Effects of Calcium Carbonate Microcapsules and Nanohydroxyapatite on Properties of Thermosensitive Chitosan/Collagen Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Calcium Carbonate Microcapsules
2.3. Preparation of Thermosensitive Calcium Carbonate Microcapsules–Nanohydroxyapatite–Chitosan/Collagen Hydrogel
2.4. Scanning Electron Microscopy and Energy Dispersive Spectroscopy
2.5. Rheological Testing
2.6. Mechanical Properties
2.7. A Mercury Intrusion Porosimetry
2.8. Swelling Test
2.9. Degradation Test
2.10. Release Profile of Flavonoids
2.11. Cytotoxicity Test of the Hydrogel
2.12. Subcutaneous Implantation in Mice
2.13. Histopathological Analysis for Biocompatibility
2.14. Statistical Analysis
3. Results
3.1. Calcium Carbonate Microcapsules
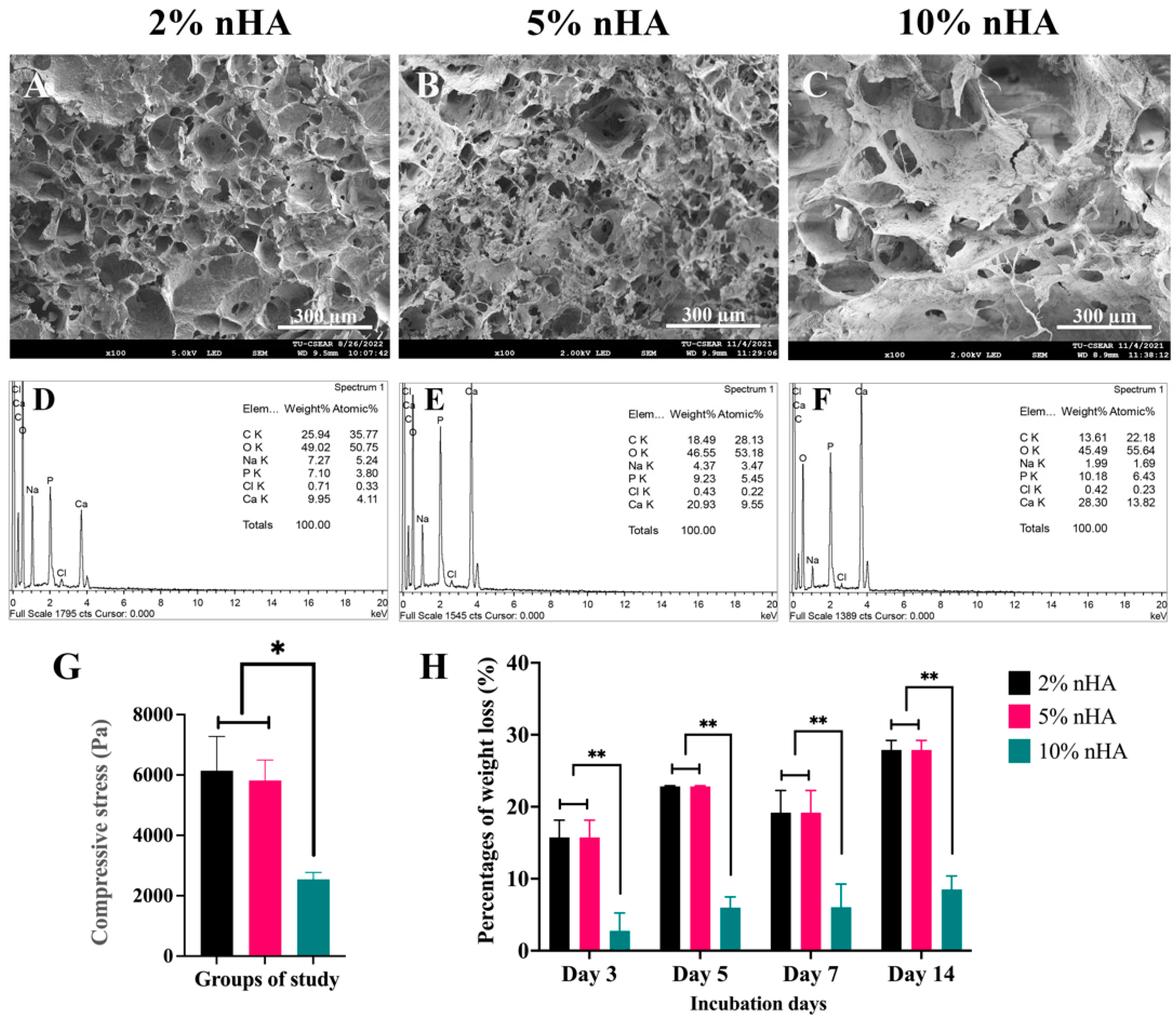
3.2. Effects of the Nanohydroxyapatite on Microstructure and Porosity of the Thermosensitive Calcium Carbonate Microcapsules–Nanohydroxyapatite–Chitosan/Collagen Hydrogel
3.3. Mechanical Strength and Degree of Degradation of the Thermosensitive Calcium Carbonate Microcapsules–Nanohydroxyapatite–Chitosan/Collagen Hydrogel
3.4. Effects of Calcium Carbonate Microcapsules on Sol–Gel Transition Time and Microstructure of the Thermosensitive Calcium Carbonate Microcapsules–Nanohydroxyapatite–Chitosan/Collagen Hydrogel
3.5. Mercury Intrusion Porosimetry of the Thermosensitive Calcium Carbonate Microcapsules–Nanohydroxyapatite–Chitosan/Collagen Hydrogel
3.6. Mechanical Strength
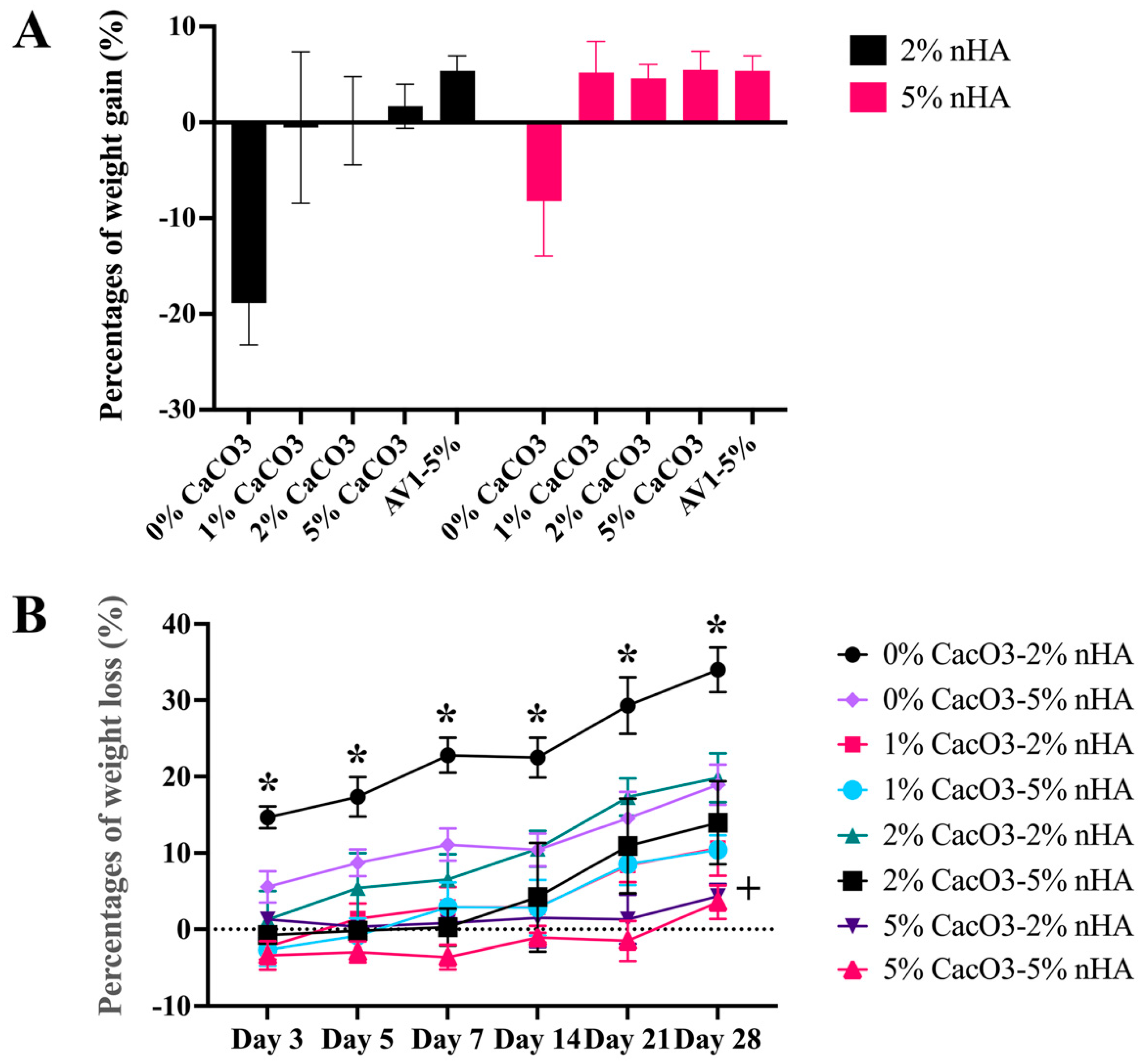
3.7. Physical Properties of the Thermosensitive Calcium Carbonate Microcapsules–Nanohydroxyapatite–Chitosan/Collagen Hydrogel
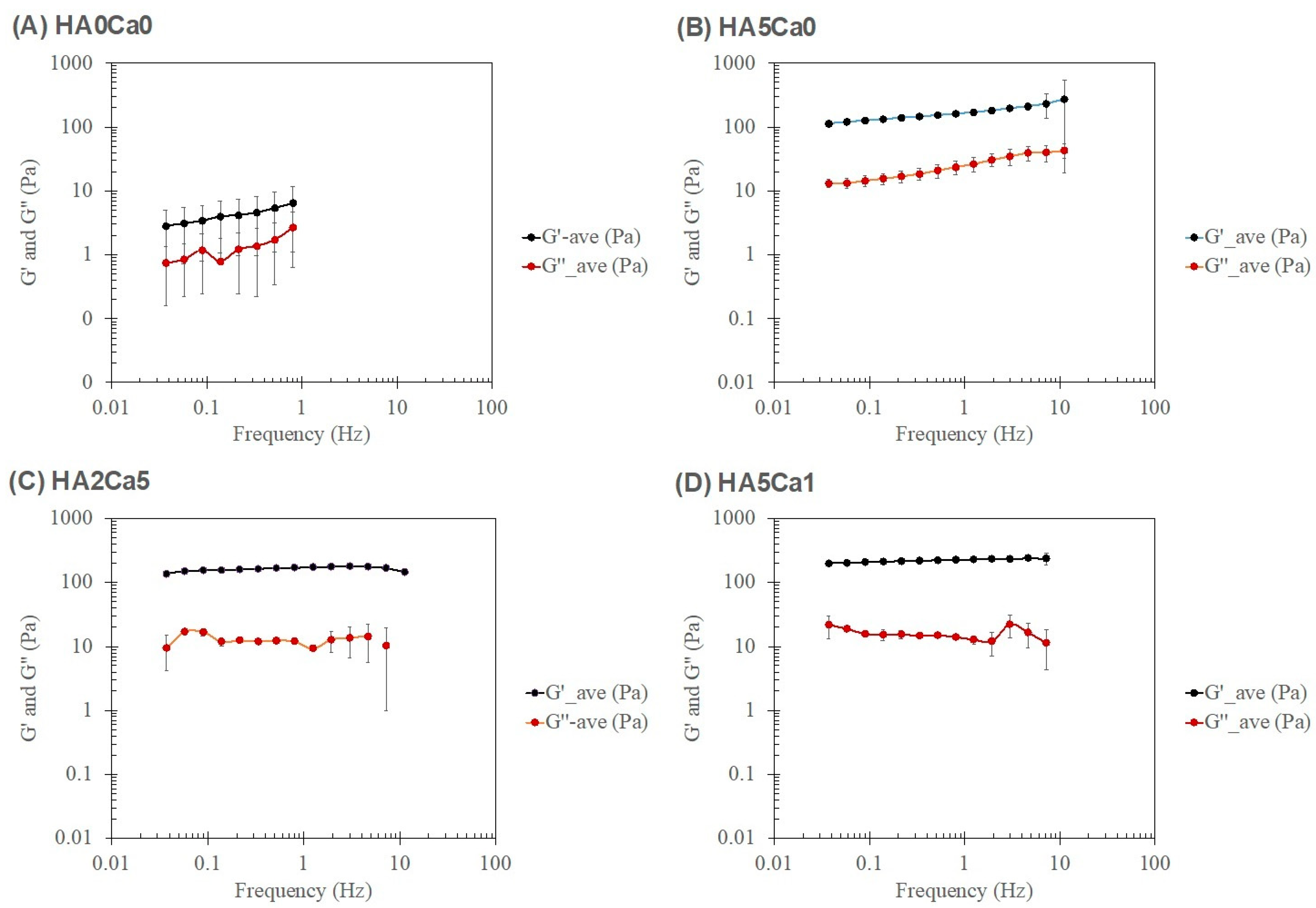
3.8. Rheological Characteristics of the Thermosensitive Calcium Carbonate Microcapsules–Nanohydroxyapatite–Chitosan/Collagen Hydrogel
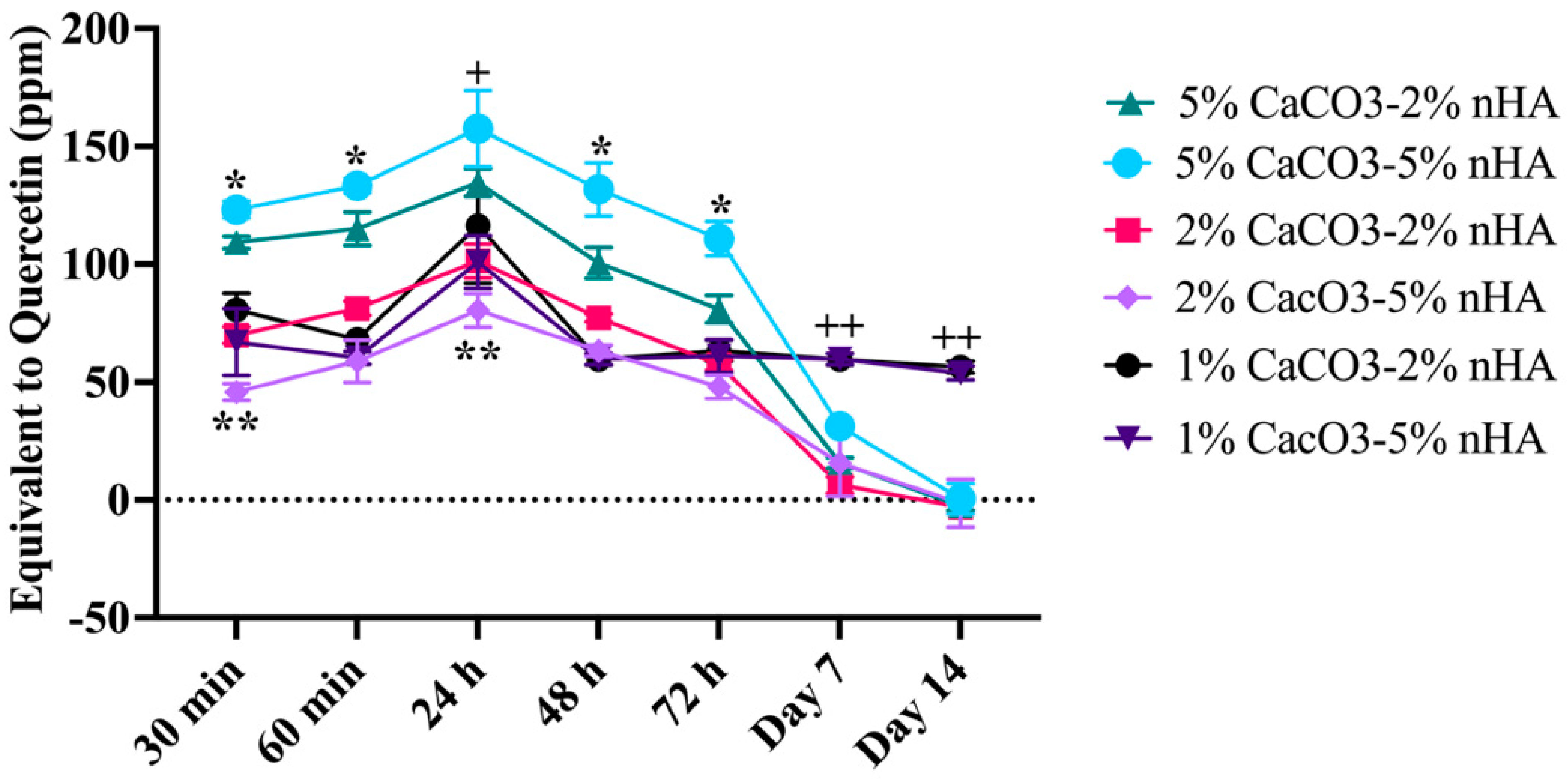
3.9. Control Release Property of the Thermosensitive Calcium Carbonate Microcapsules–Nanohydroxyapatite–Chitosan/Collagen Hydrogel
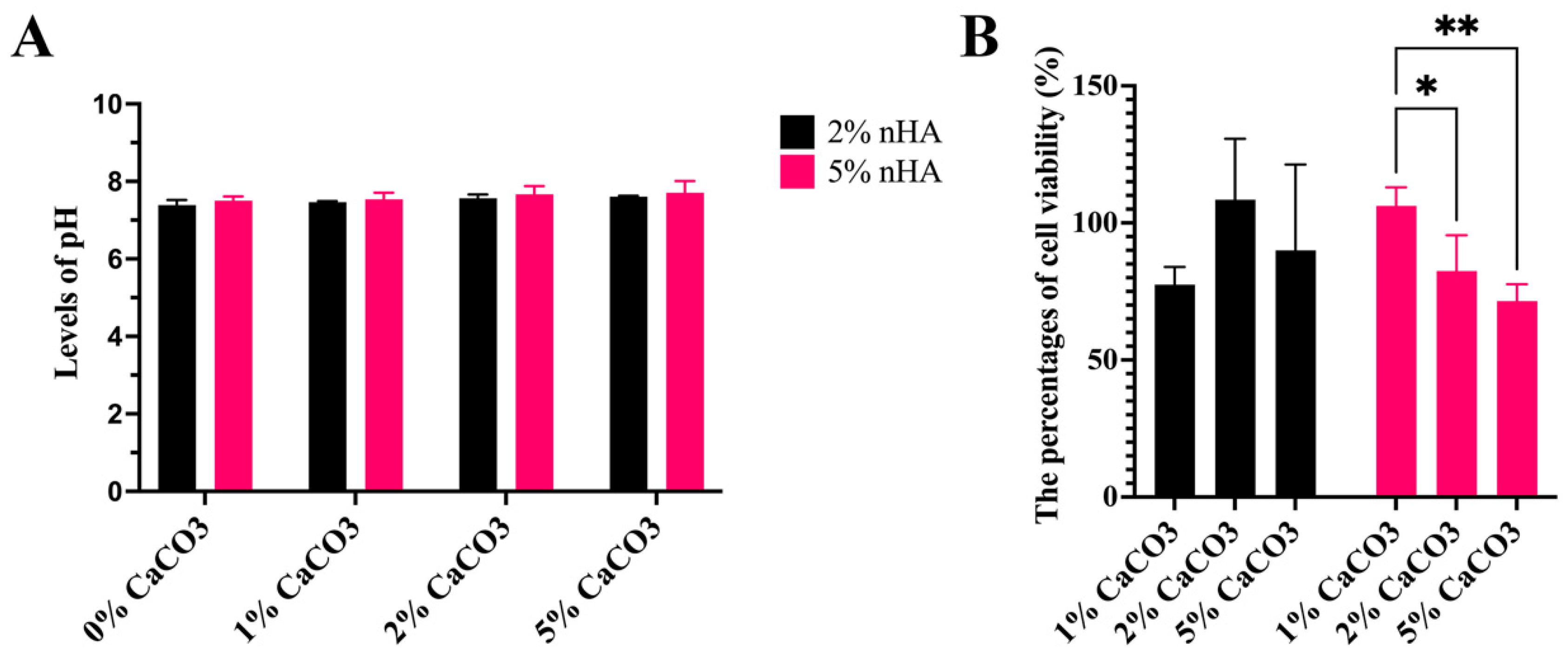
3.10. Levels of Acid–Base Balance (pH) and Cell Cytotoxicity of the Hydrogels
3.11. In Vivo Biocompatibility Analysis of the Thermosensitive Calcium Carbonate Microcapsules–Nanohydroxyapatite–Chitosan/Collagen Hydrogel
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cawood, J.I.; Howell, R.A. A classification of the edentulous jaws. Int. J. Oral Maxillofac. Surg. 1988, 17, 232–236. [Google Scholar] [CrossRef]
