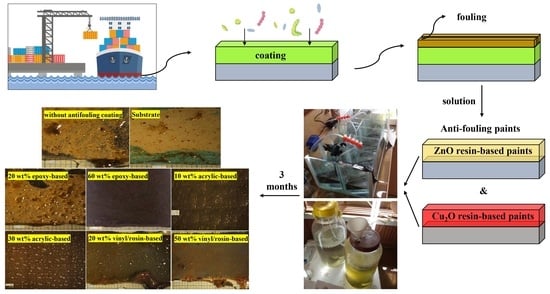
Investigation of the Release Rate of Biocide and Corrosion Resistance of Vinyl-, Acrylic-, and Epoxy-Based Antifouling Paints on Steel in Marine Infrastructures
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Materials
2.2. Paints Preparation
2.3. Curing Time
2.4. Biocide Release Rate Measurement
2.5. Antifouling Resistance Analysis
2.6. Thickness Measurement
2.7. Viscosity Measurement
2.8. Hardness Test
2.9. Bending Test
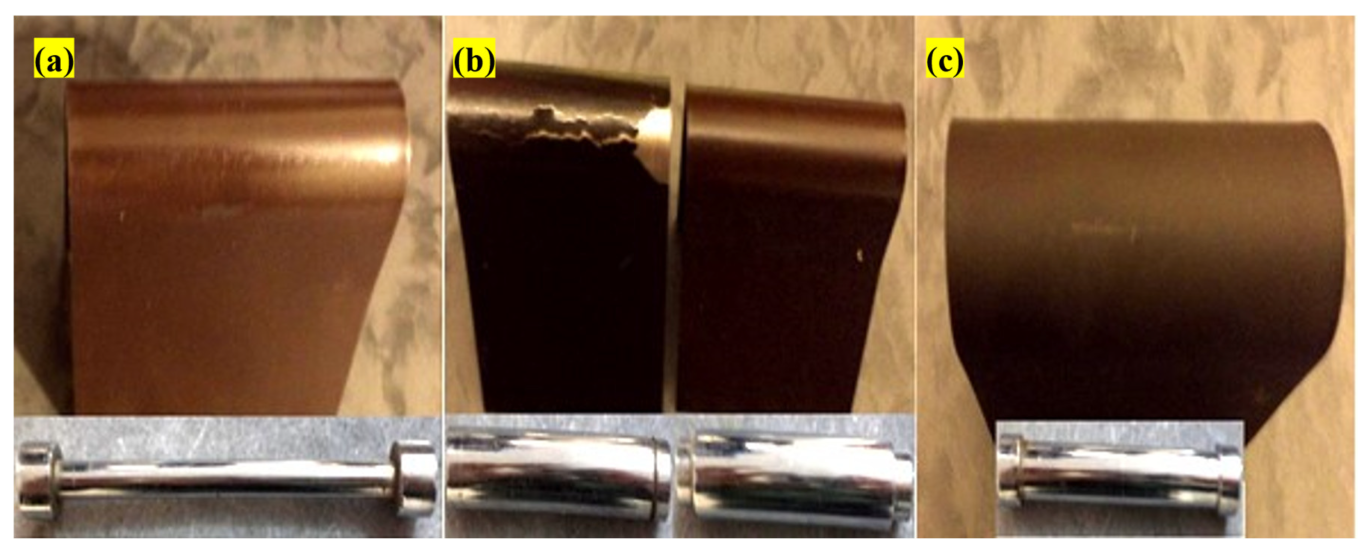
2.10. Paint Adhesion Test
2.11. Gloss Test
2.12. Impact Resistance Test
2.13. Abrasion Resistance Test
2.14. Scratch Resistance Test
2.15. Polarization Test
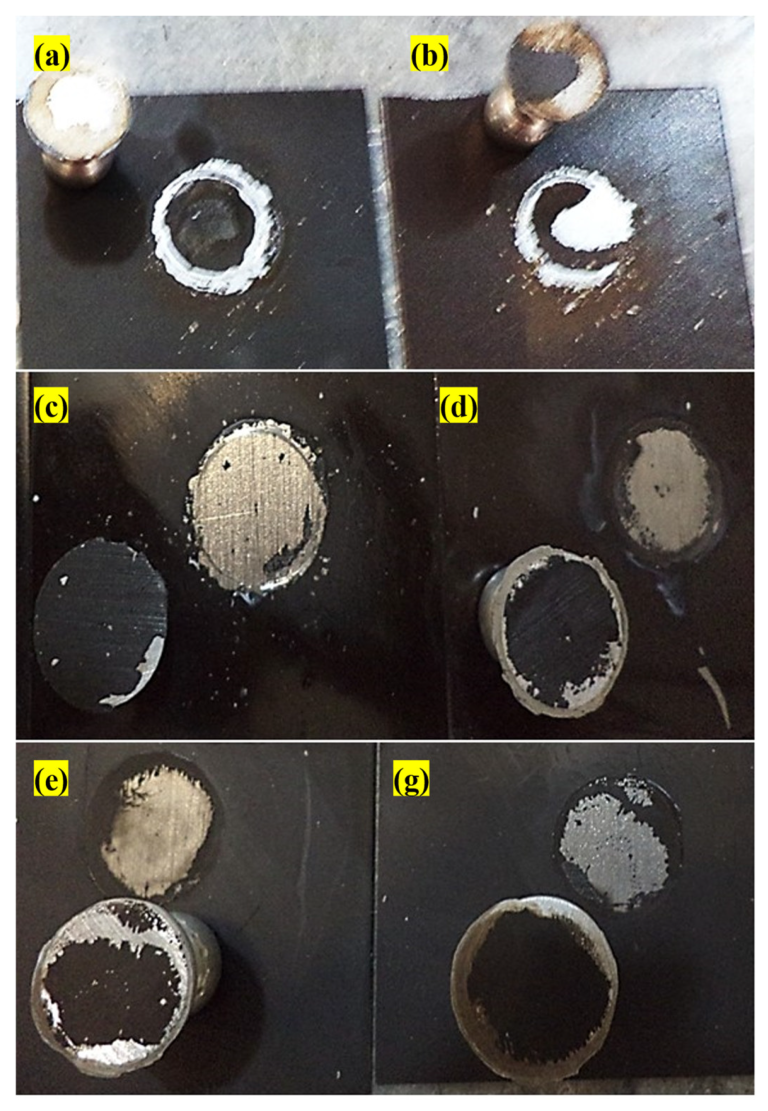
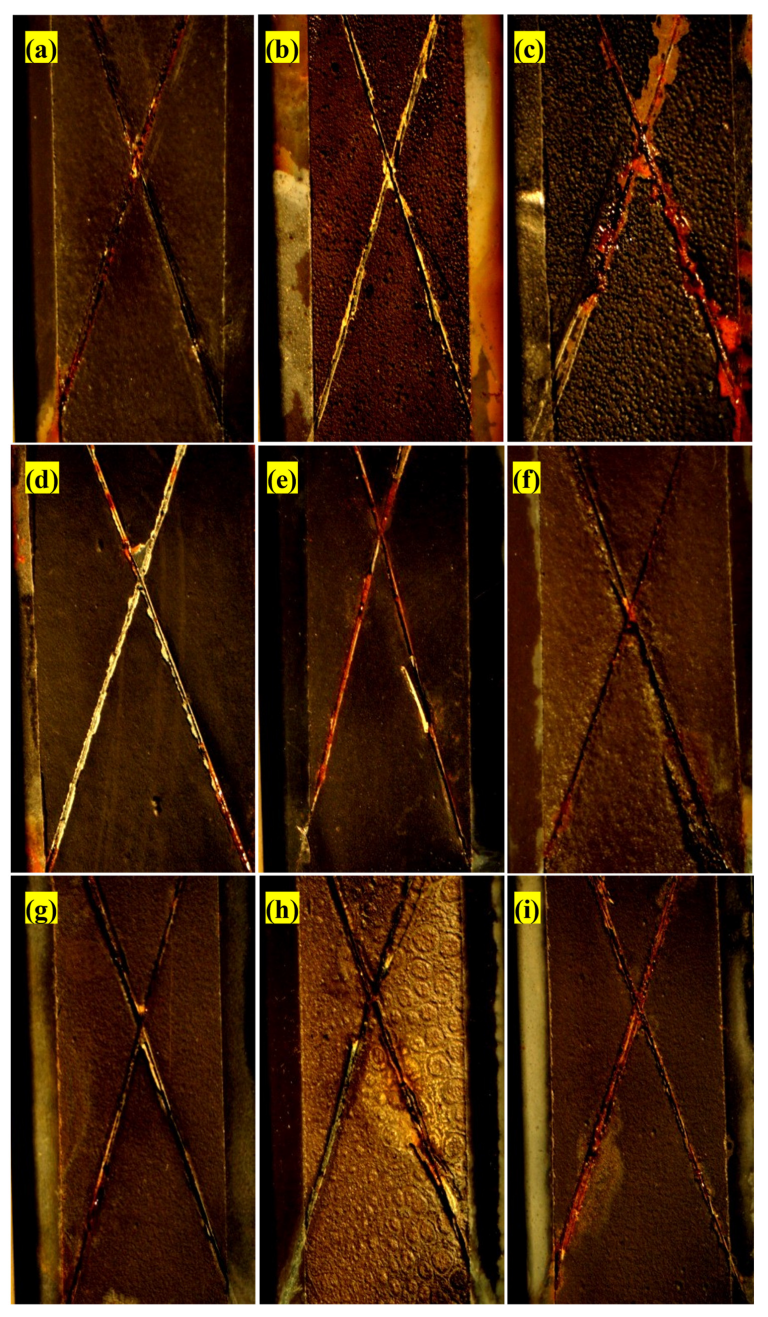
2.16. Salt Spray Test
2.17. Polyaniline Synthesis
3. Results and Discussion
3.1. Biocide Release Rate Measurement
3.1.1. Effect of Resin and Immersion Time on the Release Rate
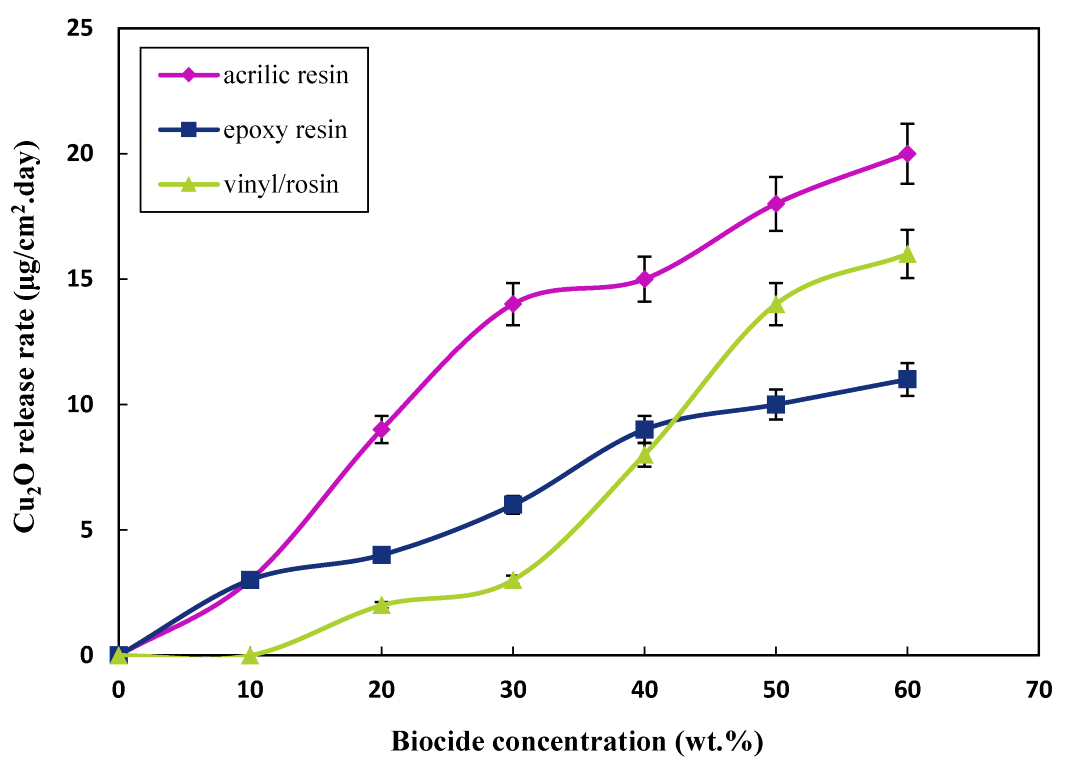
3.1.2. Effect of the Content of the Biocides on the Release Rate
3.1.3. Effect of Biocide Particle Size on the Release Rate
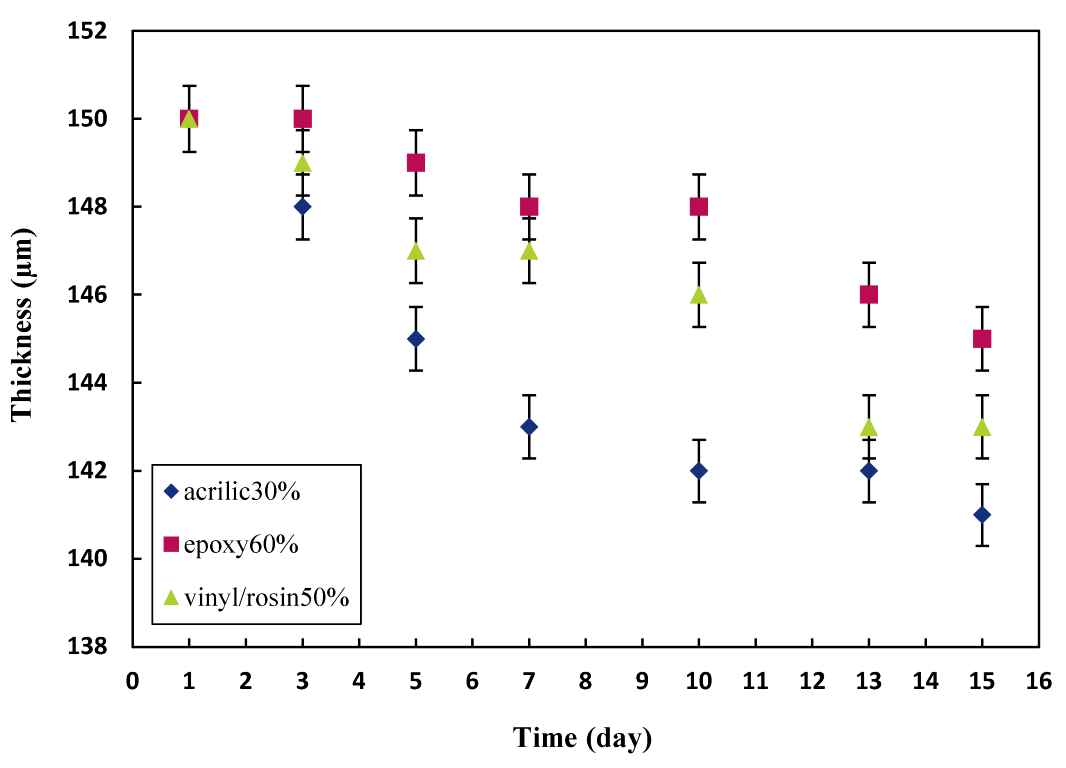
3.2. Effect of Biocide Release Rate on the Paint Thickness
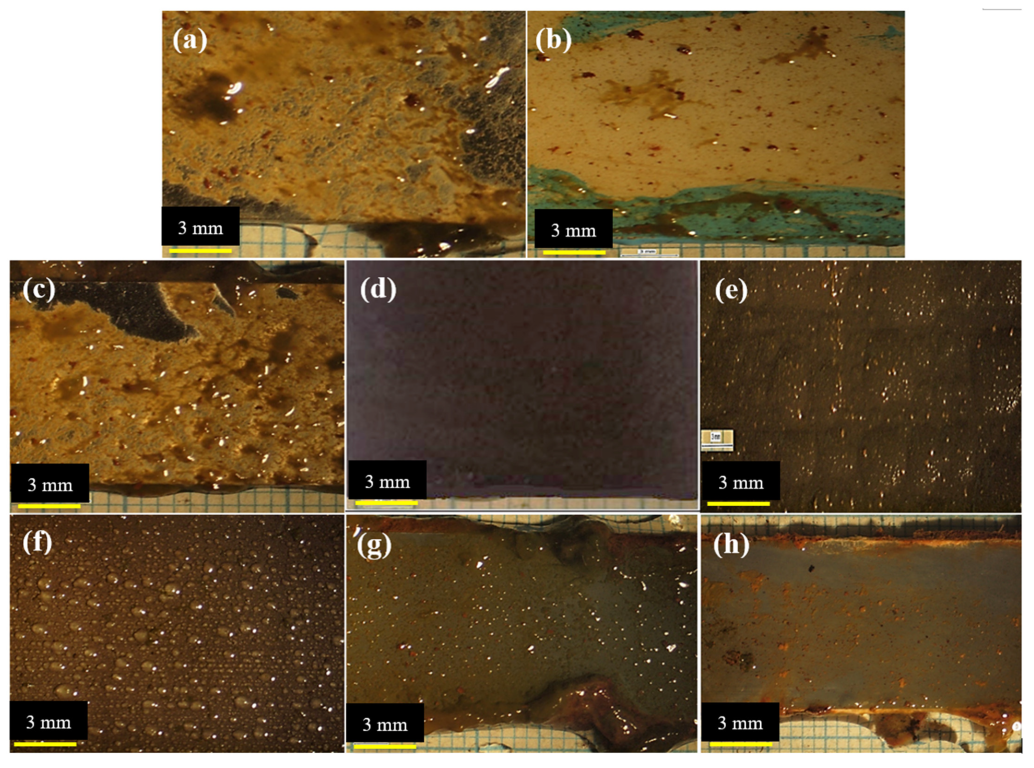
3.3. Antifouling Resistance Analysis
3.4. Curing Time
3.5. Hardness
3.6. Bending Test
3.7. Paint Adhesion Test
3.8. Gloss Test
3.9. Impact Resistance Test
3.10. Abrasion Resistance Test
3.11. Scratch Resistance Test
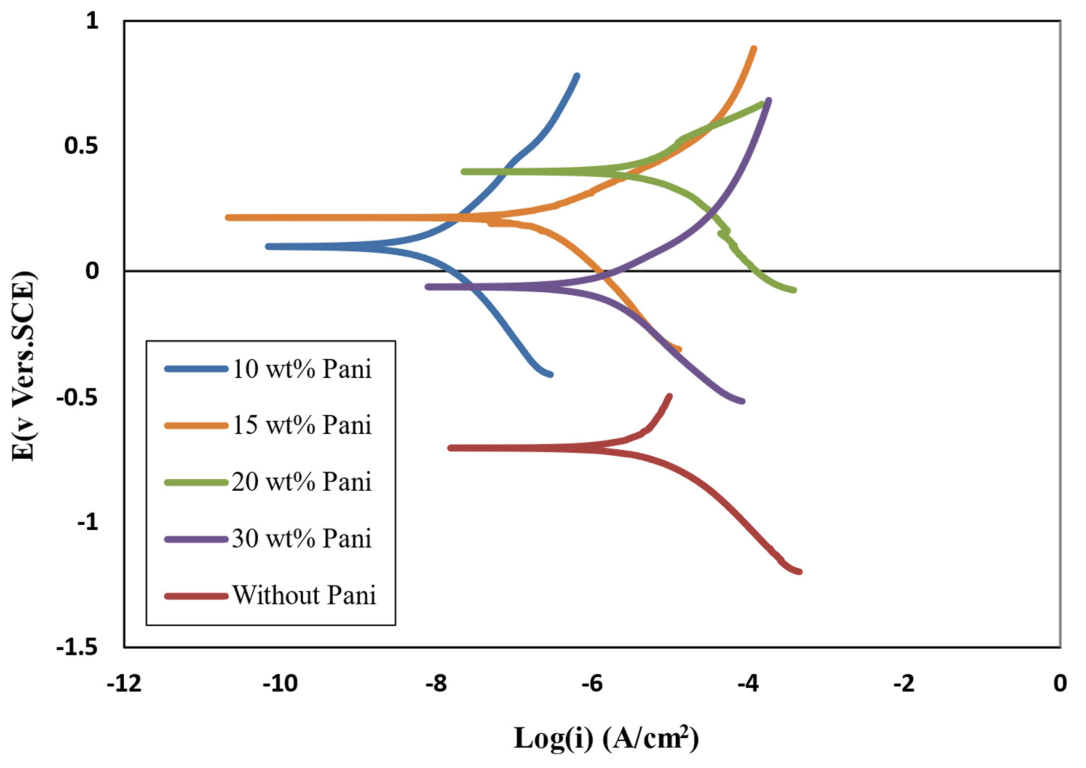
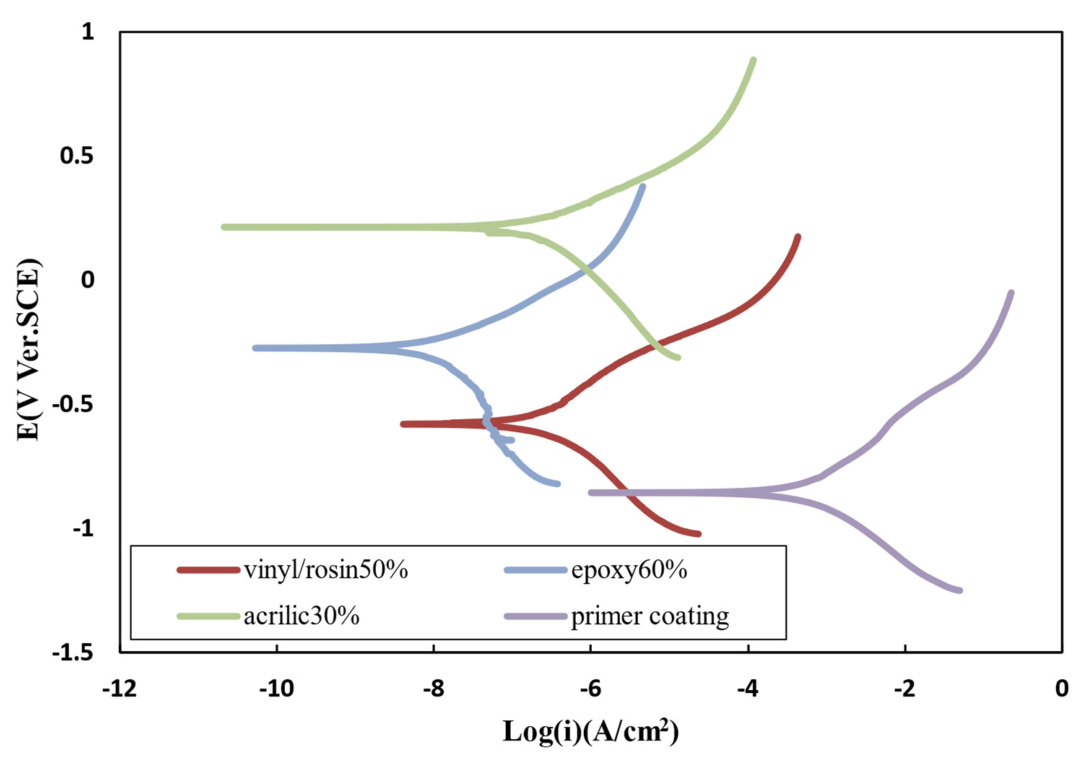
3.12. Polarization TEST
3.13. Salt Spray Test
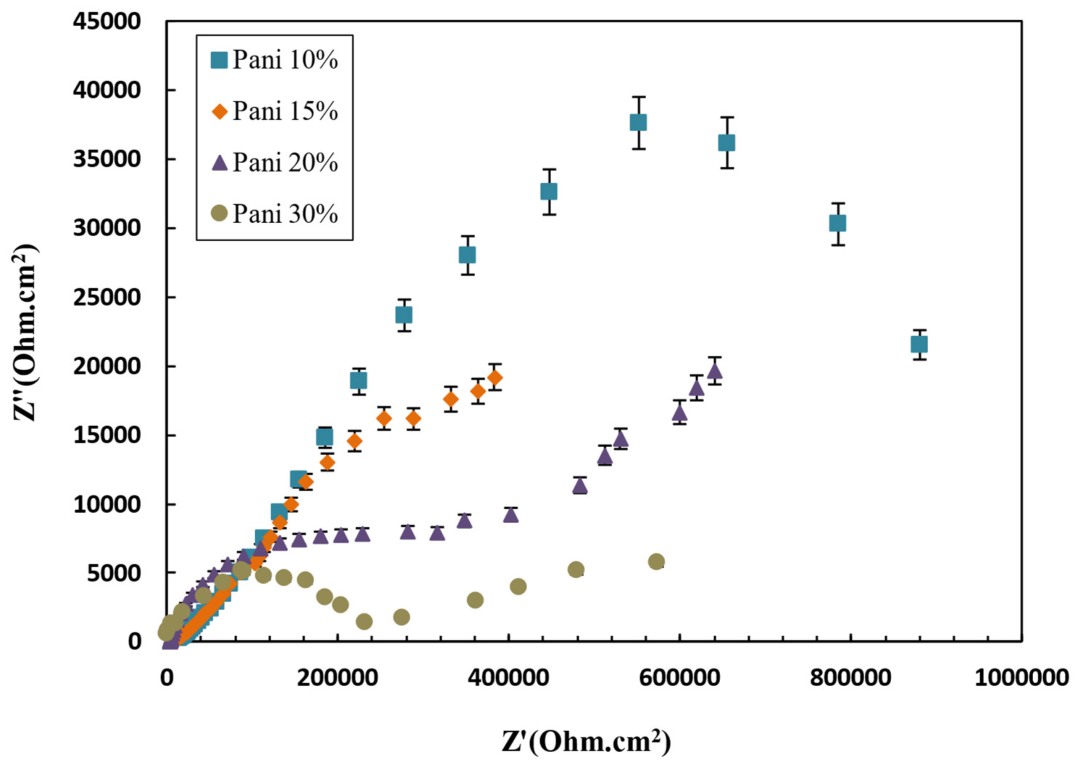
3.14. Investigation the Antifouling Property of Polyaniline in Paint and Its Efficacy on Corrosion Rate
4. Environmental Challenges and Toxicity Effects of Biocides
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chasse, K.; Scardino, A.; Swain, G. Corrosion and fouling study of copper-based antifouling coatings on 5083 aluminum alloy. Prog. Org. Coat. 2020, 141, 105555. [Google Scholar] [CrossRef]
- Tesler, A.B.; Kim, P.; Kolle, S.; Howell, C.; Ahanotu, O.; Aizenberg, J. Extremely durable biofouling-resistant metallic surfaces based on electrodeposited nanoporous tungstite films on steel. Nat. Commun. 2015, 6, 8649. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wu, J.; Zhang, X.; Shi, J.; Wei, J.; Yang, Y.; Wu, B.; Feng, Y. Special issue on advanced corrosion-resistance materials and emerging applications. The progress on antifouling organic coating: From biocide to biomimetic surface. J. Mater. Sci. Technol. 2021, 61, 46–62. [Google Scholar] [CrossRef]
- Ali, A.; Jamil, M.I.; Jiang, J.; Shoaib, M.; Amin, B.U.; Luo, S.; Zhan, X.; Chen, F.; Zhang, Q. An overview of controlled-biocide-release coating based on polymer resin for marine antifouling applications. J. Polym. Res. 2020, 27, 85. [Google Scholar] [CrossRef]
