabun, K.; Kim, S.Y.; Hiraoka, Y.; Taniike, T.; Terano, M.; Jongsomjit, B.; Praserthdam, P. Effects of various poisoning compounds on the activity and stereospecificity of heterogeneous Ziegler–Natta catalyst. Sci. Technol. Adv. Mater. 2008, 9, 024402. [Google Scholar] [CrossRef] [PubMed]
- Soga, K.; Sano, T.; Yamamoto, K.; Shiono, T. The role of additives on the im-provement of the isotacticity of polypropylene—A possible interpretation. Chem. Lett. 2006, 11, 425–428. [Google Scholar] [CrossRef]
- Quirk, R.P. Transition metal catalyzed polymerizations: Alkenes and dienes. In Proceedings of the Eleventh Midland Macromolecular Meeting, Midland, MI, USA, 17–21 August 1981; Quirk, R.P., Ed.; MMI Press by Harwood Academic Publishers: Chur, Switzerland, 1900. [Google Scholar]
- Sacchi, M.; Tritto, I.; Locatelli, P. The function of amines in conventional and supported Ziegler-Natta catalysts. Eur. Polym. J. 1988, 24, 137–140. [Google Scholar] [CrossRef]
- Tritto, I.; Sacchi, M.C.; Locatelli, P.; Zannoni, G. 13C NMR Investigation of the Interactions between Amines and Ziegler-Natta Catalysts for α-Olefin Polymerization. Macromolecules 1988, 21, 384–387. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Cano, H.; Aldas, M. Impact of Traces of Hydrogen Sulfide on the Efficiency of Ziegler–Natta Catalyst on the Final Properties of Polypropylene. Polymers 2022, 14, 3910. [Google Scholar] [CrossRef]
- Joaquin, H.-F.; Juan, L. Quantification of poisons for Ziegler Natta catalysts and effects on the production of polypropylene by gas chromatographic with simultaneous detection: Pulsed discharge helium ionization, mass spectrometry and flame ionization. J. Chromatogr. A 2019, 1614, 460736. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Castro-Suarez, J.R.; Toloza, C.A.T. Iron Oxide Powder as Responsible for the Generation of Industrial Polypropylene Waste and as a Co-Catalyst for the Pyrolysis of Non-Additive Resins. Int. J. Mol. Sci. 2022, 23, 11708. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Vivas-Reyes, R.; Toloza, C.A.T. Experimental Study of the Impact of Trace Amounts of Acetylene and Methylacetylene on the Synthesis, Mechanical and Thermal Properties of Polypropylene. Int. J. Mol. Sci. 2022, 23, 12148. [Google Scholar] [CrossRef] [PubMed]
- Kratochvíla, J.; Mejzlík, J. Retardation of the TiCl3·1/3 AlCl3/AlEt2Cl catalyzed propylene polymerization by allene and its relevance to the determination of the number of active centers. Die Makromol. Chem. 1987, 188, 1781–1794. [Google Scholar] [CrossRef]
- Variation in the Isospecific Active Sites of Internal Donor-Free MgCl2-Supported Ziegler Catalysts: Effect of External Electron Donors—Matsuoka—2001—Macromolecular Rapid Communications—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/1521-3927%2820010301%2922%3A5%3C326%3A%3AAID-MARC326%3E3.0.CO%3B2-G (accessed on 24 July 2023).
