Enhanced Electrochemical Performance of PEO-Based Composite Polymer Electrolyte with Single-Ion Conducting Polymer Grafted SiO2 Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Single-Ion Conducting Polymer through RAFT Polymerization
2.2.1. Synthesis of Lithium (4-styrenesulfonyl)(phenylsulfonyl) imide (LiSSPSI) Monomer [19]
2.2.2. Synthesis of 2-Methyl-2-[(dodecylsulfanylthiocarbonyl)sulfanyl]propanoic acid-2-(2-pyridyldithio) ethyl ester (Py-ss-DTPA) [20]
2.2.3. Synthesis of PLiSSPSI with Pyridyl Disulfide Terminal Group (py-ss-PLiSSPSI)
2.3. Preparation of PLiSSPSI Grafted SiO2 Nanoparticles (PLiSSPSI-g-SiO2)
2.3.1. Synthesis of Thiol-Functionalized Silica Particles (SiO2-SH) [22,23]
2.3.2. Synthesis of PLiSSPSI-g-SiO2 Nanoparticles
2.4. Preparation of PEO-Based Composite Solid Electrolyte with PLiSSPSI-g-SiO2 Nanoparticles
2.5. Characterization
3. Results and Discussion
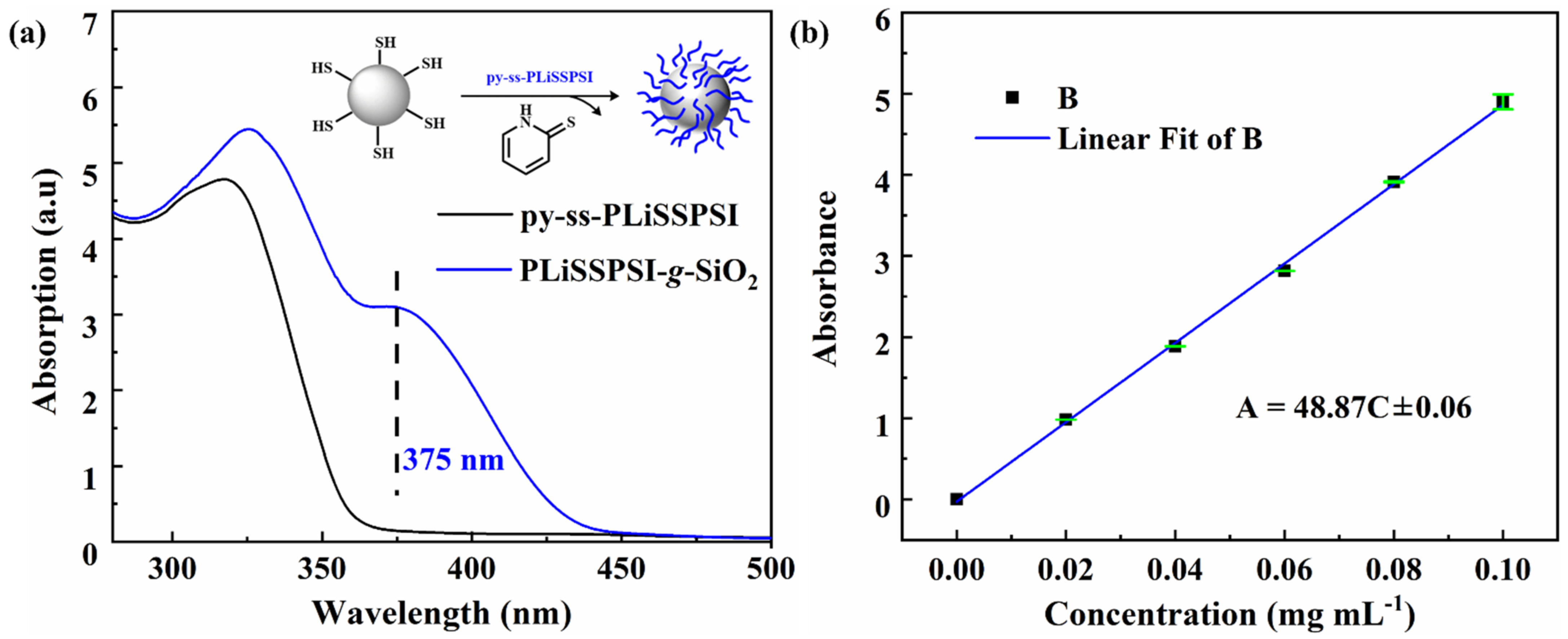
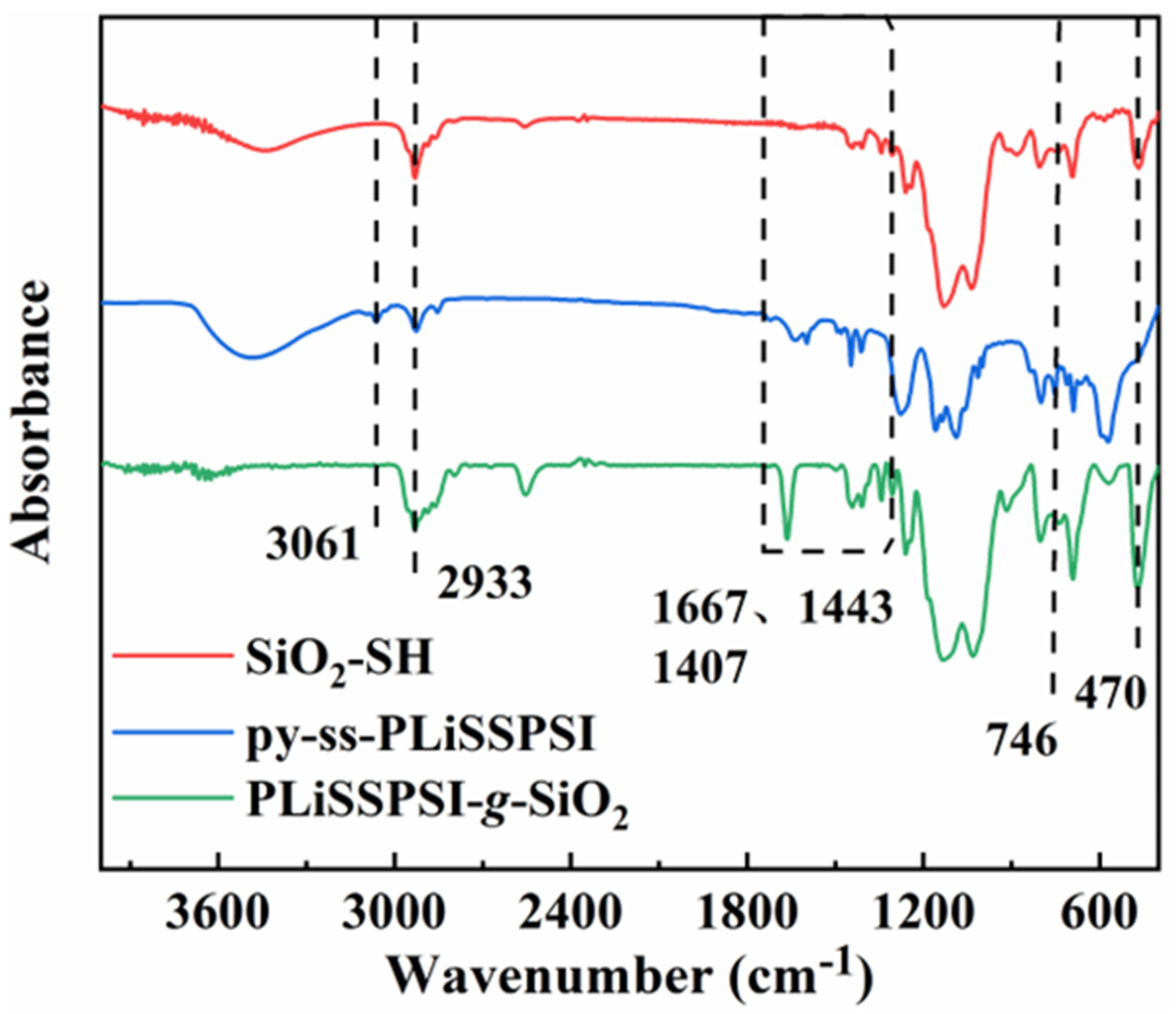
3.1. Preparation of PLiSSPSI-g-SiO2 Nanoparticles
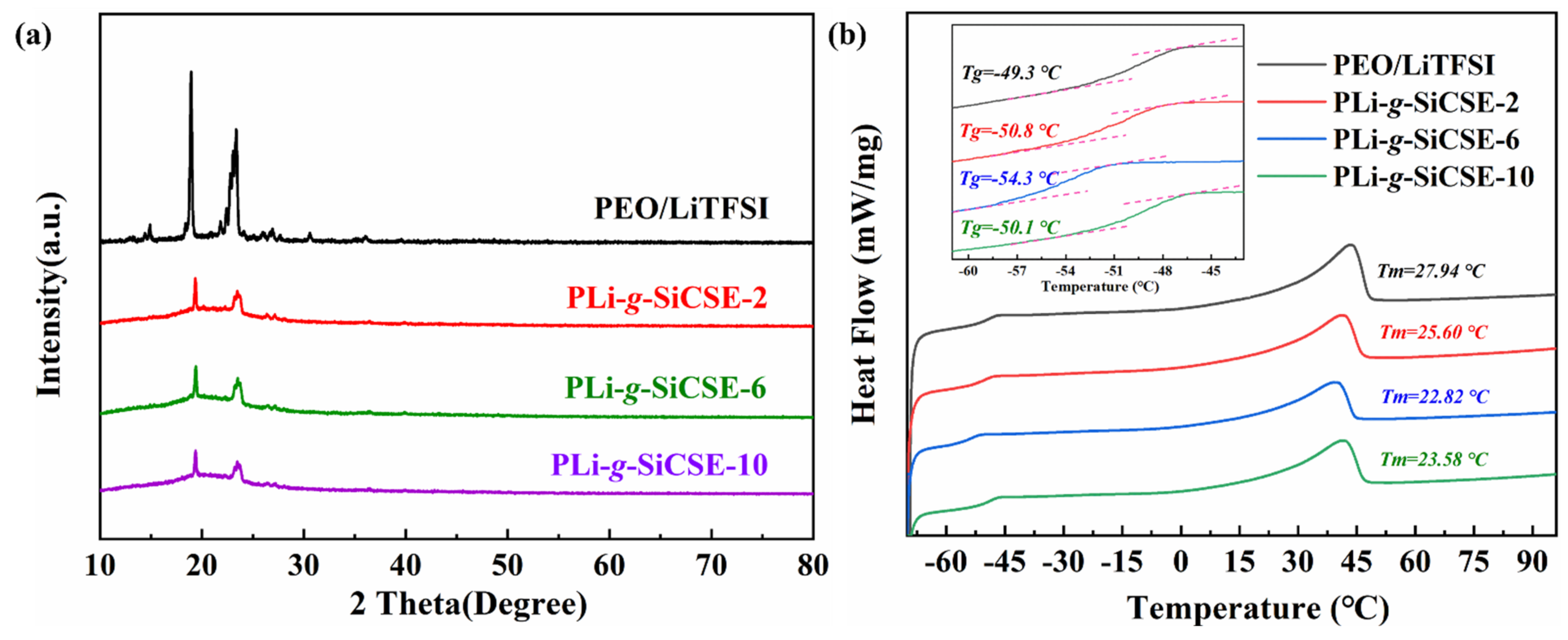
3.2. Composite Solid Polymer Electrolytes Based on Poly(ethylene oxide) and PLiSSPSI-g-SiO2 Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fenton, D.E.; Parker, J.M.; Wright, P.V. Complexes of alkali metal ions with poly (ethylene oxide). Polymer 1973, 14, 589. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, X.; Ma, Z.; Mei, P.; Xiao, W.; You, Q.; Zhang, Y. Development of the PEO based solid polymer electrolytes for all-solid state lithium ion batteries. Polymers 2018, 10, 1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, W.H. Polymer Electrolytes for Lithium-Ion Batteries. Adv. Mater. 1998, 10, 439–448. [Google Scholar] [CrossRef]
- Tan, S.J.; Zeng, X.X.; Ma, Q.; Wu, X.W.; Guo, Y.G. Recent advancements in polymer-based composite electrolytes for rechargeable lithium batteries. Electrochem. Energy Rev. 2018, 1, 113–138. [Google Scholar] [CrossRef]
- Shin, J.H.; Henderson, W.A.; Passerini, S. PEO-based polymer electrolytes with ionic liquids and their use in lithium metal-polymer electrolyte batteries. J. Electrochem. Soc. 2005, 152, A978. [Google Scholar] [CrossRef]
- Croce, F.; Appetecchi, G.B.; Persi, L.; Scrosati, B. Nanocomposite polymer electrolytes for lithium batteries. Nature 1998, 394, 456–458. [Google Scholar] [CrossRef]
- Nematdoust, S.; Najjar, R.; Bresser, D.; Passerini, S. Understanding the role of nanoparticles in PEO-based hybrid polymer electrolytes for solid-state lithium-polymer batteries. J. Phys. Chem. C 2020, 124, 27907–27915. [Google Scholar] [CrossRef]
- Rollo-Walker, G.; Malic, N.; Wang, X.; Chiefari, J.; Forsyth, M. Development and progression of polymer electrolytes for batteries: Influence of structure and chemistry. Polymers 2021, 13, 4127. [Google Scholar] [CrossRef]