- Chen, C.-L.; Maki, J.S.; Rittschof, D.; Teo, S.L.-M. Early marine bacterial biofilm on a copper-based antifouling paint. Int. Biodeterior. Biodegrad. 2013, 83, 71–76. [Google Scholar] [CrossRef]
- Shenashen, M.; El-Safty, S.A.; Higazy, S.; Selim, M.; Isago, H.; Elmarakbi, A. Recent progress in marine foul-release polymeric nanocomposite coatings. Prog. Mater. Sci. 2017, 87, 1–32. [Google Scholar]
- Long, W.; Li, H.; Yang, B.; Huang, N.; Liu, L.; Gai, Z.; Jiang, X. Superhydrophobic diamond-coated Si nanowires for application of anti-biofouling’. J. Mater. Sci. Technol. 2020, 48, 1–8. [Google Scholar] [CrossRef]
- Callow, J.A.; Callow, M.E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2011, 2, 244. [Google Scholar] [CrossRef]
- Tian, J.; Xu, K.; Hu, J.; Zhang, S.; Cao, G.; Shao, G. Durable self-polishing antifouling Cu-Ti coating by a micron-scale Cu/Ti laminated microstructure design. J. Mater. Sci. Technol. 2021, 79, 62–74. [Google Scholar] [CrossRef]
- Hadfield, M.G. Biofilms and Marine Invertebrate Larvae: What Bacteria Produce That Larvae Use to Choose Settlement Sites. Annu. Rev. Mar. Sci. 2011, 3, 453–470. [Google Scholar] [CrossRef]
- Qian, P.-Y.; Lau, S.C.K.; Dahms, H.-U.; Dobretsov, S.; Harder, T. Marine Biofilms as Mediators of Colonization by Marine Macroorganisms: Implications for Antifouling and Aquaculture. Mar. Biotechnol. 2007, 9, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Shoar, Z.K.; Pourpasha, H.; Heris, S.Z.; Mousavi, S.B.; Mohammadpourfard, M. The effect of heat transfer characteristics of macromolecule fouling on heat exchanger surface: A dynamic simulation study. Can. J. Chem. Eng. 2023, 101, 5802–5817. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, N.; Zhu, S.; Qiao, Z.; Zhang, J.; Yang, J.; Liu, W. A novel Cu-doped high entropy alloy with excellent comprehensive performances for marine application. J. Mater. Sci. Technol. 2021, 69, 48–59. [Google Scholar] [CrossRef]
- Bai, X.; Li, J.; Zhu, L.; Wang, L. Effect of Cu content on microstructure, mechanical and anti-fouling properties of TiSiN-Cu coating deposited by multi-arc ion plating. Appl. Surf. Sci. 2018, 427, 444–451. [Google Scholar] [CrossRef]
- Ma, F.; Li, J.; Zeng, Z.; Gao, Y. Tribocorrosion behavior in artificial seawater and anti-microbiologically influenced corrosion properties of TiSiN-Cu coating on F690 steel. J. Mater. Sci. Technol. 2019, 35, 448–459. [Google Scholar] [CrossRef]
- Yebra, D.M.; Kiil, S.; Weinell, C.E.; Dam-Johansen, K. Effects of marine microbial biofilms on the biocide release rate from antifouling paints—A model-based analysis. Prog. Org. Coat. 2006, 57, 56–66. [Google Scholar] [CrossRef]
- Sharma, B.K.; Gupta, A.K.; Khare, N.; Dhawan, S.; Gupta, H. Synthesis and characterization of polyaniline–ZnO composite and its dielectric behavior. Synth. Met. 2009, 159, 391–395. [Google Scholar] [CrossRef]