- Hernández-Fernández, J.; López-Martínez, J. Experimental study of the auto-catalytic effect of triethylaluminum and TiCl4 residuals at the onset of non-additive polypropylene degradation and their impact on thermo-oxidative degradation and pyrolysis. J. Anal. Appl. Pyrolysis 2021, 155, 105052. [Google Scholar] [CrossRef]
- Hernández-Fernández, J. Quantification of oxygenates, sulphides, thiols and permanent gases in propylene. A multiple linear regression model to predict the loss of efficiency in polypropylene production on an industrial scale. J. Chromatogr. A 2020, 1628, 461478. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernandez, J.; Rodríguez, E. Determination of phenolic antioxidants additives in industrial wastewater from polypropylene production using solid phase extraction with high-performance liquid chromatography. J. Chromatogr. A 2019, 1607, 460442. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, J.; Lopez-Martinez, J.; Barceló, D. Quantification and elimination of substituted synthetic phenols and volatile organic compounds in the wastewater treatment plant during the production of industrial scale polypropylene. Chemosphere 2021, 263, 128027. [Google Scholar] [CrossRef]
- Kaminsky, W. (Ed.) Metalorganic Catalysts for Synthesis and Polymerization, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar] [CrossRef]
- Kissin, Y.V. Olefin Polymers, Introduction. In Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed.; Mobil Chemical Company: Edison, NJ, USA, 2005; Volume 17, pp. 702–707. [Google Scholar] [CrossRef]
- Propene Polymerization in the Presence of MgCl2-Supported Ziegler-Natta Catalysts, 4. Effects of Lewis Bases on Polymer Stereochemistry. Available online: https://www.researchgate.net/publication/230354125_Propene_polymerization_in_the_presence_of_MgCl2-supported_Ziegler-Natta_catalysts_4_Effects_of_Lewis_bases_on_polymer_stereochemistry (accessed on 24 July 2023).
- Oro, L.A.; Carmona, D. “1 Rhodium.” The Handbook of Homogeneous Hydrogenation, Part I, Introduction, Organometallic Aspects and Mechanism of Homogeneous Hydrogenation. 2007. Available online: https://www.wiley.com/en-us/Handbook+of+Homogeneous+Hydrogenation%2C+3+Volume+Set-p-9783527311613 (accessed on 24 July 2023).
- Kissin, Y.V.; Ohnishi, R.; Konakazawa, T. Propylene Polymerization with Titanium-Based Ziegler-Natta Catalysts: Effects of Temperature and Modifiers on Molecular Weight, Molecular Weight Distribution and Stereospecificity. Macromol. Chem. Phys. 2004, 205, 284–301. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, B.; He, F.; Fu, Z.; Xu, J.; Fan, Z. Comparative Study on Kinetics of Ethylene and Propylene Polymerizations with Supported Ziegler–Natta Catalyst: Catalyst Fragmentation Promoted by Polymer Crystalline Lamellae. Polymers 2019, 11, 358. [Google Scholar] [CrossRef]
- Historical and Philosophical Remarks on Ziegler-Natta Catalysts a Discourse on Industrial Catalysis. Available online: https://www.researchgate.net/publication/282406813_Historical_and_philosophical_remarks_on_Ziegler-Natta_catalysts_a_discourse_on_industrial_catalysis (accessed on 24 July 2023).