- Zhan, H.; Wu, M.; Wang, R.; Wu, S.; Li, H.; Tian, T.; Tang, H. Excellent performances of composite polymer electrolytes with porous vinyl-functionalized SiO2 nanoparticles for lithium metal batteries. Polymers 2021, 13, 2468. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, K.; Ding, F.; Liu, X. Recent advances in solid polymer electrolytes for lithium batteries. Nano Res. 2017, 10, 4139–4174. [Google Scholar] [CrossRef]
- Bouchet, R.; Maria, S.; Meziane, R.; Aboulaich, A.; Lienafa, L.; Bonnet, J.P.; Phan, T.N.T.; Bertin, D.; Gigmes, D.; Devaux, D.; et al. Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nat. Mater. 2013, 12, 452–457. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Piszcz, M.; Coya, E.; Rojo, T.; Rodriguez-Martinez, L.M.; Armand, M.; Zhou, Z. Single lithium-ion conducting solid polymer electrolytes: Advances and perspectives. Chem. Soc. Rev. 2017, 46, 797–815. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, H.; Zhou, C.; Zheng, L.; Cheng, P.; Nie, J.; Feng, W.; Hu, Y.-S.; Li, H.; Huang, X.; et al. Single lithium-ion conducting polymer electrolytes based on a super-delocalized polyanion. Angew. Chem. Int. Ed. 2016, 55, 2521–2525. [Google Scholar] [CrossRef]
- Chazalviel, J.N. Electrochemical aspects of the generation of ramified metallic electrodeposits. Phys. Rev. A 1990, 42, 7355–7367. [Google Scholar] [CrossRef]