- Prasad, G.; Takei, T.; Yonesaki, Y.; Kumada, N.; Kinomura, N. Hybrid nanocomposite based on NbWO6 nanosheets and polyaniline. Mater. Lett. 2006, 60, 3727–3730. [Google Scholar] [CrossRef]
- Deshpande, N.; Gudage, Y.; Sharma, R.; Vyas, J.; Kim, J.; Lee, Y. Studies on tin oxide-intercalated polyaniline nanocomposite for ammonia gas sensing applications. Sens. Actuators B Chem. 2009, 138, 76–84. [Google Scholar] [CrossRef]
- Gurunathan, K.; Amalnerkar, D.; Trivedi, D. Synthesis and characterization of conducting polymer composite (PAn/TiO2) for cathode material in rechargeable battery. Mater. Lett. 2003, 57, 1642–1648. [Google Scholar] [CrossRef]
- Mostafaei, A.; Nasirpouri, F. Epoxy/polyaniline–ZnO nanorods hybrid nanocomposite coatings: Synthesis, characterization and corrosion protection performance of conducting paints. Prog. Org. Coat. 2014, 77, 146–159. [Google Scholar] [CrossRef]
- Nami, S.H.; Mousavi, S.B. Nitrate Removal Performance of Different Granular Adsorbents Using a Novel Fe-Exchanged Nanoporous Clinoptilolite. Ind. Eng. Chem. Res. 2023, 62, 3659–3671. [Google Scholar] [CrossRef]
- Hosseini, M.; Jafari, M.; Najjar, R. Effect of polyaniline–montmorillonite nanocomposite powders addition on corrosion performance of epoxy coatings on Al 5000. Surf. Coat. Technol. 2011, 206, 280–286. [Google Scholar] [CrossRef]
- Heris, S.Z.; Etemadi, M.; Mousavi, S.B.; Mohammadpourfard, M.; Ramavandi, B. Preparation and characterizations of TiO2/ZnO nanohybrid and its application in photocatalytic degradation of tetracycline in wastewater. J. Photochem. Photobiol. A Chem. 2023, 443, 114893. [Google Scholar]
- Yousefi, F.; Mousavi, S.B.; Heris, S.Z.; Naghash-Hamed, S. UV-shielding properties of a cost-effective hybrid PMMA-based thin film coatings using TiO2 and ZnO nanoparticles: A comprehensive evaluation. Sci. Rep. 2023, 13, 7116. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.B.; Heris, S.Z.; Hosseini, M.G. Experimental investigation of MoS2/diesel oil nanofluid thermophysical and rheological properties. Int. Commun. Heat Mass Transf. 2019, 108, 104298. [Google Scholar] [CrossRef]
- Naghash-Hamed, S.; Arsalani, N.; Mousavi, S.B. Facile fabrication of CuFe2O4 coated with carbon quantum dots nanocomposite as an efficient heterogeneous catalyst toward the reduction of nitroaniline compounds for management of aquatic resources. J. Photochem. Photobiol. A Chem. 2023, 443, 114822. [Google Scholar] [CrossRef]
- Naghash-Hamed, S.; Arsalani, N.; Mousavi, S.B. The catalytic performance of CuFe2O4@CQD nanocomposite as a high-perform heterogeneous nanocatalyst in nitroaniline group reduction. Sci. Rep. 2023, 13, 3329. [Google Scholar] [CrossRef]
- Mousavi, S.B.; Heidari, M.; Rahmani, F.; Sene, R.A.; Clough, P.T.; Ozmen, S. Highly robust ZrO2-stabilized CaO nanoadsorbent prepared via a facile one-pot MWCNT-template method for CO2 capture under realistic calcium looping conditions. J. Clean. Prod. 2023, 384, 135579. [Google Scholar] [CrossRef]
