Antimicrobial Wound Dressings based on Bacterial Cellulose and Independently Loaded with Nutmeg and Fir Needle Essential Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Bacterial Cellulose (BC)
2.2.2. Synthesis of the BC Membranes Loaded with Essential Oils
2.2.3. FTIR Analysis
2.2.4. SEM Analysis
2.2.5. Thermal Analysis
2.2.6. Water Absorption
2.2.7. Gas Chromatographic—Mass Spectrometric Evaluation
2.2.8. Biocompatibility
2.2.9. Antibacterial Evaluation
2.2.10. Statistical Analysis
3. Results
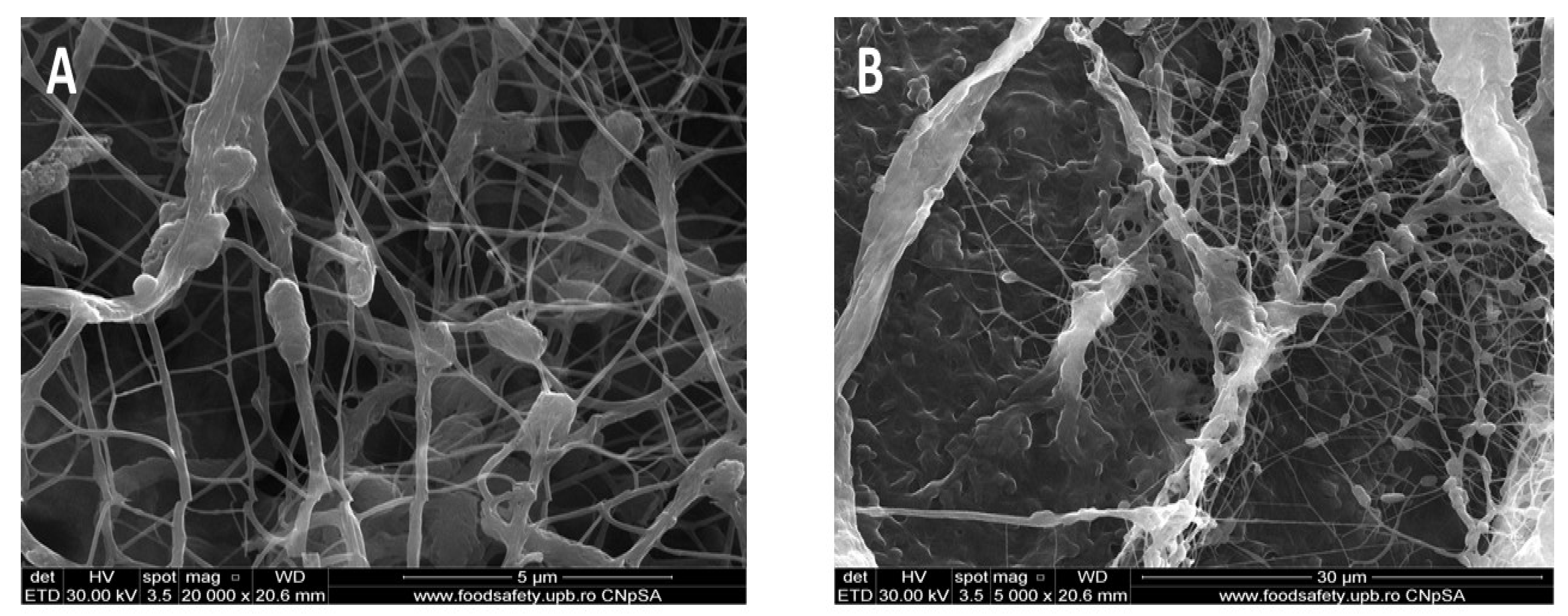
3.1. Scanning Electron Microscopy (SEM)
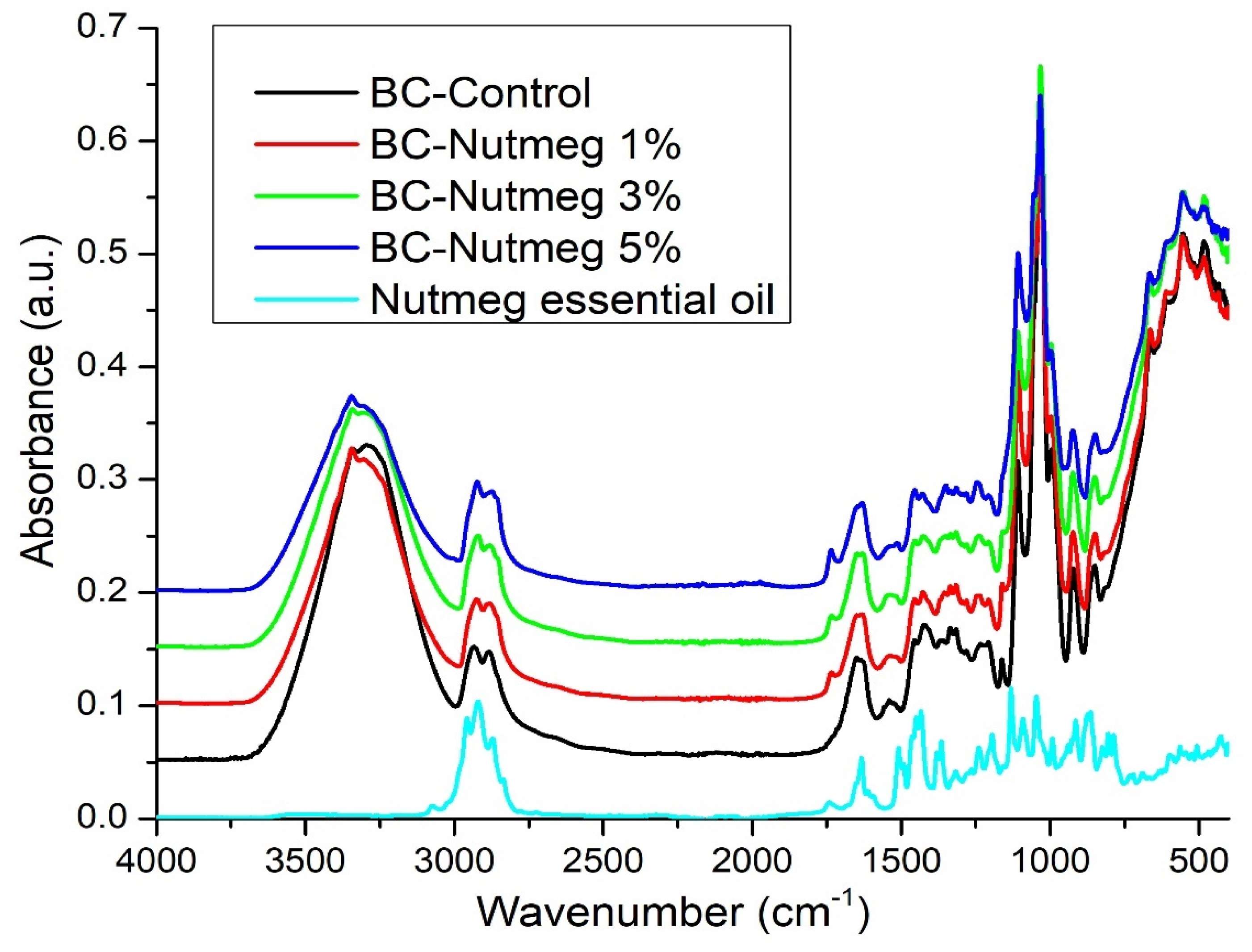
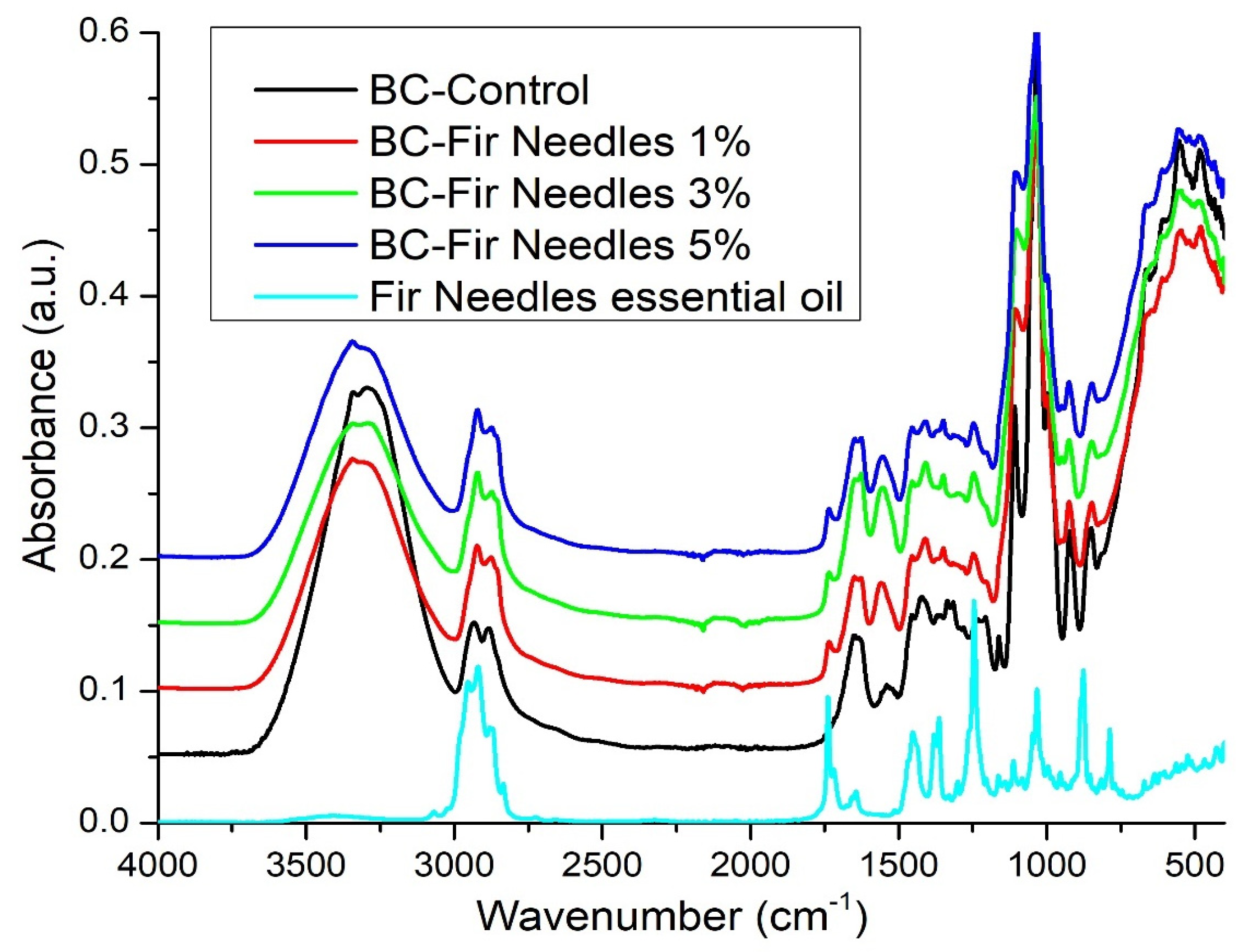
3.2. FT-IR Analysis
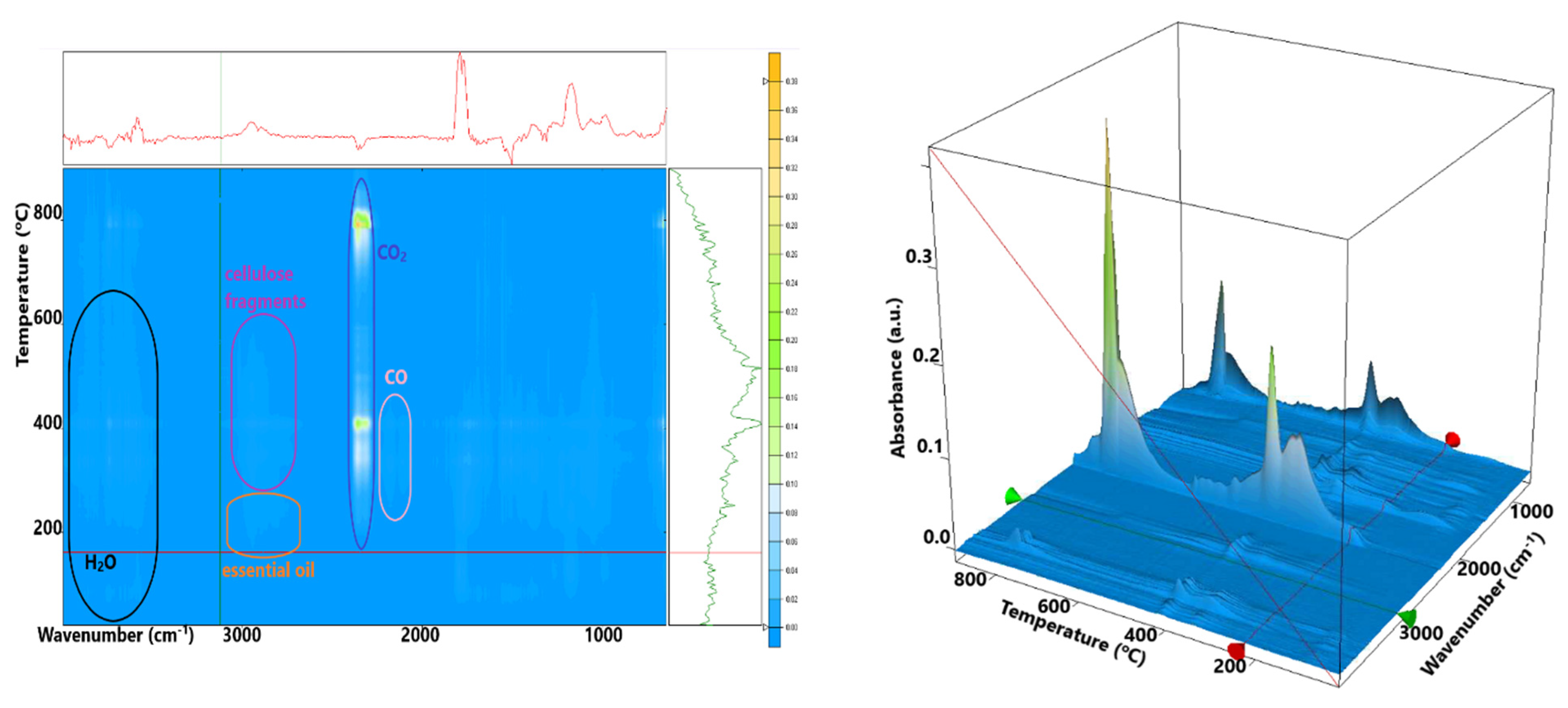
3.3. Thermal Analysis
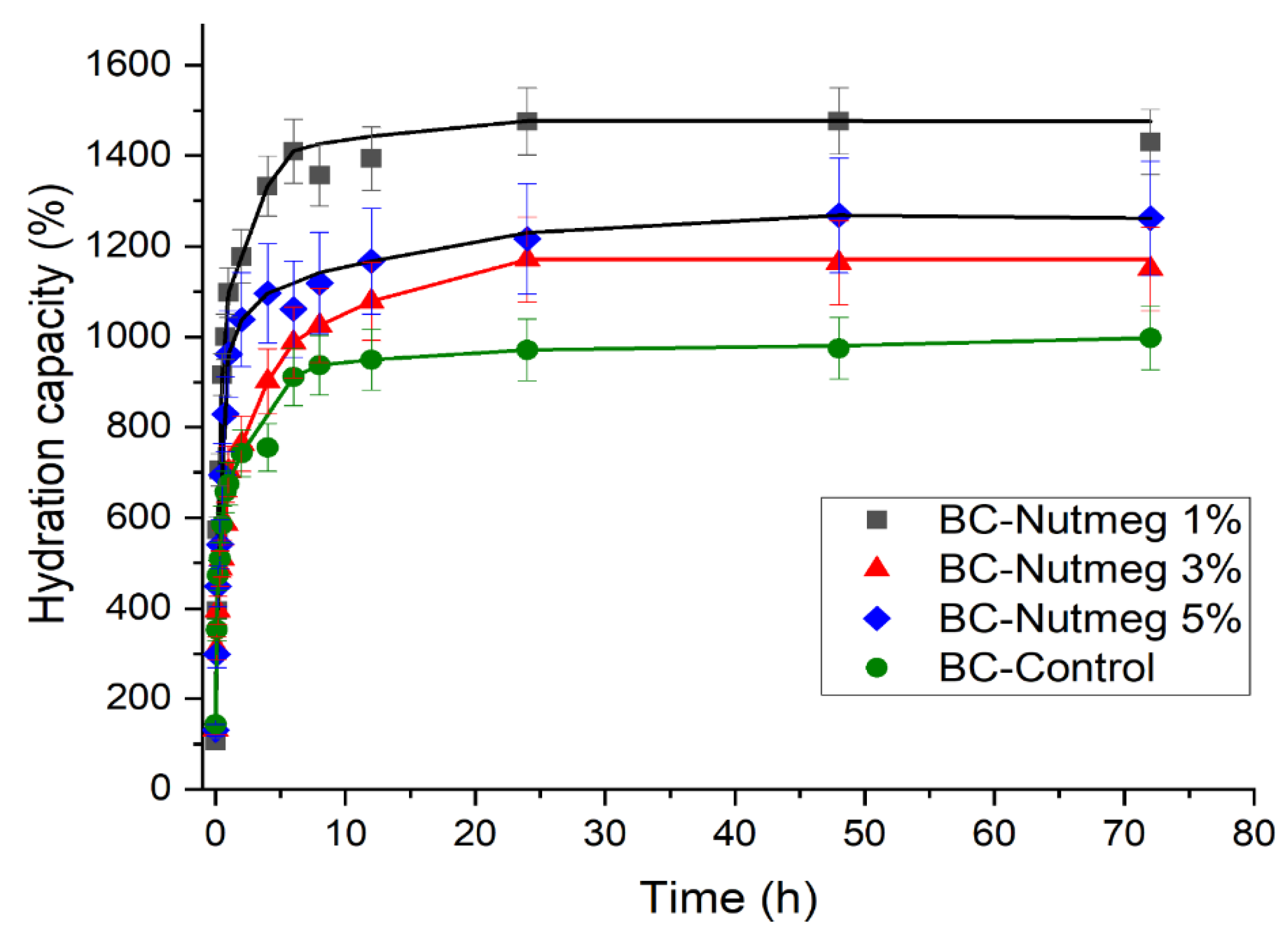
3.4. Kinetics of Water Absorption
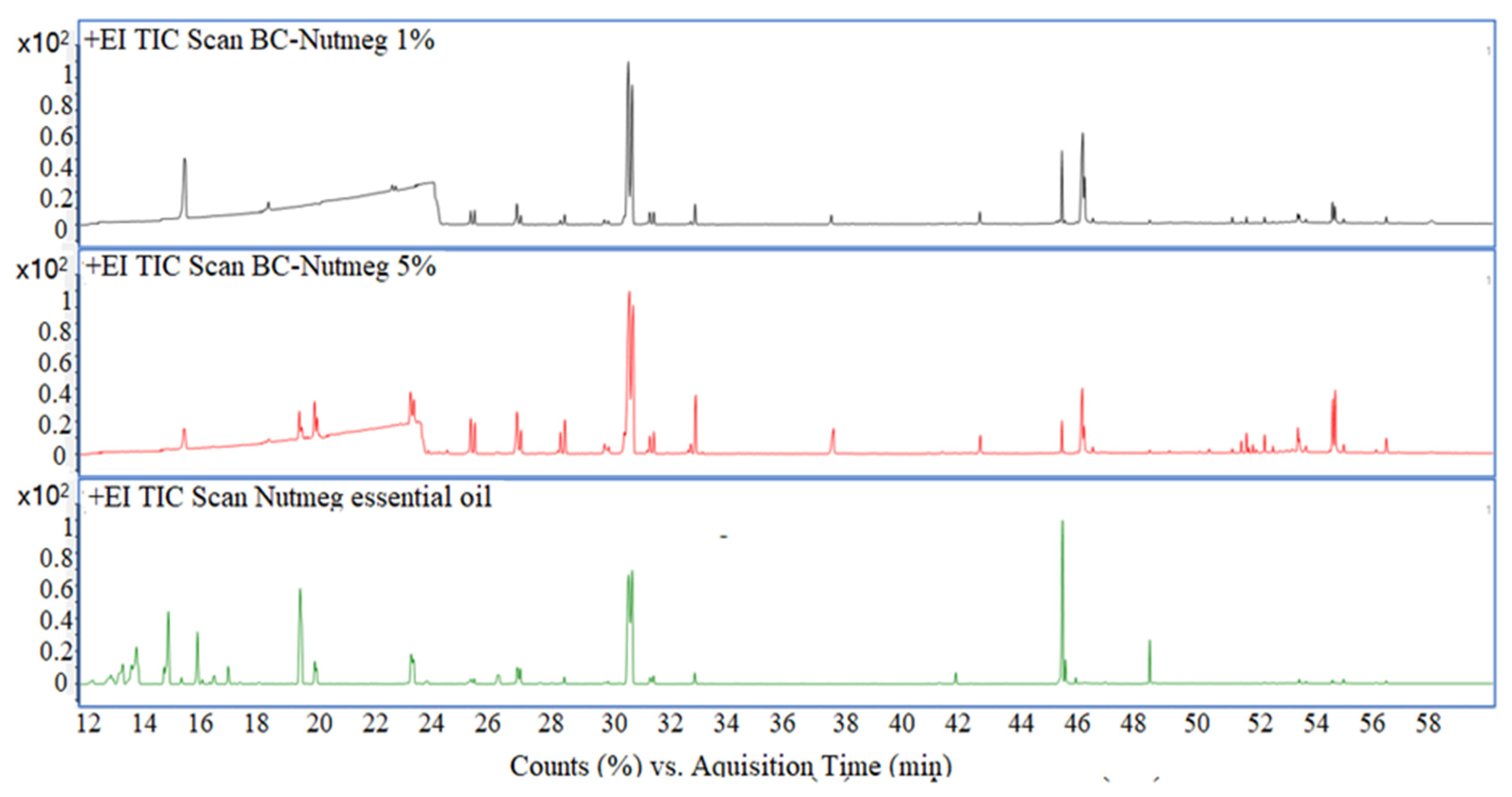
3.5. Gas Chromatographic—Mass Spectrometric Evaluation
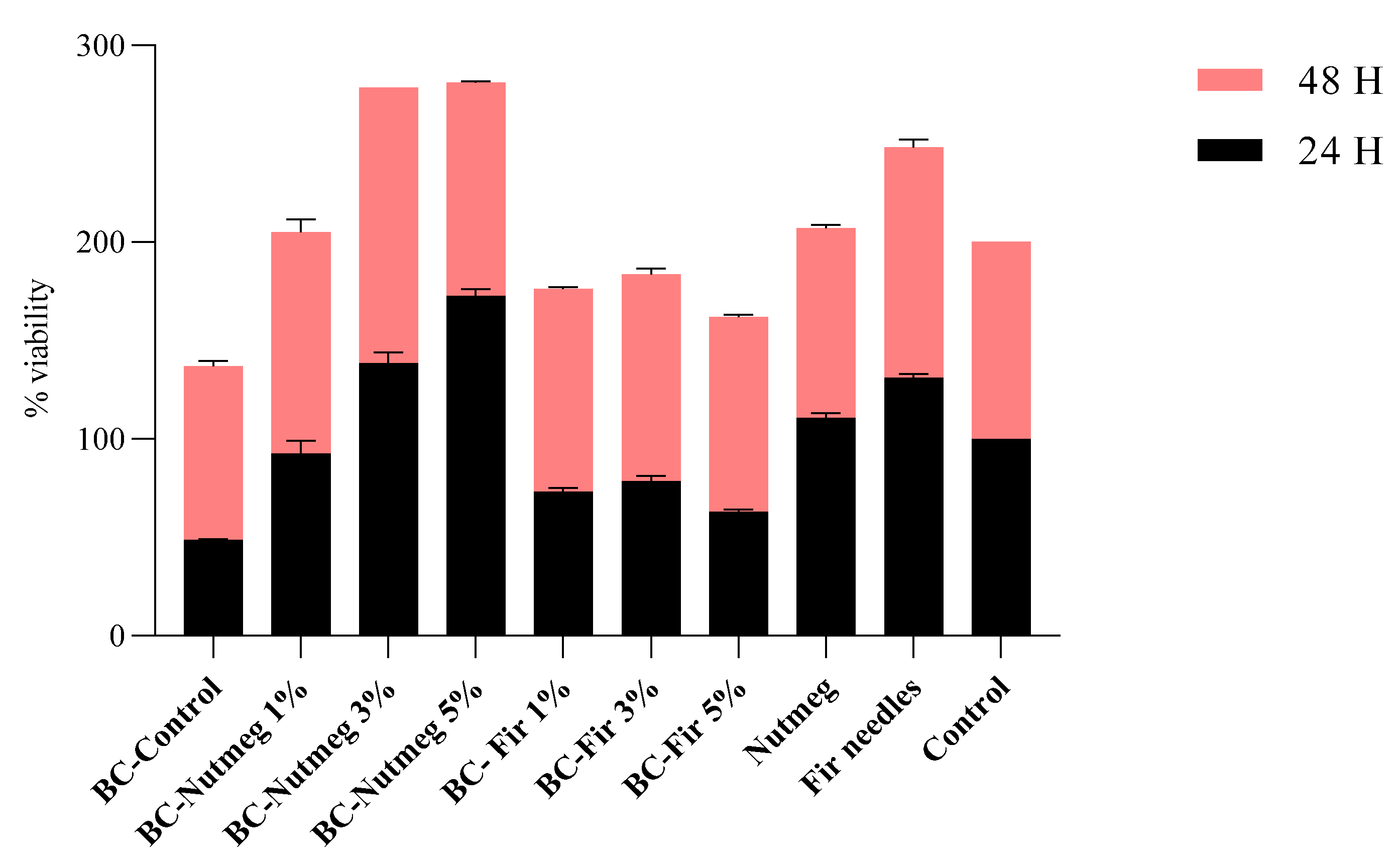
3.6. Biocompatibility
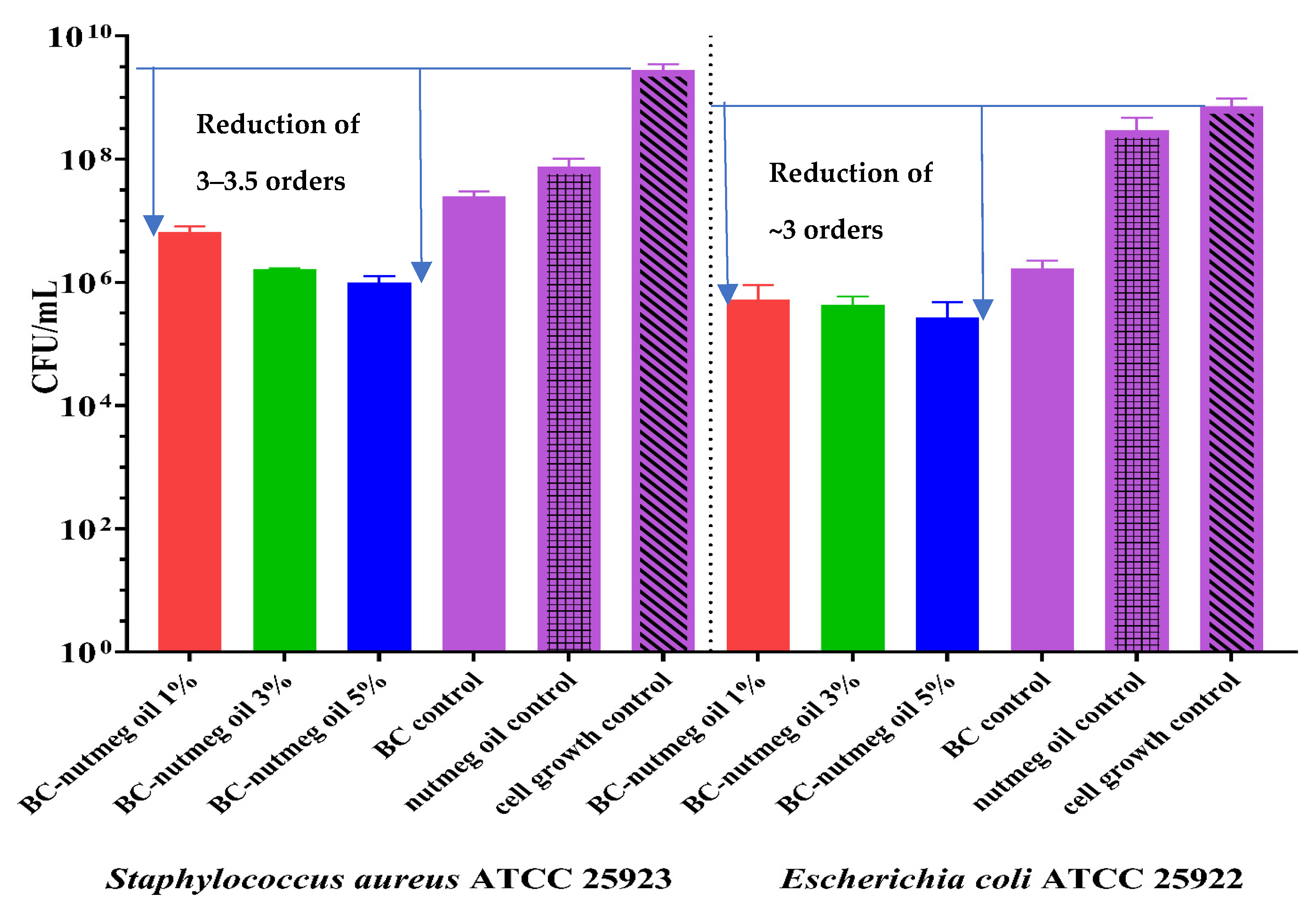
3.7. Antibacterial Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Cost Action
Conflicts of Interest
References
- Alminderej, F.M. Study of new cellulosic dressing with enhanced antibacterial performance grafted with a biopolymer of chitosan and myrrh polysaccharide extract. Arab. J. Chem. 2020, 13, 3672–3681. [Google Scholar] [CrossRef]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, S.; Zhong, L.; Wang, B.; Wang, H.; Hong, F. Zn(2+)-loaded TOBC nanofiber-reinforced biomimetic calcium alginate hydrogel for antibacterial wound dressing. Int. J. Biol. Macromol. 2020, 143, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of Appropriate Wound Dressing for Various Wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Lemnaru, G.M.; Motelica, L.; Trusca, R.D.; Ilie, C.I.; Ficai, D.; Oprea, O.; Stoica-Guzun, A.; Ficai, A.; Constantinescu, G.; Ditu, L.M. Bacterial Cellulose Membranes Loaded with Cinnamon Essential Oil. Univ. Politeh. Buchar. 2022, 84, 21–32. [Google Scholar]
- Radulescu, M.; Andronescu, E.; Dolete, G.; Popescu, R.C.; Fufa, O.; Chifiriuc, M.C.; Mogoanta, L.; Balseanu, T.A.; Mogosanu, G.D.; Grumezescu, A.M.; et al. Silver Nanocoatings for Reducing the Exogenous Microbial Colonization of Wound Dressings. Materials 2016, 9, 345. [Google Scholar] [CrossRef]