- Puhakka, E.; Pakkanen, T.T.; Pakkanen, T.A. Theoretical Investigations on Heterogeneous Ziegler−Natta Catalyst Supports: Stability of the Electron Donors at Different Coordination Sites of MgCl2. J. Phys. Chem. A 1997, 101, 6063–6068. [Google Scholar] [CrossRef]
- Joaquin, H.-F.; Juan, L.-M. Autocatalytic influence of different levels of arsine on the thermal stability and pyrolysis of polypropylene. J. Anal. Appl. Pyrolysis 2022, 161, 105385. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Guerra, Y.; Puello-Polo, E.; Marquez, E. Effects of Different Concentrations of Arsine on the Synthesis and Final Properties of Polypropylene. Polymers 2022, 14, 3123. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, J. Quantification of arsine and phosphine in industrial atmospheric emissions in Spain and Colombia. Implementation of modified zeolites to reduce the environmental impact of emissions. Atmos. Pollut. Res. 2021, 12, 167–176. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Lopez-Martinez, J.; Barceló, D. Development and validation of a methodology for quantifying parts-per-billion levels of arsine and phosphine in nitrogen, hydrogen and liquefied petroleum gas using a variable pressure sampler coupled to gas chromatography-mass spectrometry. J. Chromatogr. A 2021, 1637, 461833. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, J.; Ortega-Toro, R.; Castro-Suarez, J.R. Theoretical–Experimental Study of the Action of Trace Amounts of Formaldehyde, Propionaldehyde, and Butyraldehyde as Inhibitors of the Ziegler–Natta Catalyst and the Synthesis of an Ethylene–Propylene Copolymer. Polymers 2023, 15, 1098. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Guerra, Y.; Espinosa, E. Development and Application of a Principal Component Analysis Model to Quantify the Green Ethylene Content in Virgin Impact Copolymer Resins During Their Synthesis on an Industrial Scale. J. Polym. Environ. 2022, 30, 4800–4808. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Puello-Polo, E.; Márquez, E. Furan as Impurity in Green Ethylene and Its Effects on the Productivity of Random Ethylene–Propylene Copolymer Synthesis and Its Thermal and Mechanical Properties. Polymers 2023, 15, 2264. [Google Scholar] [CrossRef]
- Alejandro, J.; Fernandez, H. Process of Extraction, Quantification and Recovery of Additives in Polypropylene with Natural Biodegradable Solvents and Use of the Polypropylene Resulting from the Multiple Extractions. U.S. Patent 17/630,296, 25 August 2022. [Google Scholar]
- Hernández Fernández, J.; Cano, H.; Guerra, Y.; Puello Polo, E.; Ríos-Rojas, J.F.; Vivas-Reyes, R.; Oviedo, J. Identification and Quantification of Microplastics in Effluents of Wastewater Treatment Plant by Differential Scanning Calorimetry (DSC). Sustainability 2022, 14, 4920. [Google Scholar] [CrossRef]
- D’Anna, V.; Norsic, S.; Gajan, D.; Sanders, K.; Pell, A.J.; Lesage, A.; Monteil, V.; Copéret, C.; Pintacuda, G.; Sautet, P. Structural Characterization of the EtOH-TiCl4-MgCl2 Ziegler-Natta Precatalyst. J. Phys. Chem. C 2016, 120, 18075–18087. [Google Scholar] [CrossRef]
- Bahri-Laleh, N. Interaction of different poisons with MgCl2/TiCl4 based Ziegler-Natta catalysts. Appl. Surf. Sci. 2016, 379, 395–401. [Google Scholar] [CrossRef]
- ISO 16152:2005; Plastics—Determination of Xylene-Soluble Matter in Polypropylene. Available online: https://www.iso.org/standard/32127.html (accessed on 24 July 2023).
- Bremner, T.; Rudin, A.; Cook, D.G. Melt flow index values and molecular weight distributions of commercial thermoplastics. J. Appl. Polym. Sci. 1990, 41, 1617–1627. [Google Scholar] [CrossRef]