- Athari, M.-J.; Tahmasebpoor, M.; Azimi, B.; Heidari, M.; Pevida, C. Waste oleaster seed-derived activated carbon mixed with coarse particles of fluid catalytic cracking as a highly-efficient CO2 adsorbent at low temperatures. Process. Saf. Environ. Prot. 2023, 178, 580–594. [Google Scholar] [CrossRef]
- Tahmasebpoor, M.; Iranvandi, M.; Heidari, M.; Azimi, B.; Pevida, C. Development of novel waste tea-derived activated carbon promoted with SiO2 nanoparticles as highly robust and easily fluidizable sorbent for low-temperature CO2 capture. J. Environ. Chem. Eng. 2023, 11, 110437. [Google Scholar] [CrossRef]
- Kadri, Y.; Srasra, E.; Bekri-Abbes, I.; Herrasti, P. Facile and eco-friendly synthesis of polyaniline/ZnO composites for corrosion protection of AA-2024 aluminium alloy. J. Electroanal. Chem. 2021, 893, 115335. [Google Scholar] [CrossRef]
- Rui, M.; Zhu, A. The synthesis and corrosion protection mechanisms of PANI/CNT nanocomposite doped with organic phosphoric acid. Prog. Org. Coat. 2021, 153, 106134. [Google Scholar] [CrossRef]
- Najjar, R.; Katourani, S.A.; Hosseini, M.G. Self-healing and corrosion protection performance of organic polysulfide@urea-formaldehyde resin core-shell nanoparticles in epoxy/PANI/ZnO nanocomposite coatings on anodized aluminum alloy. Prog. Org. Coat. 2018, 124, 110–121. [Google Scholar] [CrossRef]
- Pagotto, J.F.; Recio, F.J.; Motheo AD, J.; Herrasti, P. Multilayers of PAni/n-TiO2 and PAni on carbon steel and welded carbon steel for corrosion protection. Surf. Coat. Technol. 2016, 289, 23–28. [Google Scholar] [CrossRef]
- Shetty, K.; Jayadev; Raj, K. Synthesized conducting Polyaniline—TiO2 based nanocomposite for corrosion control on steel 316. Mater. Today Proc. 2021, 38, 2493–2498. [Google Scholar] [CrossRef]
- Sumi, V.; Arunima, S.; Deepa, M.; Sha, M.A.; Riyas, A.; Meera, M.; Saji, V.S.; Shibli, S. PANI-Fe2O3 composite for enhancement of active life of alkyd resin coating for corrosion protection of steel. Mater. Chem. Phys. 2020, 247, 122881. [Google Scholar] [CrossRef]
- Bandgar, D.K.; Navale, S.T.; Vanalkar, S.A.; Kim, J.H.; Harale, N.S.; Patil, P.S.; Patil, V.B. Synthesis, structural, morphological, compositional and electrical transport properties of polyaniline/α-Fe2O3 hybrid nanocomposites. Synth. Met. 2014, 195, 350–358. [Google Scholar] [CrossRef]
- Gholami, A.; Mousavi, S.B.; Heris, S.Z.; Mohammadpourfard, M. Highly efficient treatment of petrochemical spent caustic effluent via electro-Fenton process for COD and TOC removal: Optimization and experimental. Biomass Convers. Biorefin. 2023, 1–17. [Google Scholar] [CrossRef]
- Ebratkhahan, M.; Naghash Hamed, S.; Zarei, M.; Jafarizad, A.; Rostamizadeh, M. Removal of neutral red dye via electro-fenton process: A response surface methodology modeling. Electrocatalysis 2021, 12, 579–594. [Google Scholar] [CrossRef]