- Isopencu, G.; Deleanu, I.; Busuioc, C.; Oprea, O.; Surdu, V.A.; Bacalum, M.; Stoica, R.; Stoica Guzun, A. Bacterial Cellulose–Carboxymethylcellulose Composite Loaded with Turmeric Extract for Antimicrobial Wound Dressing Applications. Int. J. Mol. Sci. 2023, 24, 1719. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Arcentales-Vera, B.; Estrella-Nuñez, J.; Yánez-Vega, H.; Bucio, E. Antimicrobial Activity of Composites-Based on Biopolymers. Macromol 2022, 2, 258–283. [Google Scholar] [CrossRef]
- Jiang, B.; Zhong, S.J.; Yu, H.L.; Chen, P.F.; Li, B.Y.; Li, D.M.; Liu, C.H.; Feng, Z.B. Covalent and Noncovalent Complexation of Phosvitin and Gallic Acid: Effects on Protein Functionality and In Vitro Digestion Properties. J. Agric. Food Chem. 2022, 70, 11715–11726. [Google Scholar] [CrossRef]
- Fan, G.; Yu, Z.; Tang, J.; Dai, R.; Xu, Z. Preparation of gallic acid-hydroxypropyl-β-cyclodextrin inclusion compound and study on its effect mechanism on Escherichia coli in vitro. Mater. Express 2021, 11, 655–662. [Google Scholar] [CrossRef]
- Gao, S.; Zong, L.; Zhang, Y.H.; Zhang, Y.; Guo, X.Y.; Guo, G.H.; Zhao, L.X.; Ye, F.; Fu, Y. Antifungal pentachloronitrobenzene/hydroxypropyl-beta-cyclodextrin inclusion complex nanofibers by electrospun with no polymer: Fabrication and characterization. J. Clean. Prod. 2023, 413, 137499. [Google Scholar] [CrossRef]
- Sulaeva, I.; Hettegger, H.; Bergen, A.; Rohrer, C.; Kostic, M.; Konnerth, J.; Rosenau, T.; Potthast, A. Fabrication of bacterial cellulose-based wound dressings with improved performance by impregnation with alginate. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110619. [Google Scholar] [CrossRef]
- Busuioc, C.; Isopencu, G.; Banciu, A.; Banciu, D.D.; Oprea, O.; Mocanu, A.; Deleanu, I.; Zaulet, M.; Popescu, L.; Tanasuica, R.; et al. Bacterial Cellulose Hybrid Composites with Calcium Phosphate for Bone Tissue Regeneration. Int. J. Mol. Sci. 2022, 23, 16180. [Google Scholar] [CrossRef] [PubMed]
- Singhania, R.R.; Patel, A.K.; Tseng, Y.S.; Kumar, V.; Chen, C.W.; Haldar, D.; Saini, J.K.; Dong, C.D. Developments in bioprocess for bacterial cellulose production. Bioresour. Technol. 2022, 344, 126343. [Google Scholar] [CrossRef] [PubMed]