- Shafiq-ur-Rehman; Ghafoor, S.; BiBi, S.; Kausar, A.; Ali, S.; Asim, S.; Mansha, A.; Shehzadi, S.A.; Jia, R. DFT and TDDFT Studies of Non-Fullerene Organometallic Based Acceptors for Organic Photovoltaics. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1676–1687. [Google Scholar] [CrossRef]
- Sundaram, S.; Vijayakumar, V.; Balasubramanian, V. Electronic and structure conformational analysis (HOMO-LUMO, MEP, NBO, ELF, LOL, AIM) of hydrogen bond binary liquid crystal mixture: DFT/TD-DFT approach. Comput. Theor. Chem. 2022, 1217, 113920. [Google Scholar] [CrossRef]
- Khemalapure, S.S.; Katti, V.S.; Hiremath, C.S.; Hiremath, S.M.; Basanagouda, M.; Radder, S.B. Spectroscopic (FT-IR, FT-Raman, NMR and UV-Vis), ELF, LOL, NBO, and Fukui function investigations on (5-bromo-benzofuran-3-yl)-acetic acid hydrazide (5BBAH): Experimental and theoretical approach. J. Mol. Struct. 2019, 1196, 280–290. [Google Scholar] [CrossRef]
- Bharathy, G.; Prasana, J.C.; Muthu, S.; Irfan, A.; Asif, F.B.; Saral, A.; Aayisha, S.; Devi, R.N. Evaluation of electronic and biological interactions between N-[4-(Ethylsulfamoyl)phenyl]acetamide and some polar liquids (IEFPCM solvation model) with Fukui function and molecular docking analysis. J. Mol. Liq. 2021, 340, 117271. [Google Scholar] [CrossRef]
- Pandey, A.K.; Baboo, V.; Mishra, V.N.; Singh, V.K.; Dwivedi, A. Comparative Study of Molecular Docking, Structural, Electronic, Vibrational Spectra and Fukui Function Studies of Thiadiazole Containing Schiff Base—A Complete Density Functional Study. Polycycl. Aromat. Compd. 2020, 42, 13–39. [Google Scholar] [CrossRef]
- Zülfikaroğlu, A.; Batı, H.; Dege, N. A theoretical and experimental study on isonitrosoacetophenone nicotinoyl hydrazone: Crystal structure, spectroscopic properties, NBO, NPA and NLMO analyses and the investigation of interaction with some transition metals. J. Mol. Struct. 2018, 1162, 125–139. [Google Scholar] [CrossRef]
- Shukla, B.K.; Yadava, U. DFT calculations on molecular structure, MEP and HOMO-LUMO study of 3-phenyl-1-(methyl-sulfonyl)-1H-pyrazolo[3,4-d]pyrimidine-4-amine. Mater. Today Proc. 2022, 49, 3056–3060. [Google Scholar] [CrossRef]
- Fradi, T.; Noureddine, O.; Ben Taheur, F.; Guergueb, M.; Nasri, S.; Amiri, N.; Almahri, A.; Roisnel, T.; Guerineau, V.; Issoui, N.; et al. New DMAP meso-arylporphyrin Magnesium(II) complex. Spectroscopic, Cyclic voltammetry and X-ray molecular structure characterization. DFT, DOS and MEP calculations and Antioxidant and Antifungal activities. J. Mol. Struct. 2021, 1236, 130299. [Google Scholar] [CrossRef]
- Taniike, T.; Terano, M. The Use of Donors to Increase the Isotacticity of Polypropylene. In Polyolefins: 50 years after Ziegler and Natta I; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2013; Volume 257, pp. 81–98. [Google Scholar] [CrossRef]
- Correa, A.; Piemontesi, F.; Morini, G.; Cavallo, L. Key elements in the structure and function relationship of the MgCl2/TiCl4/Lewis base Ziegler-Natta catalytic system. Macromolecules 2007, 40, 9181–9189. [Google Scholar] [CrossRef]
- Handbook of Polymer Science and Technology—Google Libros. Available online: https://books.google.es/books?hl=es&lr=&id=35PLEAAAQBAJ&oi=fnd&pg=PP1&dq=Handbook+of+Polymer+Science+and+Technology:+Synthesis+and+Properties&ots=sWBW6JFbvV&sig=Ku8akKkH3NjR0IYO1r7ttdOsyTk#v=onepage&q=Handbook%20of%20Polymer%20Science%20and%20Technology%3A%20Synthesis%20and%20Properties&f=false (accessed on 17 August 2023).