- Lago, N.; Garcia-Calvo, O.; Lopez del Amo, J.M.; Rojo, T.; Armand, M. All-solid-state lithium-ion batteries with grafted ceramic nanoparticles dispersed in solid polymer electrolytes. ChemSusChem 2015, 8, 3039–3043. [Google Scholar] [CrossRef]
- Liu, M.; Guan, X.; Liu, H.; Ma, X.; Wu, Q.; Ge, S.; Zhang, H.; Xu, J. Composite solid electrolytes containing single-ion lithium polymer grafted garnet for dendrite-free, long-life all-solid-state lithium metal batteries. Chem. Eng. J. 2022, 445, 136436. [Google Scholar] [CrossRef]
- Kim, B.; Kang, H.; Kim, K.; Wang, R.Y.; Park, M.J. All-solid-state lithium-organic batteries comprising single-ion polymer nanoparticle electrolytes. ChemSusChem 2020, 13, 2271–2279. [Google Scholar] [CrossRef]
- Porcarelli, L.; Sutton, P.; Bocharova, V.; Aguirresarobe, R.H.; Zhu, H.; Goujon, N.; Leiza, J.R.; Sokolov, A.; Forsyth, M.; Mecerreyes, D. Single-ion conducting polymer nanoparticles as functional fillers for solid electrolytes in lithium metal batteries. ACS Appl. Mater. Interfaces 2021, 13, 54354–54362. [Google Scholar] [CrossRef]
- Cao, C.; Li, Y.; Feng, Y.; Long, P.; An, H.; Qin, C.; Han, J.; Li, S.; Feng, W. A sulfonimide-based alternating copolymer as a single-ion polymer electrolyte for high-performance lithium-ion batteries. J. Mater. Chem. A 2017, 5, 22519–22526. [Google Scholar] [CrossRef]
- Hou, W.; Wang, H.; Cui, Y.; Liu, Y.; Ma, X.; Zhao, H. Surface nanostructures fabricated by polymerization-induced surface self-assembly. Macromolecules 2019, 52, 8404–8414. [Google Scholar] [CrossRef]