- Rocchietta, I.; Fontana, F.; Simion, M. Clinical outcomes of vertical bone augmentation to enable dental implant placement: A systematic review. J. Clin. Periodontol. 2008, 35, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Starch-Jensen, T.; Deluiz, D.; Deb, S.; Bruun, N.H.; Tinoco, E.M.B. Harvesting of Autogenous Bone Graft from the Ascending Mandibular Ramus Compared with the Chin Region: A Systematic Review and Meta-Analysis Focusing on Complications and Donor Site Morbidity. J. Oral Maxillofac. Res. 2020, 11, e1. [Google Scholar] [CrossRef] [PubMed]
- Clavero, J.; Lundgren, S. Ramus or chin grafts for maxillary sinus inlay and local onlay augmentation: Comparison of donor site morbidity and complications. Clin. Implant. Dent. Relat. Res. 2003, 5, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Ebied, M.; Xu, J.; Zreiqat, H. Current Approaches to Bone Tissue Engineering: The Interface between Biology and Engineering. Adv. Healthc. Mater. 2018, 7, e1701061. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Ma, P.; Wu, W.; Wei, Y.; Ren, L.; Lin, S.; Wu, J. Biomimetic gelatin/chitosan/polyvinyl alcohol/nano-hydroxyapatite scaffolds for bone tissue engineering. Mater. Des. 2022, 207, 109865. [Google Scholar] [CrossRef]
- Sun, Q.; Yu, L.; Zhang, Z.; Qian, C.; Fang, H.; Wang, J.; Wu, P.; Zhu, X.; Zhang, J.; Zhong, L.; et al. A novel gelatin/carboxymethyl chitosan/nano-hydroxyapatite/beta-tricalcium phosphate biomimetic nanocomposite scaffold for bone tissue engineering applications. Front. Chem. 2022, 10, 958420. [Google Scholar] [CrossRef]
- Li, Y.; Fang, X.; Jiang, T. Minimally traumatic alveolar ridge augmentation with a tunnel injectable thermo-sensitive alginate scaffold. J. Appl. Oral Sci. 2015, 23, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.; Rubin, J.P.; Marra, K.G. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for adipose tissue regeneration. Organogenesis 2010, 6, 173–180. [Google Scholar] [CrossRef]
- Park, H.; Temenoff, J.S.; Tabata, Y.; Caplan, A.I.; Mikos, A.G. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials 2007, 28, 3217–3227. [Google Scholar] [CrossRef] [Green Version]
- Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Ma, W.; Su, F.; Wang, Y.; Liu, J.; Wang, D.; Liu, H. The osteogenic differentiation of dog bone marrow mesenchymal stem cells in a thermo-sensitive injectable chitosan/collagen/beta-glycerophosphate hydrogel: In vitro and in vivo. J. Mater. Sci. Mater. Med. 2011, 22, 2111–2118. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Stegemann, J.P. Thermogelling chitosan and collagen composite hydrogels initiated with beta-glycerophosphate for bone tissue engineering. Biomaterials 2010, 31, 3976–3985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arpornmaeklong, P.; Sareethammanuwat, M.; Apinyauppatham, K.; Boonyuen, S. Characteristics and biologic effects of thermosensitive quercetin-chitosan/collagen hydrogel on human periodontal ligament stem cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1656–1670. [Google Scholar] [CrossRef]
