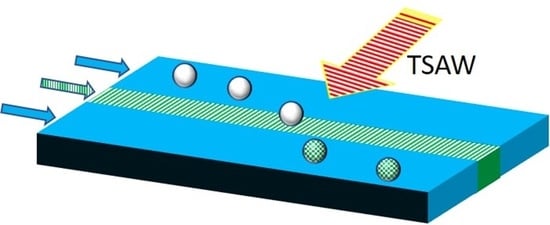
Acoustofluidics-Assisted Coating of Microparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Acoustofluidic Assembly
2.3. Conformal Coating of Microparticles
2.4. Characterization of the Coated Microparticles
3. Results and Discussion
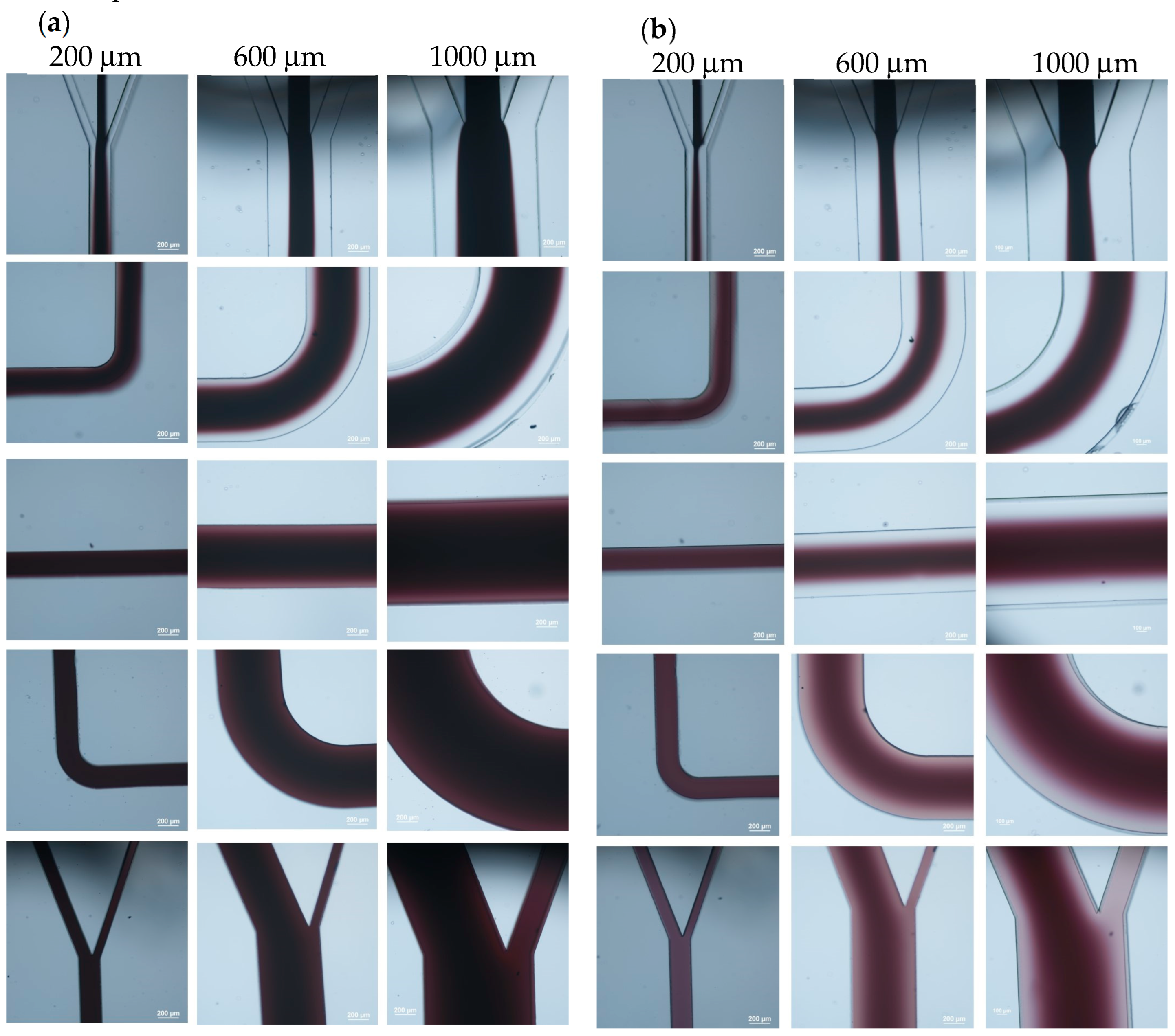
3.1. Laminar Flow Inside the Microchannel
3.2. Movement of Microparticles under Traveling Surface Acoustic Wave
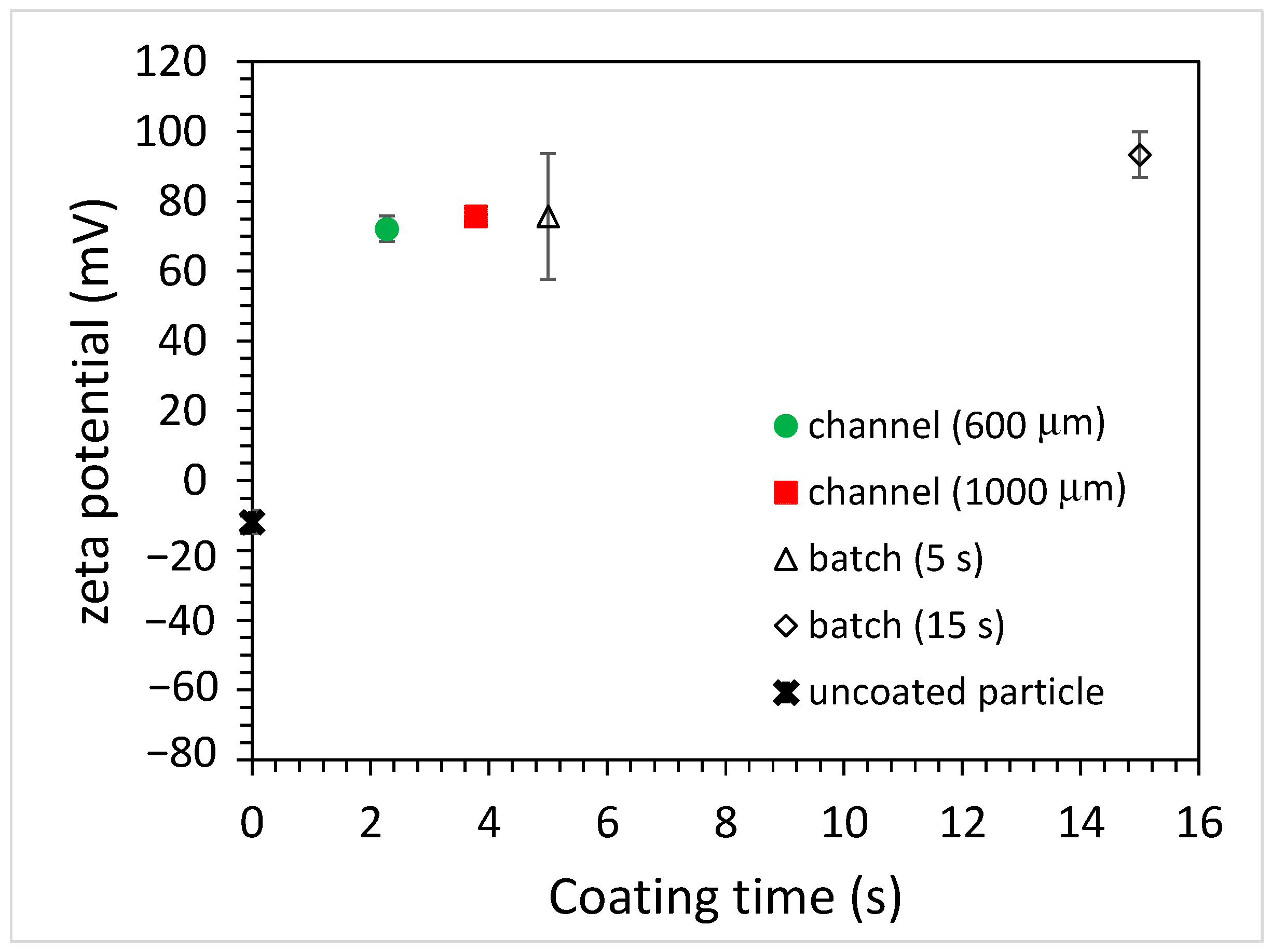
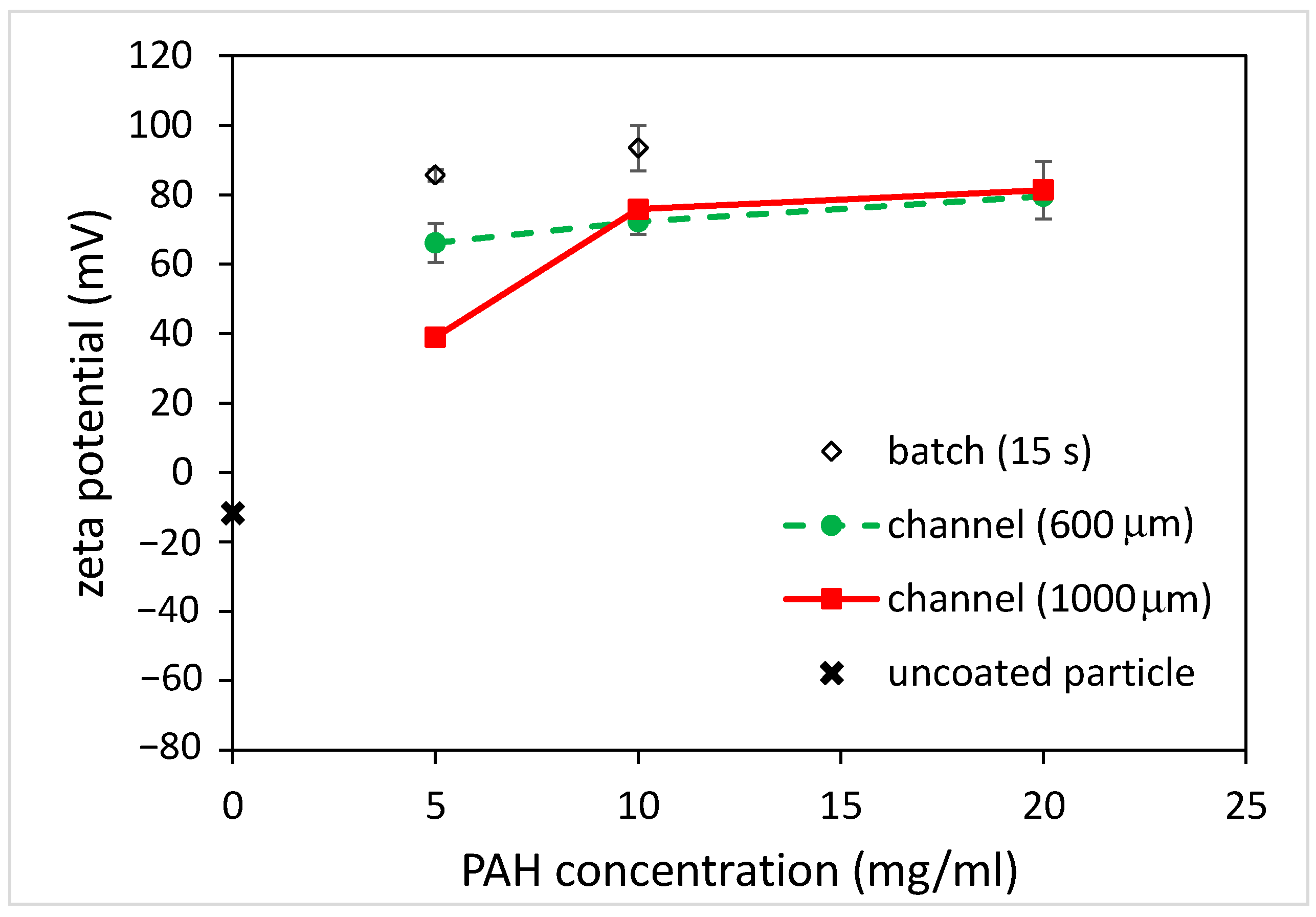
3.3. Coating of Microparticles
3.3.1. Unfunctionalized PS Particles
3.3.2. Carboxylate-Conjugated PS Microparticles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Xia, H.; Tan, X.; Song, S.; Zhang, S.; Meng, D.; Chen, Q.; Jin, Y. The potential applications of microparticles in the diagnosis, treatment, and prognosis of lung cancer. J. Transl. Med. 2022, 20, 404. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, J.; Prus, W.; Stachowiak, N. Microparticles based on natural and synthetic polymers for cosmetic applications. Int. J. Biol. Macromol. 2019, 129, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Nahum, V.; Domb, A.J. Recent Developments in Solid Lipid Microparticles for Food Ingredients Delivery. Foods 2021, 10, 400. [Google Scholar] [CrossRef]
- Zhang, Z.; Che, H.; Gao, J.; Wang, Y.; She, X.; Sun, J.; Gunawan, P.; Zhong, Z.; Su, F. Shape-controlled synthesis of Cu 2 O microparticles and their catalytic performances in the Rochow reaction. Catal. Sci. Technol. 2012, 2, 1207–1212. [Google Scholar] [CrossRef]