- Srikamut, P.; Phakkeeree, T.; Seidi, F.; Iamsaard, S.; Crespy, D. Dual-responsive polymer conjugates with enhanced anticorrosion performance. ACS Appl. Polym. Mater. 2021, 3, 5425–5433. [Google Scholar] [CrossRef]
- Lee, Y.G.; Park, J.H.; Oh, C.; Oh, S.G.; Kim, Y.C. Preparation of highly monodispersed hybrid silica spheres using a one-step sol-gel reaction in aqueous solution. Langmuir 2007, 23, 10875–10878. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Zou, H. Removal of aqueous Hg (II) by thiol-functionalized nonporous silica microspheres prepared by one-step sol-gel method. RSC Adv. 2020, 10, 18534–18542. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lin, Y.; Xu, J. Synthesis of Ag–Ho, Ag–Sm, Ag–Zn, Ag–Cu, Ag–Cs, Ag–Zr, Ag–Er, Ag–Y and Ag–Co metal organic nanoparticles for UV-Vis-NIR wide-range bio-tissue imaging. Photochem. Photobiol. Sci. 2019, 18, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hwang, H.S.; Lo, T.N.; Koh, W.G.; Park, I. Effect of Silica Size and Content on Superamphiphobic Properties of Silica-Fluoropolymer Core-Shell Coatings. Polymers 2020, 12, 2864. [Google Scholar] [CrossRef] [PubMed]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. Sulfonated surfaces by sulfur dioxide plasma surface treatment of plasma polymer films. Plasma Process. Polym. 2009, 6, 583–592. [Google Scholar] [CrossRef]