- Sareethammanuwat, M.; Boonyuen, S.; Arpornmaeklong, P. Effects of beta-tricalcium phosphate nanoparticles on the properties of a thermosensitive chitosan/collagen hydrogel and controlled release of quercetin. J. Biomed. Mater. Res. Part A 2021, 109, 1147–1159. [Google Scholar] [CrossRef]
- Arpornmaeklong, P.; Pripatnanont, P.; Suwatwirote, N. Properties of chitosan-collagen sponges and osteogenic differentiation of rat-bone-marrow stromal cells. Int. J. Oral Maxillofac. Surg. 2008, 37, 357–366. [Google Scholar] [CrossRef]
- Arpornmaeklong, P.; Suwatwirote, N.; Pripatnanont, P.; Oungbho, K. Growth and differentiation of mouse osteoblasts on chitosan-collagen sponges. Int. J. Oral Maxillofac. Surg. 2007, 36, 328–337. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, P.; Vulesevic, B.; Kuraitis, D.; Li, F.; Yang, A.F.; Griffith, M.; Ruel, M.; Suuronen, E.J. A collagen-chitosan hydrogel for endothelial differentiation and angiogenesis. Tissue Eng. Part A 2010, 16, 3099–3109. [Google Scholar] [CrossRef]
- Gkioni, K.; Leeuwenburgh, S.C.; Douglas, T.E.; Mikos, A.G.; Jansen, J.A. Mineralization of hydrogels for bone regeneration. Tissue Eng. Part B Rev. 2010, 16, 577–585. [Google Scholar] [CrossRef]
- Ghorbani, F.M.; Kaffashi, B.; Shokrollahi, P.; Akhlaghi, S.; Hedenqvist, M.S. Effect of hydroxyapatite nano-particles on morphology, rheology and thermal behavior of poly(caprolactone)/chitosan blends. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Saravanan, S.; Sastry, T.P.; Selvamurugan, N. Nanohydroxyapatite-reinforced chitosan composite hydrogel for bone tissue repair in vitro and in vivo. J. Nanobiotechnology 2015, 13, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClements, D.J. Designing biopolymer microgels to encapsulate, protect and deliver bioactive components: Physicochemical aspects. Adv. Colloid Interface Sci. 2017, 240, 31–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnelly, F.C.; Purcell-Milton, F.; Framont, V.; Cleary, O.; Dunne, P.W.; Gun′ko, Y.K. Synthesis of CaCO3 nano- and micro-particles by dry ice carbonation. Chem. Commun. 