- Khouri, O.; Goshayeshi, H.R.; Nami, S.H.; Mousavi, S.B.; Heris, S.Z.; Chaer, I. Evaluating the impact of graphene oxide nanoparticles on enhancing heat transfer characteristics within the indirect heat exchanger of a natural city gate station. Res. Sq. 2023. preprint. [Google Scholar]
- Yong, H.E.; Krishnamoorthy, K.; Hyun, K.T.; Kim, S.J. Preparation of ZnO nanopaint for marine antifouling applications. J. Ind. Eng. Chem. 2015, 29, 39–42. [Google Scholar] [CrossRef]
- Seyedi, S.S.; Shabgard, M.R.; Mousavi, S.B.; Heris, S.Z. The impact of SiC, Al2O3, and B2O3 abrasive particles and temperature on wear characteristics of 18Ni (300) maraging steel in abrasive flow machining (AFM). Int. J. Hydrogen Energy 2021, 46, 33991–34001. [Google Scholar] [CrossRef]
- Al-Belushi, M.A.; Myint MT, Z.; Kyaw, H.H.; Al-Naamani, L.; Al-Mamari, R.; Al-Abri, M.; Dobretsov, S. ZnO nanorod-chitosan composite coatings with enhanced antifouling properties. Int. J. Biol. Macromol. 2020, 162, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.; Selvaraj, M.; Palraj, S.; Subramanian, G. Studies on the anticorrosive & antifouling properties of the Gracilaria edulis extract incorporated epoxy paint in the Gulf of Mannar Coast, Mandapam, India. Prog. Org. Coat. 2016, 90, 448–454. [Google Scholar]
- Ni, C.; Feng, K.; Li, X.; Zhao, H.; Yu, L. Study on the preparation and properties of new environmentally friendly antifouling acrylic metal salt resins containing indole derivative group. Prog. Org. Coat. 2020, 148, 105824. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Oranu, E.A.; Tao, M.; Keller, A.A. Release and detection of nanosized copper from a commercial antifouling paint. Water Res. 2016, 102, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Palanichamy, S.; Subramanian, G. Antifouling properties of marine bacteriocin incorporated epoxy based paint. Prog. Org. Coat. 2017, 103, 33–39. [Google Scholar] [CrossRef]
- Quan, X.; Wang, J.; Zhao, S.; Cai, W.; Wang, Z.; Wang, S.; Cui, X. Improved antibacterial, antifouling and corrosion protective performance of epoxy coatings with poly (m-aminophenol). Prog. Org. Coat. 2018, 115, 9–17. [Google Scholar] [CrossRef]
- Quan, X.; Wang, J.; Souleyman, T.; Cai, W.; Zhao, S.; Wang, Z. Antibacterial and antifouling performance of bisphenol-A/Poly (ethylene glycol) binary epoxy coatings containing bromine-benzyl-disubstituted polyaniline. Prog. Org. Coat. 2018, 124, 61–70. [Google Scholar] [CrossRef]
- Tonelli, M.; Perini, I.; Ridi, F.; Baglioni, P. Improving the properties of antifouling hybrid composites: The use of Halloysites as nano-containers in epoxy coatings. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126779. [Google Scholar] [CrossRef]