- Garcia-Calvo, O.; Lago, N.; Devaraj, S.; Armand, M. Cross-linked solid polymer electrolyte for all-solid-state rechargeable lithium batteries. Electrochim. Acta 2016, 220, 587–594. [Google Scholar]
- Chan, C.H.; Kammer, H.-W. Low frequency dielectric relaxation and conductance of solid polymer electrolytes with PEO and blends of PEO and PMMA. Polymers 2020, 12, 1009. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Ding, S.P.; Ye, Z.; Xia, D.L.; Xu, J.T. PEO-based block copolymer electrolytes containing double conductive phases with improved mechanical and electrochemical properties. Materials 2022, 15, 7930. [Google Scholar] [CrossRef]
- Butzelaar, A.J.; Roring, P.; Mach, T.P.; Hoffmann, M.; Jeschull, F.; Wilhelm, M.; Winter, M.; Brunklaus, G.; Théato, P. Styrene-based poly(ethylene oxide) side-chain block copolymers as solid polymer electrolytes for high-voltage lithium-metal batteries. ACS Appl. Mater. Interfaces 2021, 13, 39257–39270. [Google Scholar] [CrossRef]
- Lin, D.; Liu, W.; Liu, Y.; Lee, H.R.; Hsu, P.C.; Liu, K.; Cui, Y. High ionic conductivity of composite solid polymer electrolyte via in situ synthesis of monodispersed SiO2 nanospheres in poly (ethylene oxide). Nano Lett. 2016, 16, 459–465. [Google Scholar] [CrossRef]
- Lee, F.; Tsai, M.C.; Lin, M.H.; Ni’mah, Y.L.; Hy, S.; Kuo, C.Y.; Cheng, J.-H.; Rick, J.; Su, W.-N.; Hwang, B.-J. Capacity retention of lithium sulfur batteries enhanced with nano-sized TiO2-embedded polyethylene oxide. J. Mater. Chem. A 2017, 5, 6708–6715. [Google Scholar] [CrossRef]