2017, 53, 6657–6660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Som, A.; Raliya, R.; Tian, L.; Akers, W.; Ippolito, J.E.; Singamaneni, S.; Biswas, P.; Achilefu, S. Monodispersed calcium carbonate nanoparticles modulate local pH and inhibit tumor growth in vivo. Nanoscale 2016, 8, 12639–12647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalmykova, T.P.; Kostina, Y.V.; Ilyin, S.O.; Bogdanova, Y.G.; Severin, A.V.; Ivanov, P.L.; Antonov, S.V. Effect of Synthesis Medium on the Structure and Physicochemical Properties of Biomineral Composites Based on Hydroxyapatite and Hyaluronic Acid. Polym. Sci. Ser. B 2020, 62, 61–71. [Google Scholar] [CrossRef]
- Badila, A.E.; Radulescu, D.M.; Ilie, A.; Niculescu, A.G.; Grumezescu, A.M.; Radulescu, A.R. Bone Regeneration and Oxidative Stress: An Updated Overview. Antioxidants 2022, 11, 318. [Google Scholar] [CrossRef]
- Balestrin, L.A.; Back, P.I.; Marques, M.D.S.; Araujo, G.M.S.; Carrasco, M.C.F.; Batista, M.M.; Silveira, T.; Rodrigues, J.L.; Fachel, F.N.S.; Koester, L.S.; et al. Effect of Hydrogel Containing Achyrocline satureioides (Asteraceae) Extract-Loaded Nanoemulsions on Wound Healing Activity. Pharmaceutics 2022, 14, 2726. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Y.; Jiang, X.; Zhang, X.; Xia, L.; Lin, K.; Xu, Y. The Effect of Quercetin on the Osteogenesic Differentiation and Angiogenic Factor Expression of Bone Marrow-Derived Mesenchymal Stem Cells. PLoS ONE 2015, 10, e0129605. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Li, M.; Wei, Y.; Xue, C.; Chen, M.; Fei, Y.; Tan, L.; Luo, Z.; Cai, K.; Hu, Y. ROS-activatable biomimetic interface mediates in-situ bioenergetic remodeling of osteogenic cells for osteoporotic bone repair. Biomaterials 2022, 291, 121878. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, D.; Li, Y.; Zou, J.; Han, R.; Li, H.; Zhang, J. Effect of Puerarin on Osteogenic Differentiation in vitro and on New Bone Formation in vivo. Drug Des. Dev. Ther. 2022, 16, 2885–2900. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Chen, X.; Du, S.; Ma, Y.; Chen, H.; Yuan, G.; Li, J.; Xiong, D.; Tan, H.; Ling, Z.; et al. Injectable polysaccharide hydrogel embedded with hydroxyapatite and calcium carbonate for drug delivery and bone tissue engineering. Int. J. Biol. Macromol. 2018, 118, 1257–1266. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Ko, Y.C.; Chang, Y.F.; Huang, S.H.; Liu, C.J. Thermosensitive chitosan-gelatin-based hydrogel containing curcumin-loaded nanoparticles and latanoprost as a dual-drug delivery system for glaucoma treatment. Exp. Eye Res. 2019, 179, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Moreira, C.D.; Carvalho, S.M.; Mansur, H.S.; Pereira, M.M. Thermogelling chitosan-collagen-bioactive glass nanoparticle hybrids as potential injectable systems for tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Pan, P.; Zhang, Y.; Zhong, S.; Zhang, Q. Preparation of chitosan/nano hydroxyapatite organic-inorganic hybrid microspheres for bone repair. Colloids Surf. B Biointerfaces 2015, 134, 401–407. [Google Scholar] [CrossRef]