- Grasso, G.; Colella, F.; Forciniti, S.; Onesto, V.; Iuele, H.; Siciliano, A.C.; Carnevali, F.; Chandra, A.; Gigli, G.; del Mercato, L.L. Fluorescent nano-and microparticles for sensing cellular microenvironment: Past, present and future applications. Nanoscale Adv. 2023, 5, 4311–4336. [Google Scholar] [CrossRef] [PubMed]
- Li, C.M.; Wang, X.P.; Jiao, Z.H.; Zhang, Y.S.; Yin, X.B.; Cui, X.M.; Wei, Y.Z. Functionalized porous silica-based nano/micro particles for environmental remediation of hazard ions. Nanomaterials 2019, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.S.; Wexler, J.S.; Wan, J.; Stone, H.A. Conformal coating of particles in microchannels by magnetic forcing. Appl. Phys. Lett. 2011, 99, 15. [Google Scholar] [CrossRef]
- Moon, B.U.; Hakimi, N.; Hwang, D.K.; Tsai, S.S. Microfluidic conformal coating of non-spherical magnetic particles. Biomicrofluidics 2014, 8, 5. [Google Scholar] [CrossRef]
- Tarn, M.D.; Fakhrullin, R.F.; Paunov, V.N.; Pamme, N. Microfluidic device for the rapid coating of magnetic cells with polyelectrolytes. Mater. Lett. 2013, 95, 182–185. [Google Scholar] [CrossRef]
- de Hemptinne, A.; Gelin, P.; Ziemecka, I.; De Malsche, W. Microfluidic device for multilayer coating of magnetic microparticles. Powder Technol. 2023, 416, 118223. [Google Scholar] [CrossRef]