- Hernández-Fernández, J.; Rayón, E.; López, J.; Arrieta, M.P. Enhancing the Thermal Stability of Polypropylene by Blending with Low Amounts of Natural Antioxidants. Macromol. Mater. Eng. 2019, 304, 1900379. [Google Scholar] [CrossRef]







| Propanol (ppm) | MT of PP Produced | Productivity Ziegler–Natta (TM/kg) | MFI | Soluble in Xylene (%Xs) |
|---|---|---|---|---|
| 0 | 46 | 46 | 20 | 3.5 |
| 0 | 46 | 46 | 20 | 3.5 |
| 0 | 46 | 46 | 20 | 3.5 |
| 2.1 | 45.72 | 45.72 | 20 | 3.5 |
| 2 | 45.74 | 45.74 | 20 | 3.5 |
| 2.1 | 45.76 | 45.76 | 20 | 3.5 |
| 5.2 | 45.53 | 45.53 | 20 | 3.5 |
| 5.3 | 45.37 | 45.37 | 20 | 3.5 |
| 5.2 | 45.43 | 45.43 | 20 | 3.5 |
| 11 | 44.85 | 44.85 | 20 | 3.5 |
| 12 | 44.84 | 44.84 | 20 | 3.5 |
| 11 | 44.87 | 44.87 | 20 | 3.5 |
| 20 | 43.47 | 43.47 | 20.3 | 3.47 |
| 21 | 43.46 | 43.46 | 20.3 | 3.48 |
| 20 | 43.49 | 43.49 | 20.3 | 3.48 |
| 35 | 41.23 | 41.23 | 20.7 | 3.47 |
| 36 | 41.15 | 41.15 | 20.7 | 3.47 |
| 35 | 41.19 | 41.19 | 20.7 | 3.47 |
| 45 | 38.57 | 38.57 | 21 | 3.45 |
| 46 | 38.45 | 38.45 | 21 | 3.45 |
| 46 | 38.57 | 38.57 | 21 | 3.45 |
| 62 | 36.56 | 36.56 | 21.5 | 3.43 |
| 62 | 36.49 | 36.49 | 21.5 | 3.42 |
| 63 | 36.36 | 36.36 | 21.5 | 3.43 |
| Arsine (ppm) | MT of PP Produced | Productivity Ziegler–Natta (TM/kg) | MFI | Soluble in Xylene (%Xs) |
|---|---|---|---|---|
| 0 | 46 | 46 | 20 | 3.5 |
| 0 | 46 | 46 | 20 | 3.5 |
| 0 | 46 | 46 | 20 | 3.5 |
| 0.00095 | 45.68 | 45.68 | 20 | 3.5 |
| 0.00096 | 45.67 | 45.67 | 20 | 3.5 |
| 0.0096 | 45.69 | 45.69 | 20 | 3.5 |
| 0.0032 | 45.48 | 45.48 | 20 | 3.5 |
| 0.0033 | 45.47 | 45.47 | 20 | 3.5 |
| 0.0031 | 45.49 | 45.49 | 20 | 3.5 |
| 0.006 | 44.79 | 44.79 | 20 | 3.5 |
| 0.005 | 44.78 | 44.78 | 20 | 3.5 |
| 0.006 | 44.79 | 44.79 | 20 | 3.5 |
| 0.01 | 43.51 | 43.51 | 20.3 | 3.48 |
| 0.012 | 43.55 | 43.55 | 20.3 | 3.48 |
| 0.011 | 43.62 | 43.62 | 20.3 | 3.48 |
| 0.017 | 41.33 | 41.33 | 20.7 | 3.46 |
| 0.019 | 41.35 | 41.35 | 20.7 | 3.46 |
| 0.018 | 41.36 | 41.36 | 20.7 | 3.46 |
| 0.023 | 38.65 | 38.65 | 21 | 3.45 |
| 0.024 | 38.62 | 38.62 | 21 | 3.45 |
| 0.023 | 38.63 | 38.63 | 21 | 3.45 |
| 0.035 | 36.68 | 36.68 | 21.5 | 3.43 |
| 0.034 | 36.65 | 36.65 | 21.5 | 3.43 |
| 0.036 | 36.71 | 36.71 | 21.5 | 3.43 |