- Jia, Z.; Tang, M.; Wu, J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Juhasz, J.A.; Best, S.M.; Bonfield, W. Preparation of novel bioactive nano-calcium phosphate-hydrogel composites. Sci. Technol. Adv. Mater. 2010, 11, 014103. [Google Scholar] [CrossRef]
- Adams, S.; Pacharinsak, C. Mouse anesthesia and analgesia. Curr. Protoc. Mouse Biol. 2015, 5, 51–63. [Google Scholar] [CrossRef]
- Arpornmaeklong, P.; Brown, S.E.; Wang, Z.; Krebsbach, P.H. Phenotypic characterization, osteoblastic differentiation, and bone regeneration capacity of human embryonic stem cell-derived mesenchymal stem cells. Stem Cells Dev. 2009, 18, 955–968. [Google Scholar] [CrossRef]
- Saulacic, N.; Fujioka-Kobayashi, M.; Kobayashi, E.; Schaller, B.; Miron, R.J. Guided bone regeneration with recombinant human bone morphogenetic protein 9 loaded on either deproteinized bovine bone mineral or a collagen barrier membrane. Clin. Implant. Dent. Relat. Res. 2017, 19, 600–607. [Google Scholar] [CrossRef]
- Chantawong, P.; Tanaka, T.; Uemura, A.; Shimada, K.; Higuchi, A.; Tajiri, H.; Sakura, K.; Murakami, T.; Nakazawa, Y.; Tanaka, R. Silk fibroin-Pellethane(R) cardiovascular patches: Effect of silk fibroin concentration on vascular remodeling in rat model. J. Mater. Sci. Mater. Med. 2017, 28, 191. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Ciprioti, S.V. Characterization of Hybrid Materials Prepared by Sol-Gel Method for Biomedical Implementations. A Critical Review. Materials 2021, 14, 1788. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Paiva, S.S.; Caffrey, D.; Cavanagh, B.L.; Murphy, C.M. Injectable chitosan/collagen hydrogels nano-engineered with functionalized single wall carbon nanotubes for minimally invasive applications in bone. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112340. [Google Scholar] [CrossRef]
- Yu, H.D.; Zhang, Z.Y.; Win, K.Y.; Chan, J.; Teoh, S.H.; Han, M.Y. Bioinspired fabrication of 3D hierarchical porous nanomicrostructures of calcium carbonate for bone regeneration. Chem. Commun. 2010, 46, 6578–6580. [Google Scholar] [CrossRef] [PubMed]