- Borchers, A.; Pieler, T. Programming pluripotent precursor cells derived from Xenopus embryos to generate specific tissues and organs. Genes 2010, 1, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Gea, S.; Reynolds, C.T.; Roohpour, N.; Wirjosentono, B.; Soykeabkaew, N.; Bilotti, E.; Peijs, T. Investigation into the structural, morphological, mechanical and thermal behaviour of bacterial cellulose after a two-step purification process. Bioresour. Technol. 2011, 102, 9105–9110. [Google Scholar] [CrossRef] [PubMed]
- Cubas, A.L.V.; Provin, A.P.; Dutra, A.R.A.; Mouro, C.; Gouveia, I.C. Advances in the Production of Biomaterials through Kombucha Using Food Waste: Concepts, Challenges, and Potential. Polymers 2023, 15, 1701. [Google Scholar] [CrossRef]
- El Fawal, G.F.; Abu-Serie, M.M.; Hassan, M.A.; Elnouby, M.S. Hydroxyethyl cellulose hydrogel for wound dressing: Fabrication, characterization and in vitro evaluation. Int. J. Biol. Macromol. 2018, 111, 649–659. [Google Scholar] [CrossRef]
- Keshk, S.M.A.S. Bacterial Cellulose Production and its Industrial Applications. J. Bioprocess. Biotech. 2014, 4, 150. [Google Scholar] [CrossRef]
- Marquele-Oliveira, F.; da Silva Barud, H.; Torres, E.C.; Machado, R.T.A.; Caetano, G.F.; Leite, M.N.; Frade, M.A.C.; Ribeiro, S.J.L.; Berretta, A.A. Development, characterization and pre-clinical trials of an innovative wound healing dressing based on propolis (EPP-AF(R))-containing self-microemulsifying formulation incorporated in biocellulose membranes. Int. J. Biol. Macromol. 2019, 136, 570–578. [Google Scholar] [CrossRef]
- Zheng, L.; Li, S.; Luo, J.; Wang, X. Latest Advances on Bacterial Cellulose-Based Antibacterial Materials as Wound Dressings. Front. Bioeng. Biotechnol. 2020, 8, 593768. [Google Scholar] [CrossRef]
- Cacicedo, M.L.; Pacheco, G.; Islan, G.A.; Alvarez, V.A.; Barud, H.S.; Castro, G.R. Chitosan-bacterial cellulose patch of ciprofloxacin for wound dressing: Preparation and characterization studies. Int. J. Biol. Macromol. 2020, 147, 1136–1145. [Google Scholar] [CrossRef]
- Thielmann, J.; Muranyi, P.; Kazman, P. Screening essential oils for their antimicrobial activities against the foodborne pathogenic bacteria Escherichia coli and Staphylococcus aureus. Heliyon 2019, 5, e01860. [Google Scholar] [CrossRef] [PubMed]