| Parameters | Energy Propanol | Energy Arsine |
|---|---|---|
| EHOMO-1 (eV) | −8.5354 | −10.1596 |
| EHOMO (eV) | −7.0964 | −7.5799 |
| ΔE (eV) | 9.0056 | 8.1356 |
| ELUMO (eV) | 1.9091 | 0.5557 |
| ELUMO+1 (eV) | 2.5565 | 0.5576 |
| η = ½ (ELUMO − EHOMO) (eV) | 4.5028 | 4.0678 |
| χ = −1/2 (ELUMO + EHOMO) (eV) | 2.5936 | 3.5121 |
| S = 1/η (eV) | 0.2221 | 0.2458 |
| µ = 1/2(ELUMO + EHOMO) (eV) | −2.5936 | −3.5121 |
| ω = µ2/2η (eV) | 0.7495 | 1.5162 |
| N = EHOMO(PR) − EHOMO(TCE) (eV) | 2.0645 | 1.581 |
| Parameters | Energy TiCl4 | Energy MgCl2 |
|---|---|---|
| EHOMO-1 (eV) | −95.773 | −78.576 |
| EHOMO (eV) | −95.767 | −78.576 |
| ΔE (eV) | 49.772 | 49.525 |
| ELUMO (eV) | −45.925 | −29.051 |
| ELUMO+1 (eV) | −45.925 | −28.301 |
| η = ½ (ELUMO − EHOMO) (eV) | 24.886 | 24.762 |
| χ = −1/2 (ELUMO + EHOMO) (eV) | 70.846 | 53.813 |
| S = 1/η (eV) | 0.4018 | 0.4038 |
| µ = ½ (ELUMO + EHOMO) (eV) | −70.846 | −53.813 |
| ω = µ2/2η (eV) | 100.843 | 51.161 |
| N = 1/ω (eV) | 0.0992 | 0.1954 |
| Number | f+ | f− | f0 | Δf |
|---|---|---|---|---|
| 1 | 0.0868 | 0.0019 | 0.0443 | 0.0849 |
| 2 | 0.0011 | 0.0001 | 0.0006 | 0.001 |
| 3 | 0.0011 | 0.0001 | 0.0006 | 0.001 |
| 4 | 0.0286 | 0 | 0.0143 | 0.0286 |
| 5 | 0.0229 | 0.0019 | 0.0124 | 0.021 |
| 6 | 0.0009 | 0.0102 | 0.0055 | −0.0093 |
| 7 | 0.0009 | 0.0102 | 0.0055 | −0.0093 |
| 8 | 0.2116 | 0.0317 | 0.1217 | 0.1798 |
| 9 | 0.0442 | 0.089 | 0.0666 | −0.0448 |
| 10 | 0.0443 | 0.089 | 0.0666 | −0.0447 |
| 11 | 0.4912 | 0.766 | 0.6286 | −0.2748 |
| 12 | 0.0666 | 0 | 0.0333 | 0.0666 |
| Number | f+ | f− | f0 | Δf |
|---|---|---|---|---|
| 1 | 0.6351 | 0.8507 | 0.7429 | −0.2156 |
| 2 | 0.0232 | 0.0565 | 0.0399 | −0.0334 |
| 3 | 0.2428 | 0.0565 | 0.1496 | 0.1862 |
| 4 | 0.099 | 0.0362 | 0.0676 | 0.0627 |
| Number | f+ | f− | f0 | Δf |
|---|---|---|---|---|
| 1 | 0.7969 | 0.0001 | 0.3985 | 0.7969 |
| 2 | 0.0507 | 0.3742 | 0.2124 | −0.3235 |
| 3 | 0.0504 | 0.164 | 0.1072 | −0.1136 |
| 4 | 0.0512 | 0.374 | 0.2126 | −0.3228 |
| 5 | 0.0508 | 0.0877 | 0.0693 | −0.0369 |
| Number | f+ | f− | f0 | Δf |
|---|---|---|---|---|
| 1 | 0.0807 | 0.0019 | 0.0413 | 0.0788 |
| 2 | 0.0166 | 0.0071 | 0.0119 | 0.0095 |