- Rogina, A.; Rico, P.; Gallego Ferrer, G.; Ivankovic, M.; Ivankovic, H. In Situ Hydroxyapatite Content Affects the Cell Differentiation on Porous Chitosan/Hydroxyapatite Scaffolds. Ann. Biomed. Eng. 2016, 44, 1107–1119. [Google Scholar] [CrossRef]
- Hassani, L.N.; Hindre, F.; Beuvier, T.; Calvignac, B.; Lautram, N.; Gibaud, A.; Boury, F. Lysozyme encapsulation into nanostructured CaCO3 microparticles using a supercritical CO2 process and comparison with the normal route. J. Mater. Chem. B 2013, 1, 4011–4019. [Google Scholar] [CrossRef] [Green Version]
- Schliephake, H. Application of bone growth factors--the potential of different carrier systems. Oral Maxillofac. Surg. 2010, 14, 17–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.; Dima, C.; Huang, M.; Assadpour, E.; Wang, J.; Sun, B.; Kharazmi, M.S.; Jafari, S.M. Advanced CaCO3-derived delivery systems for bioactive compounds. Adv. Colloid Interface Sci. 2022, 309, 102791. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Feng, Q.; Wang, M.; Guo, X.; Zheng, Q. Porous nano-HA/collagen/PLLA scaffold containing chitosan microspheres for controlled delivery of synthetic peptide derived from BMP-2. J. Control. Release 2009, 134, 111–117. [Google Scholar] [CrossRef]
- Cunico, L.P.; Cobo, A.M.; Al-Hamimi, S.; Turner, C. Solubility and Thermal Degradation of Quercetin in CO2-Expanded Liquids. Molecules 2020, 25, 5582. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.K.; Bhartiya, P.; Singh, A.; Dutta, P.K. Preparation, physicochemical and biological evaluation of quercetin based chitosan-gelatin film for food packaging. Carbohydr. Polym. 2020, 227, 115348. [Google Scholar] [CrossRef]
- Arpornmaeklong, P.; Pripatnanont, P.; Kittidumkerng, W.; Mitarnun, W. Effects of autogenous growth factors on heterotopic bone formation of osteogenic cells in small animal model. J. Cranio-Maxillofac. Surg. 2012, 40, 332–340. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.W.; Suh, H. Sustained release of ascorbate-2-phosphate and dexamethasone from porous PLGA scaffolds for bone tissue engineering using mesenchymal stem cells. Biomaterials 2003, 24, 4671–4679. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fan, H.; Deng, X.; Wu, L.; Yi, T.; Gu, L.; Zhou, C.; Fan, Y.; Zhang, X. Scaffold Structural Microenvironmental Cues to Guide Tissue Regeneration in Bone Tissue Applications. Nanomaterials 2018, 8, 960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daugela, P.; Pranskunas, M.; Juodzbalys, G.; Liesiene, J.; Baniukaitiene, O.; Afonso, A.; Sousa Gomes, P. Novel cellulose/hydroxyapatite scaffolds for bone tissue regeneration: In vitro and in vivo study. J. Tissue Eng. Regen. Med. 2018, 12, 1195–1208. [Google Scholar] [CrossRef]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L.J. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 2005, 26, 433–441. [Google Scholar] [CrossRef]