- Ahmed, H.T.; Abdullah, O.G. Preparation and composition optimization of PEO:MC polymer blend films to enhance electrical conductivity. Polymers 2019, 11, 853. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Xu, H.; Wang, A.; Liu, X.; Chen, J.; Wang, Z.; Feng, D.; Zeng, Q.; Zhang, L. Nanocomposite all-solid-state polymer electrolyte for high-performance lithium batteries. Energy Technol. 2019, 7, 122–130. [Google Scholar] [CrossRef]
- Hu, J.; Wang, W.; Peng, H.; Guo, M.; Feng, Y.; Xue, Z.; Ye, Y.; Xie, X. Flexible organic-inorganic hybrid solid electrolytes formed via thiol-acrylate photopolymerization. Macromolecules 2017, 50, 1970–1980. [Google Scholar] [CrossRef]
- Jamalpour, S.; Ghahramani, M.; Ghaffarian, S.R.; Javanbakht, M. Improved performance of lithium ion battery by the incorporation of novel synthesized organic-inorganic hybrid nanoparticles SiO2-poly (methyl methacrylate-co-ureidopyrimidinone) in gel polymer electrolyte based on poly (vinylidene fluoride). Polymer 2021, 228, 123924. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P. Electrolytes for solid-state lithium rechargeable batteries: Recent advances and perspectives. Chem. Soc. Rev. 2011, 40, 2525–2540. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Wang, Y.; Li, Z.; Peng, C.; Feng, Y.; Feng, W. Cross-linked single-ion solid polymer electrolytes with alternately distributed lithium sources and ion-conducting segments for lithium metal batteries. Macromolecules 2021, 54, 9135–9144. [Google Scholar] [CrossRef]