- Ma, J.; Ma, C.; Yang, Y.; Xu, W.; Zhang, G. Biodegradable polyurethane carrying antifoulants for inhibition of marine biofouling. Ind. Eng. Chem. Res. 2014, 53, 12753–12759. [Google Scholar] [CrossRef]
- Shtykova, L.; Fant, C.; Handa, P.; Larsson, A.; Berntsson, K.; Blanck, H.; Simonsson, R.; Nydén, M.; Härelind, H.I. Adsorption of antifouling booster biocides on metal oxide nanoparticles: Effect of different metal oxides and solvents. Prog. Org. Coat. 2009, 64, 20–26. [Google Scholar] [CrossRef]
- Razmjoo, F.; Sadeghi, E.; Alizadeh-Sani, M.; Noroozi, R.; Azizi-Lalabadi, M. Fabrication and application of functional active packaging material based on carbohydrate biopolymers formulated with Lemon verbena/Ferulago angulata extracts for the preservation of raw chicken meat. Food Process. Preserv. 2022, 46, e16830. [Google Scholar] [CrossRef]
- ISIRI6456; Paints and Varnishes; Surface-Drying Test; Ballotini Method. Institute of Standards and Industrial Research of Iran: Tehran, Iran, 2014.
- Takahashi, K. Release rate of biocides from antifouling paints. Ecotoxicol. Antifouling Biocides 2009, 3–22. [Google Scholar]
- ASTM D6442-06; Standard Test Method for Determination of Copper Release Rate From Antifouling Coatings in Substitute Ocean Water. ASTM International: West Conshohocken, PA, USA, 2020.
- Singh, S.; Singh, R.; Sharma, N.R.; Singh, A. Ethanolic Extract of Cockroach Wing Powder as Corrosion Inhibitor for N80 Steel in an ASTM D1141-98(2013) Standard Artificial Seawater Solution. Int. J. Electrochem. Sci. 2021, 16, 210841. [Google Scholar] [CrossRef]
- ASTM D1141; Standard Practice for Preparation of Substitute Ocean Water. ASTM International: West Conshohocken, PA, USA, 2021.
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Brady, R.F. Clean hulls without poisons: Devising and testing nontoxic marine coatings. J. Coat. Technol. 2000, 72, 45–56. [Google Scholar] [CrossRef]
- Sapozhnikova, Y.; Wirth, E.; Schiff, K.; Fulton, M. Antifouling biocides in water and sediments from California marinas. Mar. Pollut. Bull. 2013, 69, 189–194. [Google Scholar] [CrossRef]
- Beitelman, A.D.; Marsh, C.P.; Carlson, T.A. Low heat-transfer coatings in heat distribution systems. In Proceedings of the CORROSION 2009, Atlanta, GA, USA, 22–26 March 2009. NACE–09496 NACE CORROSION. [Google Scholar]
- ASTM D1186; Standard Test Methods for Nondestructive Measurement of Dry Film Thickness of Nonmagnetic Coatings Applied to a Ferrous Base. ASTM International: West Conshohocken, PA, USA, 2021.
- Patel, C.J.; Mannari, V. Air-drying bio-based polyurethane dispersion from cardanol: Synthesis and characterization of coatings. Prog. Org. Coat. 2014, 77, 997–1006. [Google Scholar] [CrossRef]
- ASTM D562; Standard Test Method for Consistency of Paints Measuring Krebs Unit (KU) Viscosity Using a Stormer-Type Viscometer. ASTM International: West Conshohocken, PA, USA, 2010.