- Valdivieso-Ugarte, M.; Plaza-Diaz, J.; Gomez-Llorente, C.; Lucas Gómez, E.; Sabés-Alsina, M.; Gil, Á. In vitro examination of antibacterial and immunomodulatory activities of cinnamon, white thyme, and clove essential oils. J. Funct. Foods 2021, 81, 104436. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Kairytė, A.; Kremensas, A. Nutmeg filler as a natural compound for the production of polyurethane composite foams with antibacterial and anti-aging properties. Polym. Test. 2020, 86, 106479. [Google Scholar] [CrossRef]
- Gupta, A.D.; Rajpurohit, D. Antioxidant and Antimicrobial Activity of Nutmeg (Myristica fragrans). In Nuts and Seeds in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar] [CrossRef]
- Mickus, R.; Janciuke, G.; Raskevicius, V.; Mikalayeva, V.; Matulyte, I.; Marksa, M.; Maciunas, K.; Bernatoniene, J.; Skeberdis, V.A. The effect of nutmeg essential oil constituents on Novikoff hepatoma cell viability and communication through Cx43 gap junctions. Biomed. Pharmacother. 2021, 135, 111229. [Google Scholar] [CrossRef] [PubMed]
- Šojić, B.; Tomović, V.; Kocić-Tanackov, S.; Škaljac, S.; Ikonić, P.; Džinić, N.; Živković, N.; Jokanović, M.; Tasić, T.; Kravić, S. Effect of nutmeg (Myristica fragrans) essential oil on the oxidative and microbial stability of cooked sausage during refrigerated storage. Food Control. 2015, 54, 282–286. [Google Scholar] [CrossRef]
- Beckerman, B.; Persaud, H. Nutmeg overdose: Spice not so nice. Complement. Ther. Med. 2019, 46, 44–46. [Google Scholar] [CrossRef]
- Zubaid Ul, K.; Yatoo, G.N.; Wani, H.; Shah, S.A.; Zargar, M.I.; Rather, M.A.; Banday, J.A. Gas Chromatographic-Mass Spectrometric Analysis, Antibacterial, Antioxidant and Antiproliferative Activities of the Needle Essential Oil of Abies pindrow growing wild in Kashmir, India. Microb. Pathog. 2021, 158, 105013. [Google Scholar] [CrossRef]
- Hofmann, T.; Visi-Rajczi, E.; Albert, L. Antioxidant properties assessment of the cones of conifers through the combined evaluation of multiple antioxidant assays. Ind. Crops Prod. 2020, 145, 111935. [Google Scholar] [CrossRef]
- Lemnaru Popa, G.M.; Trusca, R.D.; Ilie, C.I.; Tiplea, R.E.; Ficai, D.; Oprea, O.; Stoica-Guzun, A.; Ficai, A.; Ditu, L.M. Antibacterial Activity of Bacterial Cellulose Loaded with Bacitracin and Amoxicillin: In Vitro Studies. Molecules 2020, 25, 4069. [Google Scholar] [CrossRef] [PubMed]
- Meftahi, A.; Nasrolahi, D.; Babaeipour, V.; Alibakhshi, S.; Shahbazi, S. Investigation of Nano Bacterial Cellulose Coated by Sesamum Oil for Wound Dressing Application. Procedia Mater. Sci. 2015, 11, 212–216. [Google Scholar] [CrossRef]