- Chao, L.; Jiao, C.; Liang, H.; Xie, D.; Shen, L.; Liu, Z. Analysis of Mechanical Properties and Permeability of Trabecular-Like Porous Scaffold by Additive Manufacturing. Front. Bioeng. Biotechnol. 2021, 9, 779854. [Google Scholar] [CrossRef]
- Chimene, D.; Lennox, K.K.; Kaunas, R.R.; Gaharwar, A.K. Advanced Bioinks for 3D Printing: A Materials Science Perspective. Ann. Biomed. Eng. 2016, 44, 2090–2102. [Google Scholar] [CrossRef] [PubMed]
- Accadbled, F.; Ambard, D.; de Gauzy, J.S.; Swider, P. A measurement technique to evaluate the macroscopic permeability of the vertebral end-plate. Med. Eng. Phys. 2008, 30, 116–122. [Google Scholar] [CrossRef]
- Ryan, A.J.; Gleeson, J.P.; Matsiko, A.; Thompson, E.M.; O’Brien, F.J. Effect of different hydroxyapatite incorporation methods on the structural and biological properties of porous collagen scaffolds for bone repair. J. Anat. 2015, 227, 732–745. [Google Scholar] [CrossRef]
- Syahrom, A.; Abdul Kadir, M.R.; Harun, M.N.; Ochsner, A. Permeability study of cancellous bone and its idealised structures. Med. Eng. Phys. 2015, 37, 77–86. [Google Scholar] [CrossRef]
- Wang, C.; Shan, S.; Wang, C.; Wang, J.; Li, J.; Hu, G.; Dai, K.; Li, Q.; Zhang, X. Mechanical stimulation promote the osteogenic differentiation of bone marrow stromal cells through epigenetic regulation of Sonic Hedgehog. Exp. Cell Res. 2017, 352, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Shirakata, Y.; Imafuji, T.; Nakamura, T.; Kawakami, Y.; Shinohara, Y.; Noguchi, K.; Pilloni, A.; Sculean, A. Periodontal wound healing/regeneration of two-wall intrabony defects following reconstructive surgery with cross-linked hyaluronic acid-gel with or without a collagen matrix: A preclinical study in dogs. Quintessence Int. 2021, 4, 308–316. [Google Scholar] [CrossRef]
- Keaveny, T.M.; Morgan, E.F.; Niebur, G.L.; Yeh, O.C. Biomechanics of trabecular bone. Annu. Rev. Biomed. Eng. 2001, 3, 307–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junge, K.; Binnebosel, M.; von Trotha, K.T.; Rosch, R.; Klinge, U.; Neumann, U.P.; Lynen Jansen, P. Mesh biocompatibility: Effects of cellular inflammation and tissue remodelling. Langenbeck’s Arch. Surg. 2012, 397, 255–270. [Google Scholar] [CrossRef]






| Sample Groups | G′@ LVR (Pa) | G″@ LVR (Pa) | Humpof G″ | σ @′ = G″ (Pa) |
|---|---|---|---|---|
| System 1 | ||||
| Chitosan/collagen hydrogel alone | 9 ± 2 | 5 ± 1 | None | None |