- ASTM D3363; Standard Test Method for Film Hardness by Pencil Test. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D4366; Standard Test Methods for Hardness of Organic Coatings by Pendulum Damping Tests. ASTM International: West Conshohocken, PA, USA, 2021.
- Azadi, M.; Bahrololoom, M.E.; Heidari, F. Enhancing the mechanical properties of an epoxy coating with rice husk ash, a green product. J. Coat. Technol. Res. 2011, 8, 117–123. [Google Scholar] [CrossRef]
- Kurt, Ş.; Özçifçi, A. Effect of various fire retardants on Brinell hardness of some wood. BioResources 2009, 4, 960–969. [Google Scholar] [CrossRef]
- ASTM D522; Standard Test Methods for Mandrel Bend Test of Attached Organic Coatings. ASTM International: West Conshohocken, PA, USA, 2008.
- ASTM D4541; Standard Test Method for Pull-Off Strength of Coatings Using Portable Adhesion Testers. ASTM International: West Conshohocken, PA, USA, 2022.
- Schilling, M. Coating Adhesion Testing in Accordance with ASTM D4541 Sticky Business; Corrosion Probe, Inc.: Centerbrook, CT, USA, 2004. [Google Scholar]
- Leloup, F.B.; Audenaert, J.; Obein, G.; Ged, G.; Hanselaer, P. Repeatability and reproducibility of specular gloss meters in theory and practice. J. Coat. Technol. Res. 2016, 13, 941–951. [Google Scholar] [CrossRef]
- ASTM D523; Standard Test Method for Specular Gloss. ASTM International: West Conshohocken, PA, USA, 1999.
- ASTM D1794; Standard Test Method for Alcohol-Benzene Soluble Matter in Cellulose. ASTM International: West Conshohocken, PA, USA, 1991.
- Gore, P.M.; Balakrishnan, S.; Kandasubramanian, B. Superhydrophobic corrosion inhibition polymer coatings. In Superhydrophobic Polymer Coatings; Elsevier: Amsterdam, The Netherlands, 2019; pp. 223–243. [Google Scholar]
- ASTM D4060; Standard Test Method for Abrasion Resistance of Organic Coatings by the Taber Abraser. ASTM International: West Conshohocken, PA, USA, 2019.
- Browning, R.; Lim, G.-T.; Moyse, A.; Sue, H.-J.; Chen, H.; Earls, J. Quantitative evaluation of scratch resistance of polymeric coatings based on a standardized progressive load scratch test. Surf. Coat. Technol. 2006, 201, 2970–2976. [Google Scholar] [CrossRef]
- ASTM D7027; Standard Test Method for Evaluation of Scratch Resistance of Polymeric Coatings and Plastics Using an Instrumented Scratch Machine. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM G102-89; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM International: West Conshohocken, PA, USA, 2010.
- Vitelaru, C.; Ghiban, N.; Parau, A.C.; Balaceanu, M.; Miculescu, F.; Vladescu, A. Corrosion behaviour of Ti6Al4V alloy in artificial saliva solution with fluoride content and low pH value: Korrosionsverhalten der Legierung Ti6Al4V in künstlicher Speichelflüssigkeit mit Fluoridanteilen und geringem pH-Wert. Mater. Werkst. 2014, 45, 91–98. [Google Scholar] [CrossRef]
- Krishnan, R.; Manivannan, S.; Patel, P.B.; Patil, P.P.; Venkatesh, R.; Thanappan, S. Studies on Corrosion Behavior of Mg-Al-Zn-RE Cast Alloy with Powder-Coated Al and CED Mg by Salt Spray Test, Immersion Test, and Electrochemical Test. Int. J. Chem. Eng. 2022, 2022, 1891419. [Google Scholar] [CrossRef]
- ASTM B117; Standard Practice for Operating Salt Spray (Fog) Apparatus. ASTM International: West Conshohocken, PA, USA, 2019.
- Wang, X.-H.; Li, J.; Zhang, J.-Y.; Sun, Z.-C.; Yu, L.; Jing, X.-B.; Sun, Z.-X.; Ye, Z.-J. Polyaniline as marine antifouling and corrosion-prevention agent. Synth. Met. 1999, 102, 1377–1380. [Google Scholar] [CrossRef]
- Mostafaei, A.; Nasirpouri, F. Preparation and characterization of a novel conducting nanocomposite blended with epoxy coating for antifouling and antibacterial applications. J. Coat. Technol. Res. 2013, 10, 679–694. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, J.; Zhou, T.; He, B.; Huang, W.; Wang, B. Preparation and properties study of polyaniline conductive anti-fouling coatings. Int. J. Electrochem. Sci. 2012, 7, 8170. [Google Scholar] [CrossRef]
- Gitlitz, M.H.; Leiner, H.H. Erodible Ship-Bottom Paints for Control of Marine Fouling. U.S. Patent 4,593,055, 3 June 1986. [Google Scholar]
- Yebra, D.M.; Kiil, S.; Weinell, C.E.; Dam-Johansen, K. Dissolution rate measurements of sea water soluble pigments for antifouling paints: ZnO. Prog. Org. Coat. 2006, 56, 327–337. [Google Scholar] [CrossRef]
- Candries, M. Drag, Boundary-Layer and Roughness Characteristics of Marine Surfaces Coated with Antifoulings. Ph.D. Thesis, University of Newcastle upon Tyne, Newcastle upon Tyne, UK, 2001. [Google Scholar]
- Almeida, E.; Diamantino, T.C.; de Sousa, O. Marine paints: The particular case of antifouling paints. Prog. Org. Coat. 2007, 59, 2–20. [Google Scholar] [CrossRef]
- Kiil, S.; Dam-Johansen, K.; Weinell, C.E.; Pedersen, M.S. Seawater-soluble pigments and their potential use in self-polishing antifouling paints: Simulation-based screening tool. Prog. Org. Coat. 2002, 45, 423–434. [Google Scholar] [CrossRef]
- Sobhani, E.; Heris, S.Z.; Mousavi, S.B. The Synergistic Effect of Intumescent Fire-Resistive Paint Containing TiO2 Nanoparticles and Chlorinated Paraffin onto Atmospheric-Metallic Substrates. ChemistrySelect 2022, 7, e202203513. [Google Scholar] [CrossRef]
- Tahmasebpoor, M.; Hosseini Nami, S.; Khatamian, M.; Sanaei, L. Arsenate removal from contaminated water using Fe2O3-clinoptilolite powder and granule. Environ. Technol. 2022, 43, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Khatamian, M.; Nami, S.H.; Mosayeb, S.G.H.; Divband, B. Preparation and characterization of nano- Fe(OH)3, its composites with two natural zeolites, and granulation of them for nitrate removal from polluted water. Phys. Scr. 2023, 98, 035024. [Google Scholar] [CrossRef]