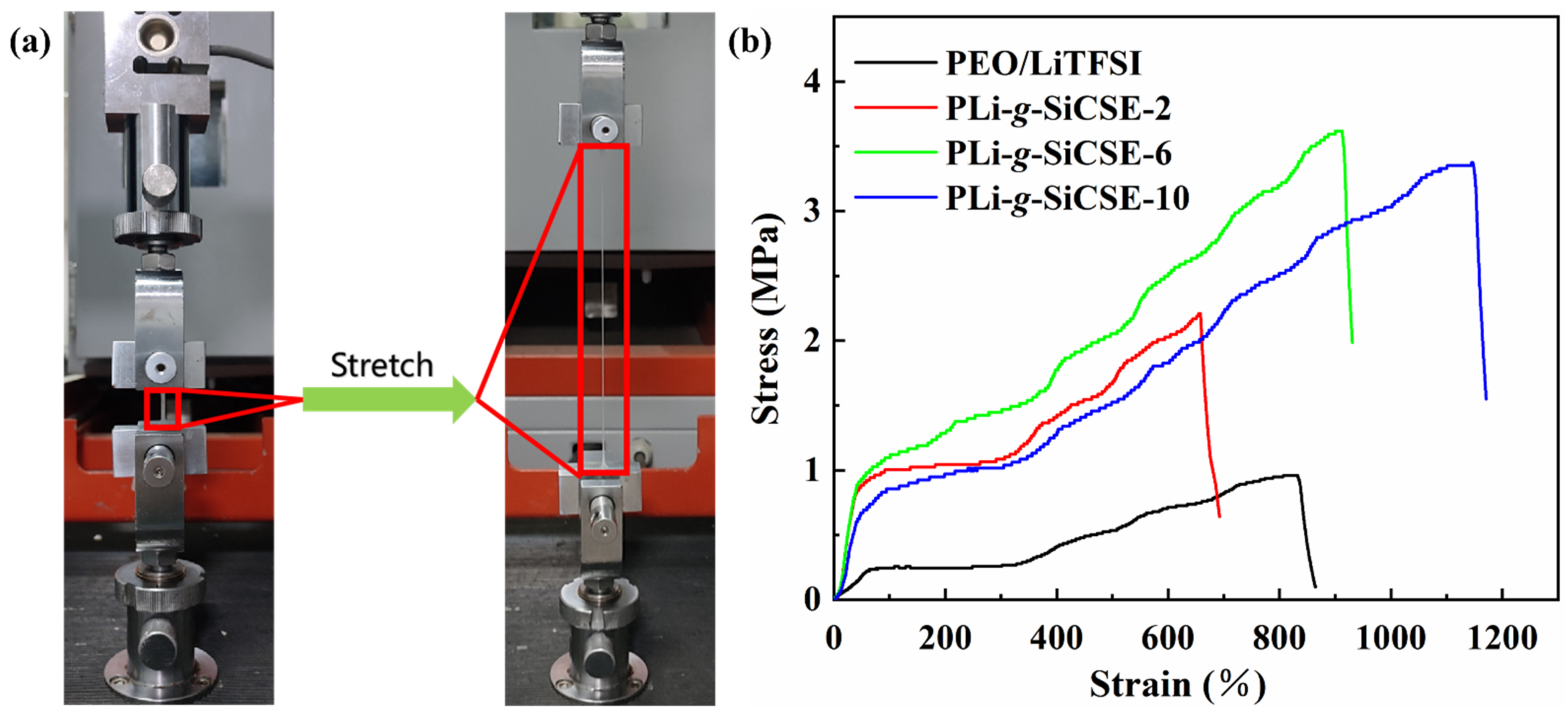
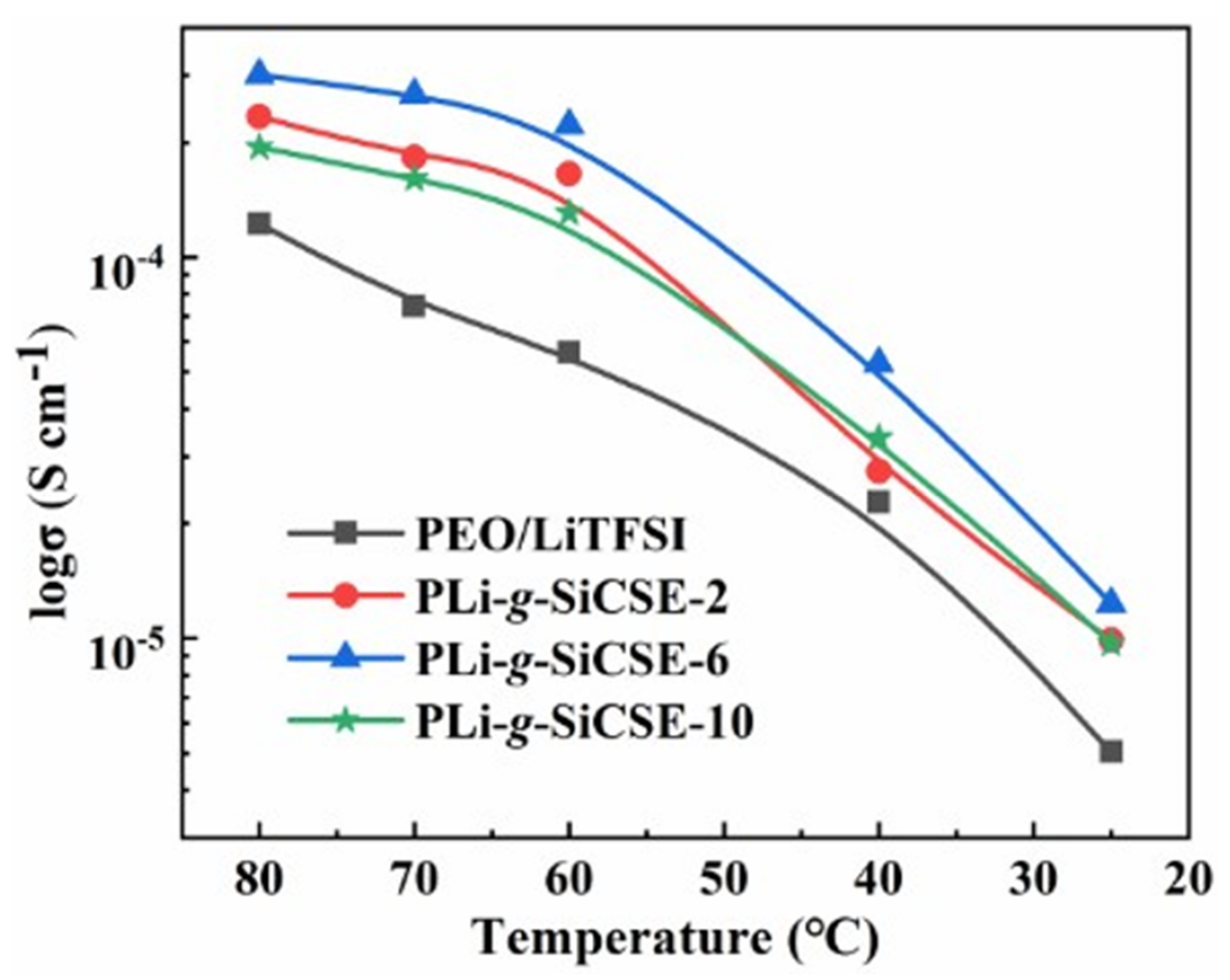



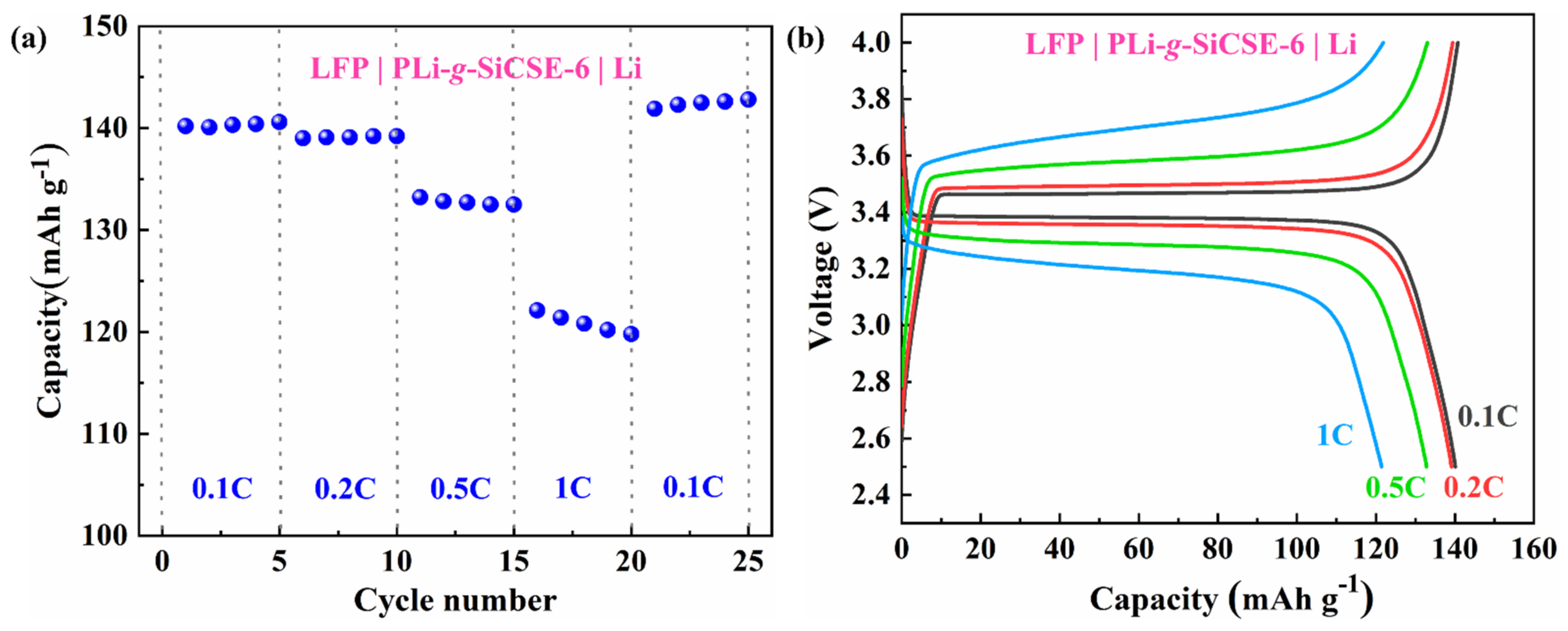

| PEO/LiTFSI | PLi-g-SiCSE-2 | PLi-g-SiCSE-6 | PLi-g-SiCSE-10 | |
|---|---|---|---|---|
| Stress (MPa) | 0.97 | 2.22 | 3.63 | 3.37 |
| Strain (%) | 830 | 657 | 911 | 1147 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Mao, W.; Gong, J.; Liu, H.; Shao, Y.; Sun, L.; Wang, H.; Wang, C. Enhanced Electrochemical Performance of PEO-Based Composite Polymer Electrolyte with Single-Ion Conducting Polymer Grafted SiO2 Nanoparticles. Polymers 2023, 15, 394. https://doi.org/10.3390/polym15020394
Liu X, Mao W, Gong J, Liu H, Shao Y, Sun L, Wang H, Wang C. Enhanced Electrochemical Performance of PEO-Based Composite Polymer Electrolyte with Single-Ion Conducting Polymer Grafted SiO2 Nanoparticles. Polymers. 2023; 15(2):394. https://doi.org/10.3390/polym15020394
Chicago/Turabian StyleLiu, Xuan, Wanning Mao, Jie Gong, Haiyu Liu, Yanming Shao, Liyu Sun, Haihua Wang, and Chao Wang. 2023. "Enhanced Electrochemical Performance of PEO-Based Composite Polymer Electrolyte with Single-Ion Conducting Polymer Grafted SiO2 Nanoparticles" Polymers 15, no. 2: 394. https://doi.org/10.3390/polym15020394