| System 2 | ||||
| 0%CaCO3-2%nHA | 24 ± 1 | 8 ± 1 | None | None |
| 0%CaCO3-5%nHA | 132 ± 7 | 20 ± 2 | None | None |
| System 3 | ||||
| 1%CaCO3-2%nHA | 418 ± 56 | 55 ± 6 | Yes | 26 ± 11 |
| 2%CaCO3-2%nHA | 578 ± 181 | 65 ± 20 | Yes | 70 ± 48 |
| 5%CaCO3-2%nHA | 495 ± 33 | 79 ± 11 | Yes | 48 ± 19 |
| System 4 | ||||
| 1%CaCO3-5%nHA | 600 ± 74 | 53 ± 8 | Yes | 62 ± 14 |
| 2%CaCO3-5%nHA | 623 ± 95 | 89 ± 11 | Yes | 81 ± 8 |
| 5%CaCO3-5%nHA | 568 ± 79 | 91 ± 9 | Yes | 76 ± 15 |
| Sample Groups | F (Power Law) (Hz) | Power Law Parameters | Characteristic Responses of the Hydrogels | |||
|---|---|---|---|---|---|---|
| G′ | G″ | |||||
| A′ | n′ | A″ | n″ | |||
| System 1 | ||||||
| Chitosan/collagen hydrogel alone | 0.2–1.0 | 3 ± 2 | 1.0 ± 0.2 | 0.8 ± 0.5 | 1.5 ± 0.1 | Weak gel |
| System 2 | ||||||
| 0%CaCO3-2%nHA | 0.1–10.0 | 40 ± 12 | 0.07 ± 0.01 | 11 ± 3 | 0.09 ± 0.01 | Weak–Strong gel |
| 0%CaCO3-5%nHA | 0.1–10.0 | 140 ± 20 | 0.06 ± 0.01 | 17 ± 3 | 0.09 ± 0.01 | Strong gel |
| System 3 | ||||||
| 1%CaCO3-2%nHA | 0.4–1.2 | 137 ± 7 | 0.12 ± 0.02 | 11 ± 1 | −0.1 ± 0.2 | Strong particulate gel |
| 2%CaCO3-2%nHA | 0.4–3.0 | 151 ± 11 | 0.08 ± 0.01 | 11 ± 2 | −0.1 ± 0.2 | Strong particulate gel |
| 5%CaCO3-2%nHA | 0.4–4.6 | 330 ± 30 | 0.04 ± 0.01 | 26 ± 24 | 0.0 ± 0.1 | Strong particulate gel |
| System 4 | ||||||
| 1%CaCO3-5%nHA | 0.2–1.9 | 202 ± 15 | 0.10 ± 0.02 | 19 ± 2 | −0.3 ± 0.1 | Strong particulate gel |
| 2%CaCO3-5%nHA | 0.2–7.2 | 193 ± 12 | 0.03 ± 0.02 | 17 ± 1 | −0.1 ± 0.1 | Strong particulate gel |
| 5%CaCO3-5%nHA | 0.6–7.2 | 226 ± 53 | 0.03 ± 0.01 | 17 ± 4 | −0.1 ± 0.1 | Strong particulate gel |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arpornmaeklong, P.; Jaiman, N.; Apinyauppatham, K.; Fuongfuchat, A.; Boonyuen, S. Effects of Calcium Carbonate Microcapsules and Nanohydroxyapatite on Properties of Thermosensitive Chitosan/Collagen Hydrogels. Polymers 2023, 15, 416. https://doi.org/10.3390/polym15020416
Arpornmaeklong P, Jaiman N, Apinyauppatham K, Fuongfuchat A, Boonyuen S. Effects of Calcium Carbonate Microcapsules and Nanohydroxyapatite on Properties of Thermosensitive Chitosan/Collagen Hydrogels. Polymers. 2023; 15(2):416. https://doi.org/10.3390/polym15020416
Chicago/Turabian StyleArpornmaeklong, Premjit, Natthaporn Jaiman, Komsan Apinyauppatham, Asira Fuongfuchat, and Supakorn Boonyuen. 2023. "Effects of Calcium Carbonate Microcapsules and Nanohydroxyapatite on Properties of Thermosensitive Chitosan/Collagen Hydrogels" Polymers 15, no. 2: 416. https://doi.org/10.3390/polym15020416