- Alorabi, A.Q.; Tarn, M.D.; Gómez-Pastora, J.; Bringas, E.; Ortiz, I.; Paunov, V.N.; Pamme, N. On-chip polyelectrolyte coating onto magnetic droplets—Towards continuous flow assembly of drug delivery capsules. Lab A Chip 2017, 17, 3785–3795. [Google Scholar] [CrossRef] [PubMed]
- Kantak, C.; Beyer, S.; Yobas, L.; Bansal, T.; Trau, D. A ‘microfluidic pinball’ for on-chip generation of layer-by-layer polyelectrolyte microcapsules. Lab A Chip 2011, 11, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Choi, S. Continuous medium exchange and cell isolation by size-selective passage through slanted micro-obstacles. J. Micromech. Microeng. 2014, 24, 025007. [Google Scholar] [CrossRef]
- Pan, D.; Liu, M.; Chen, Q.; Huang, W.; Li, B. Effects of flow rates and density matching on the integrity of solid particles coated by water phase compound droplets during the transport process. Coatings 2018, 8, 191. [Google Scholar] [CrossRef]
- Ayan, B.; Ozcelik, A.; Bachman, H.; Tang, S.Y.; Xie, Y.; Wu, M.; Li, P.; Huang, T.J. Acoustofluidic coating of particles and cells. Lab A Chip 2016, 16, 4366–4372. [Google Scholar] [CrossRef]
- Rasouli, R.; Villegasa, K.M.; Maryam Tabrizian, M. Acoustofluidics—Changing paradigm in tissue engineering, therapeutics development, and biosensing. Lab A Chip 2023, 23, 1300–1338. [Google Scholar] [CrossRef]