- Khatamian, M.; Afshar No, N.; Hosseini Nami, S.; Fazli-Shokouhi, S. Synthesis and characterization of zeolite A, Fe3O4/zeolite A, and Fe2O3/zeolite A nanocomposites and investigation of their arsenic removal performance. J. Iran. Chem. Soc. 2023, 20, 1657–1670. [Google Scholar] [CrossRef]




| Element | Mn | P | S | C | Fe |
|---|---|---|---|---|---|
| Percent (wt.%) | 0.6 | 0.03 | 0.02 | 0.17 | 0.18 |
| Material | Role | wt.% |
|---|---|---|
| Resin (binder) | Film-forming component of paint | 10, 20, 30, 40, 50, 60, and 70 0.1 |
| Cu2O particles | Biocide activity | 0, 7, 14, 20, 27, 33, and 40 0.1 |
| ZnO particles | Biocide activity | 0, 3, 6, 10, 13, 17, and 20 0.1 |
| Titanium dioxide (pigment) | Providing the color of the paint | 5 0.1 |
| Iron (III) oxide red (pigment) | Providing the color of the paint | 5 0.1 |
| Dioctyl phthalate | Providing better pigment dispersion | 5 0.1 |
| Talc | Providing the color of the paint | 5 0.1 |
| Efka FA 4644 | Dispersing agent | 2 0.1 |
| BYK310 | Providing uniform dispersion | 2 0.1 |
| Resin | Total Biocide Content (wt.%) | Cu2O Content in Paint (wt.%) | ZnO Content in Paint (wt.%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 Days | 5–10 Days | 10–15 Days | |||||||
| Acrylic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 7 | 3 | 7 | 9 | 3 | 3 | 3 | 2 | |
| 20 | 14 | 6 | 20 | 19 | 10 | 9 | 11 | 9 | |
| 30 | 20 | 10 | 31 | 29 | 13 | 14 | 12 | 14 | |
| 40 | 27 | 13 | 49 | 41 | 18 | 15 | 22 | 23 | |
| 50 | 33 | 17 | 53 | 60 | 25 | 18 | 25 | 20 | |
| 60 | 40 | 20 | 70 | 64 | 29 | 21 | 27 | 24 | |
| Epoxy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 7 | 3 | 5 | 0 | 5 | 3 | 5 | 0 | |
| 20 | 14 | 6 | 12 | 7 | 10 | 4 | 8 | 5 | |
| 30 | 20 | 10 | 13 | 6 | 9 | 6 | 7 | 5 | |
| 40 | 27 | 13 | 18 | 10 | 9 | 9 | 9 | 7 | |
| 50 | 33 | 17 | 25 | 14 | 12 | 10 | 10 | 8 | |
| 60 | 40 | 20 | 34 | 20 | 15 | 11 | 16 | 12 | |
| Vinyl | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 7 | 3 | 5 | 3 | 2 | 0 | 2 | 2 | |
| 20 | 14 | 6 | 10 | 7 | 4 | 2 | 4 | 0 | |
| 30 | 20 | 10 | 16 | 9 | 9 | 3 | 8 | 3 | |
| 40 | 27 | 13 | 23 | 14 | 12 | 8 | 10 | 10 | |
| 50 | 33 | 17 | 35 | 21 | 18 | 14 | 16 | 15 | |
| 60 | 40 | 20 | 47 | 26 | 23 | 16 | 26 | 15 | |
| Resin | Total Biocide Content (wt.%) | ||
|---|---|---|---|
| Acrylic | 30 | 31 | 29 |
| Epoxy | 60 | 34 | 20 |
| Vinyl | 50 | 35 | 21 |
| Resin | Total Biocide Content (wt.%) | Particle Size (µm) | ||||||
|---|---|---|---|---|---|---|---|---|
| 5 Days | 5–10 Days | 10–15 Days | ||||||
| Acrylic | 20 | 20 | 9 | 11 | 9 | 10 | 19 | 20 |
| 50 | 4 | 5 | 8 | 8 | 16 | 20 | ||
| 40 | 20 | 23 | 22 | 15 | 18 | 41 | 49 | |
| 50 | 19 | 15 | 12 | 14 | 29 | 40 | ||
| Epoxy | 20 | 20 | 5 | 8 | 4 | 10 | 7 | 12 |
| 50 | 2 | 5 | 2 | 4 | 5 | 5 | ||
| 40 | 20 | 7 | 9 | 9 | 9 | 10 | 18 | |
| 50 | 3 | 4 | 3 | 3 | 4 | 8 | ||
| Vinyl/rosin | 20 | 20 | 0 | 4 | 2 | 4 | 7 | 10 |
| 50 | 0 | 3 | 0 | 3 | 5 | 6 | ||
| 40 | 20 | 10 | 10 | 8 | 12 | 14 | 23 | |
| 50 | 3 | 6 | 5 | 7 | 10 | 19 | ||
| Resin | Total Biocide Content (wt.%) | Pencil Hardness Degree | König Pendulum Oscillation Time (s) |
|---|---|---|---|
| Acrylic | 10 | B | 65 |
| 30 | H | 67 | |
| 40 | 3H | 70 | |
| Epoxy | 20 | 5H | 91 |
| 40 | 5H | 127 | |
| 60 | 6H | 165 | |
| Vinyl/rosin | 20 | 4H | 78 |
| 40 | 4H | 109 | |
| 50 | B | 65 |
| Resin | Total Biocide Content (wt.%) | Pin (Dolly) Separation Force (MPa) |
|---|---|---|
| Acrylic | 30 | 12/4 |
| 40 | 61/4 | |
| Epoxy | 40 | 58/9 |
| 60 | 41/8 | |
| Vinyl/rosin | 40 | 61/6 |
| 50 | 17/7 |
| Resin | Total Biocide Content (wt.%) | Lux |
|---|---|---|
| Acrylic | 30 | 26 |
| 40 | 20 | |
| Epoxy | 40 | 42 |
| 60 | 40 | |
| Vinyl/rosin | 40 | 45 |
| 50 | 38 |
| Resin | Total Biocide Content (wt.%) | Initial Weight (g) | Final Weight (g) | Mass Loss (g) |
|---|---|---|---|---|
| Acrylic | 30 | 62.847 | 62.83 | 0.017 |
| Epoxy | 60 | 64.196 | 64.18 | 0.016 |
| Vinyl/rosin | 50 | 63.301 | 63.30 | 0.001 |
| Resin | Total Biocide Content (wt.%) | Applied Weight (g) | Photo of Formed Scratch |
|---|---|---|---|
| Acrylic | 30 | 1500 |  |
| Epoxy | 60 | 3000 | No scratch |
| Vinyl/rosin | 50 | 2000 |  |
| Resin | Ecorr (V) | () | |
|---|---|---|---|
| Primer coating | −0.83 | 79 | 3.61 |
| Acrylic 30 wt.% | −0.39 | 3.2 | 0.128 |
| Epoxy 60 wt.% | −0.27 | 0.082 | 0.013 |
| Vinyl/rosin 50 wt.% | −0.58 | 0.47 | 0.0188 |
| Polyaniline Concentration (wt.%) | Ecorr (V) | ||
|---|---|---|---|
| 0 | −0.73 | 6.3 | 0.24 |
| 10 | 0.11 | 0.0093 | 0.00035 |
| 15 | 0.23 | 0.37 | 0.0148 |
| 20 | 0.47 | 8.7 | 0.348 |
| 30 | −0.07 | 3.1 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalaie, A.; Afshaar, A.; Mousavi, S.B.; Heidari, M. Investigation of the Release Rate of Biocide and Corrosion Resistance of Vinyl-, Acrylic-, and Epoxy-Based Antifouling Paints on Steel in Marine Infrastructures. Polymers 2023, 15, 3948. https://doi.org/10.3390/polym15193948
Jalaie A, Afshaar A, Mousavi SB, Heidari M. Investigation of the Release Rate of Biocide and Corrosion Resistance of Vinyl-, Acrylic-, and Epoxy-Based Antifouling Paints on Steel in Marine Infrastructures. Polymers. 2023; 15(19):3948. https://doi.org/10.3390/polym15193948
Chicago/Turabian StyleJalaie, Adel, Abdolah Afshaar, Seyed Borhan Mousavi, and Mohammad Heidari. 2023. "Investigation of the Release Rate of Biocide and Corrosion Resistance of Vinyl-, Acrylic-, and Epoxy-Based Antifouling Paints on Steel in Marine Infrastructures" Polymers 15, no. 19: 3948. https://doi.org/10.3390/polym15193948