- Gupta, A.; Keddie, D.J.; Kannappan, V.; Gibson, H.; Khalil, I.R.; Kowalczuk, M.; Martin, C.; Shuai, X.; Radecka, I. Production and characterisation of bacterial cellulose hydrogels loaded with curcumin encapsulated in cyclodextrins as wound dressings. Eur. Polym. J. 2019, 118, 437–450. [Google Scholar] [CrossRef]
- Kwak, M.H.; Kim, J.E.; Go, J.; Koh, E.K.; Song, S.H.; Son, H.J.; Kim, H.S.; Yun, Y.H.; Jung, Y.J.; Hwang, D.Y. Bacterial cellulose membrane produced by Acetobacter sp. A10 for burn wound dressing applications. Carbohydr. Polym. 2015, 122, 387–398. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyai, P. Fabrication of microporous bacterial cellulose embedded with magnetite and hydroxyapatite nanocomposite scaffold for bone tissue engineering. Mater. Chem. Phys. 2019, 237, 121868. [Google Scholar] [CrossRef]
- Ahmed, J.; Gultekinoglu, M.; Edirisinghe, M. Bacterial cellulose micro-nano fibres for wound healing applications. Biotechnol. Adv. 2020, 41, 107549. [Google Scholar] [CrossRef]
- Zmejkoski, D.; Spasojevic, D.; Orlovska, I.; Kozyrovska, N.; Sokovic, M.; Glamoclija, J.; Dmitrovic, S.; Matovic, B.; Tasic, N.; Maksimovic, V.; et al. Bacterial cellulose-lignin composite hydrogel as a promising agent in chronic wound healing. Int. J. Biol. Macromol. 2018, 118, 494–503. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Gelatin/agar-based functional film integrated with Pickering emulsion of clove essential oil stabilized with nanocellulose for active packaging applications. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127220. [Google Scholar] [CrossRef]
- Singh, J.; Tan, N.C.S.; Mahadevaswamy, U.R.; Chanchareonsook, N.; Steele, T.W.J.; Lim, S. Bacterial cellulose adhesive composites for oral cavity applications. Carbohydr. Polym. 2021, 274, 118403. [Google Scholar] [CrossRef]
- Asanarong, O.; Minh Quan, V.; Boonrungsiman, S.; Sukyai, P. Bioactive wound dressing using bacterial cellulose loaded with papain composite: Morphology, loading/release and antibacterial properties. Eur. Polym. J. 2021, 143, 110224. [Google Scholar] [CrossRef]
- Ghozali, M.; Meliana, Y.; Chalid, M. Synthesis and characterization of bacterial cellulose by Acetobacter xylinum using liquid tapioca waste. Mater. Today Proc. 2021, 44, 2131–2134. [Google Scholar] [CrossRef]