- Devendran, C.; Gunasekara, N.R.; Collinsb, D.J.; Neild, A. Batch process particle separation using surface acoustic waves (SAW): Integration of travelling and standing SAW. RSC Adv. 2016, 6, 5856–5864. [Google Scholar] [CrossRef]
- Chang, L.Y.; Liao, T.W.; Ye, M.L.; Juang, Y.J. Utilization of n-dodecane as coupling layer for reusable acoustofluidic microchips. J. Micromech. Microeng. 2021, 31, 127001. [Google Scholar] [CrossRef]
- Szilagyi, I.; Trefalt, G.; Tiraferri, A.; Maroni, P.; Borkovec, M. Polyelectrolyte adsorption, interparticle forces, and colloidal aggregation. Soft Matter 2014, 10, 2479–2502. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, C.K. Methods for Characterization of Nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Woodhead Publishing: Sawston, UK, 2017; pp. 43–58. [Google Scholar]
- Park, J.; Choi, Y.W.; Kim, K.; Chung, H.; Sohn, D. Aggregation processes of a weak polyelectrolyte, poly (allylamine) hydrochloride. Bull. Korean Chem. Soc. 2008, 29, 104. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, M.-L.; Chang, G.-M.; Juang, Y.-J. Acoustofluidics-Assisted Coating of Microparticles. Polymers 2023, 15, 4033. https://doi.org/10.3390/polym15194033
Yeh M-L, Chang G-M, Juang Y-J. Acoustofluidics-Assisted Coating of Microparticles. Polymers. 2023; 15(19):4033. https://doi.org/10.3390/polym15194033
Chicago/Turabian StyleYeh, Ming-Lin, Geng-Ming Chang, and Yi-Je Juang. 2023. "Acoustofluidics-Assisted Coating of Microparticles" Polymers 15, no. 19: 4033. https://doi.org/10.3390/polym15194033