| 3 | 0.0581 | 0.0001 | 0.0291 | 0.058 |
| 4 | 0.0148 | 0 | 0.0074 | 0.0148 |
| 5 | 0.0174 | 0 | 0.0087 | 0.0174 |
| 6 | 0.0581 | 0 | 0.0291 | 0.0581 |
| 7 | 0.0139 | 0 | 0.007 | 0.0139 |
| 8 | 0.0139 | 0 | 0.007 | 0.0139 |
| 9 | 0.0807 | 0.0001 | 0.0404 | 0.0806 |
| 10 | 0.0174 | 0 | 0.0087 | 0.0174 |
| 11 | 0.0148 | 0 | 0.0074 | 0.0148 |
| 12 | 0.0166 | 0.0004 | 0.0085 | 0.0162 |
| 13 | 0.0027 | 0.9096 | 0.4561 | −0.9069 |
| 14 | 0.0123 | 0.0183 | 0.0153 | −0.0059 |
| 15 | 0.0095 | 0.0002 | 0.0049 | 0.0092 |
| 16 | 0.0068 | 0 | 0.0034 | 0.0068 |
| 17 | 0.0352 | 0.0006 | 0.0179 | 0.0346 |
| 18 | 0.008 | 0 | 0.004 | 0.008 |
| 19 | 0.0274 | 0 | 0.0137 | 0.0274 |
| 20 | 0.0087 | 0 | 0.0043 | 0.0087 |
| 21 | 0.008 | 0 | 0.004 | 0.008 |
| 22 | 0.0352 | 0 | 0.0176 | 0.0352 |
| 23 | 0.0068 | 0 | 0.0034 | 0.0068 |
| 24 | 0.0095 | 0 | 0.0047 | 0.0094 |
| 25 | 0.0123 | 0.001 | 0.0067 | 0.0113 |
| 26 | 0.0027 | 0.0507 | 0.0267 | −0.048 |
| 27 | 0.0087 | 0 | 0.0043 | 0.0087 |
| 28 | 0.0807 | 0.0019 | 0.0413 | 0.0788 |
| 29 | 0.0166 | 0.0071 | 0.0119 | 0.0095 |
| 30 | 0.0581 | 0.0001 | 0.0291 | 0.058 |
| 31 | 0.0148 | 0 | 0.0074 | 0.0148 |
| 32 | 0.0174 | 0 | 0.0087 | 0.0174 |
| 33 | 0.0581 | 0 | 0.029 | 0.0581 |
| 34 | 0.0139 | 0 | 0.007 | 0.0139 |
| 35 | 0.0139 | 0 | 0.007 | 0.0139 |
| 36 | 0.0807 | 0.0001 | 0.0404 | 0.0806 |
| 37 | 0.0174 | 0 | 0.0087 | 0.0174 |
| 38 | 0.0148 | 0 | 0.0074 | 0.0148 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Fernández, J.; González-Cuello, R.; Ortega-Toro, R. Parts per Million of Propanol and Arsine as Responsible for the Poisoning of the Propylene Polymerization Reaction. Polymers 2023, 15, 3619. https://doi.org/10.3390/polym15173619
Hernández-Fernández J, González-Cuello R, Ortega-Toro R. Parts per Million of Propanol and Arsine as Responsible for the Poisoning of the Propylene Polymerization Reaction. Polymers. 2023; 15(17):3619. https://doi.org/10.3390/polym15173619
Chicago/Turabian StyleHernández-Fernández, Joaquín, Rafael González-Cuello, and Rodrigo Ortega-Toro. 2023. "Parts per Million of Propanol and Arsine as Responsible for the Poisoning of the Propylene Polymerization Reaction" Polymers 15, no. 17: 3619. https://doi.org/10.3390/polym15173619