- Dupuy, N.; Molinet, J.; Mehl, F.; Nanlohy, F.; Le Dréau, Y.; Kister, J. Chemometric analysis of mid infrared and gas chromatography data of Indonesian nutmeg essential oils. Ind. Crops Prod. 2013, 43, 596–601. [Google Scholar] [CrossRef]
- Kumar, V.; Nanda, M.; Verma, M.; Singh, A. An integrated approach for extracting fuel, chemicals, and residual carbon using pine needles. Biomass Convers. Biorefinery 2018, 8, 447–454. [Google Scholar] [CrossRef]
- Acquah, G.E.; Via, B.K.; Fasina, O.O.; Eckhardt, L.G. Rapid Quantitative Analysis of Forest Biomass Using Fourier Transform Infrared Spectroscopy and Partial Least Squares Regression. J. Anal. Methods Chem. 2016, 2016, 1839598. [Google Scholar] [CrossRef] [PubMed]
- Boukir, A.; Fellak, S.; Doumenq, P. Structural characterization of Argania spinosa Moroccan wooden artifacts during natural degradation progress using infrared spectroscopy (ATR-FTIR) and X-Ray diffraction (XRD). Heliyon 2019, 5, e02477. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Xing, D.; Lia, J. FTIR Studies of the Changes in Wood Chemistry from Wood Forming Tissue under Inclined Treatment. Energy Procedia 2012, 16, 758–762. [Google Scholar] [CrossRef]
- Faisul Aris, F.A.; Mohd Fauzi, F.N.A.; Tong, W.Y.; Syed Abdullah, S.S. Interaction of silver sulfadiazine wıth bacterial cellulose via ex-situ modification method as an alternative diabetic wound healing. Biocatal. Agric. Biotechnol. 2019, 21, 101332. [Google Scholar] [CrossRef]
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular stress responses: Cell survival and cell death. Int. J. Cell Biol. 2010, 2010, 214074. [Google Scholar] [CrossRef] [PubMed]
- Suntar, I.; Tumen, I.; Ustun, O.; Keles, H.; Akkol, E.K. Appraisal on the wound healing and anti-inflammatory activities of the essential oils obtained from the cones and needles of Pinus species by in vivo and in vitro experimental models. J. Ethnopharmacol. 2012, 139, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Gheorghita, D.; Grosu, E.; Robu, A.; Ditu, L.M.; Deleanu, I.M.; Gradisteanu Pircalabioru, G.; Raiciu, A.-D.; Bita, A.-I.; Antoniac, A.; Antoniac, V.I. Essential Oils as Antimicrobial Active Substances in Wound Dressings. Materials 2022, 15, 6923. [Google Scholar] [CrossRef]
- Matulyte, I.; Jekabsone, A.; Jankauskaite, L.; Zavistanaviciute, P.; Sakiene, V.; Bartkiene, E.; Ruzauskas, M.; Kopustinskiene, D.M.; Santini, A.; Bernatoniene, J. The Essential Oil and Hydrolats from Myristica fragrans Seeds with Magnesium Aluminometasilicate as Excipient: Antioxidant, Antibacterial, and Anti-inflammatory Activity. Foods 2020, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, A.; Sangole, P. Alternative Approaches to Wound Healing; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Zhang, H.H.; Zhang, C.M.; Xu, Q.Q.; Zhang, C.H. Effective Treatment of Small Uncomplicated Skin Abscesses with Fire Needle: A Case Series. Infect Drug Resist. 2021, 14, 4085–4090. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Lai, F.; Offerta, A.; Sarpietro, M.G.; Micicchè, L.; Maccioni, A.M.; Valenti, D.; Fadda, A.M. From nanoemulsions to nanostructured lipid carriers: A relevant development in dermal delivery of drugs and cosmetics. J. Drug Deliv. Sci. Technol. 2016, 32, 100–112. [Google Scholar] [CrossRef]
- Okugawa, A.; Sakaino, M.; Yuguchi, Y.; Yamane, C. Relaxation phenomenon and swelling behavior of regenerated cellulose fibers affected by water. Carbohydr. Polym. 2020, 231, 115663. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Gupta, M.K. Water absorption behavior of cellulosic fibres polymer composites: A review on its effects and remedies. J. Ind. Text. 2020, 51, 7480S–7512S. [Google Scholar] [CrossRef]
- Piaru, S.P.; Mahmud, R.; Abdul Majid, A.M.S.; Mahmoud Nassar, Z.D. Antioxidant and antiangiogenic activities of the essential oils of Myristica fragrans and Morinda citrifolia. Asian Pac. J. Trop. Med. 2012, 5, 294–298. [Google Scholar] [CrossRef]
- Jaric, S.; Kostic, O.; Mataruga, Z.; Pavlovic, D.; Pavlovic, M.; Mitrovic, M.; Pavlovic, P. Traditional wound-healing plants used in the Balkan region (Southeast Europe). J. Ethnopharmacol. 2018, 211, 311–328. [Google Scholar] [CrossRef]


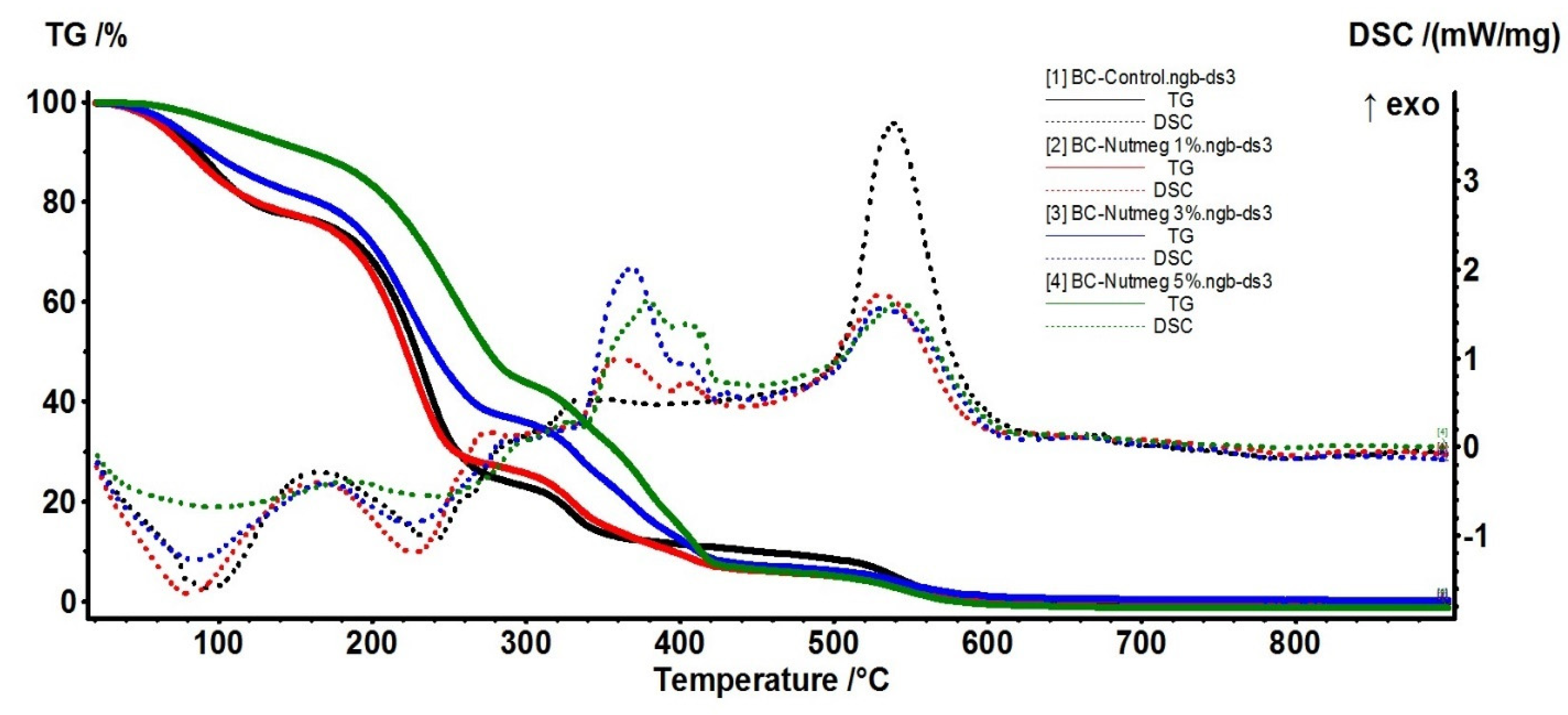




| Sample | Temperature (°C) | Mass Loss (%) |
|---|---|---|
| BC-Control | 160 | 23.51 |
| 160–300 | 53.51 | |
| 300–440 | 12.60 | |
| >440 | 10.67 | |
| BC-Nutmeg 1% | 160 | 23.63 |
| 160–300 | 50.71 | |
| 300–440 | 19.21 | |
| >440 | 6.34 | |
| BC-Nutmeg 3% | 160 | 19.39 |
| 160–300 | 44.74 | |
| 300–440 | 28.33 | |
| >440 | 7.23 | |
| BC-Nutmeg 5% | 160 | 10.17 |
| 160–300 | 45.97 | |
| 300–440 | 37.15 | |
| >440 | 7.95 |
| Sample | Temperature (°C) | Mass Loss (%) |
|---|---|---|
| BC-Control | 160 | 23.51 |
| 160–300 | 53.51 | |
| 300–440 | 12.60 | |
| >440 | 10.67 | |
| BC-Fir needle 1% | 160 | 55.96 |
| 160–300 | 17.26 | |
| 300–440 | 18.73 | |
| >440 | 5.70 | |
| BC-Fir needle 3% | 160 | 73.24 |
| 160–300 | 13.22 | |
| 300–440 | 9.49 | |
| >440 | 3.49 | |
| BC-Fir needle 5% | 160 | 39.27 |
| 160–300 | 25.67 | |
| 300–440 | 22.86 | |
| >440 | 10.16 |
| Samples | Compounds | Retention Time (RT) |
|---|---|---|
| BC/Fir needle essential oil | D-Limonene | 13.698 |
| Pinene epoxide | 16.299 | |
| Fenchol | 17.058 | |
| Sabinol | 17.849 | |
| Endo-Borneol | 18.973 | |
| Bornyl acetate | 22.936 | |
| D-Verbenone | 23.654 | |
| Longifolene | 27.191 | |
| Caryophyllene oxide | 32.341 | |
| 2-Methylisoborneol | 30.666 | |
| Palmitic acid, methyl ester | 41.641 | |
| Oleic acid, methyl ester | 45.282 | |
| Methyl stearate | 45.730 | |
| BC/Nutmeg essential oil | γ-Terpinene | 14.794 |
| Terpinolene | 15.782 | |
| Terpineol | 19.288 | |
| Safrole | 23.065 | |
| Eugenol | 25.218 | |
| Methyleugenol | 26.685 | |
| trans-Isoeugenol | 28.163 | |
| Myristicin | 30.607 | |
| Elemicin | 31.346 | |
| Methoxyeugenol | 32.772 | |
| Ricinoleic acid methyl ester | 48.261 | |
| Licarin A | 53.308 | |
| 6-Methoxyeugenyl isovalerate | 54.577 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemnaru, G.-M.; Motelica, L.; Trusca, R.D.; Ilie, C.I.; Croitoru, A.-M.; Ficai, D.; Oprea, O.; Stoica-Guzun, A.; Ficai, A.; Ditu, L.-M.; et al. Antimicrobial Wound Dressings based on Bacterial Cellulose and Independently Loaded with Nutmeg and Fir Needle Essential Oils. Polymers 2023, 15, 3629. https://doi.org/10.3390/polym15173629
Lemnaru G-M, Motelica L, Trusca RD, Ilie CI, Croitoru A-M, Ficai D, Oprea O, Stoica-Guzun A, Ficai A, Ditu L-M, et al. Antimicrobial Wound Dressings based on Bacterial Cellulose and Independently Loaded with Nutmeg and Fir Needle Essential Oils. Polymers. 2023; 15(17):3629. https://doi.org/10.3390/polym15173629
Chicago/Turabian StyleLemnaru (Popa), Georgiana-Madalina, Ludmila Motelica, Roxana Doina Trusca, Cornelia Ioana Ilie, Alexa-Maria Croitoru, Denisa Ficai, Ovidiu Oprea, Anicuta Stoica-Guzun, Anton Ficai, Lia-Mara Ditu, and et al. 2023. "Antimicrobial Wound Dressings based on Bacterial Cellulose and Independently Loaded with Nutmeg and Fir Needle Essential Oils" Polymers 15, no. 17: 3629. https://doi.org/10.3390/polym15173629







