A Comparative Study of Resistant Dextrins and Resistant Maltodextrins from Different Tuber Crop Starches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
2.2.1. Preparation of RDs
2.2.2. Preparation of RMDs
2.3. Structure Characterization
2.3.1. Scanning Electron Microscopy (SEM)
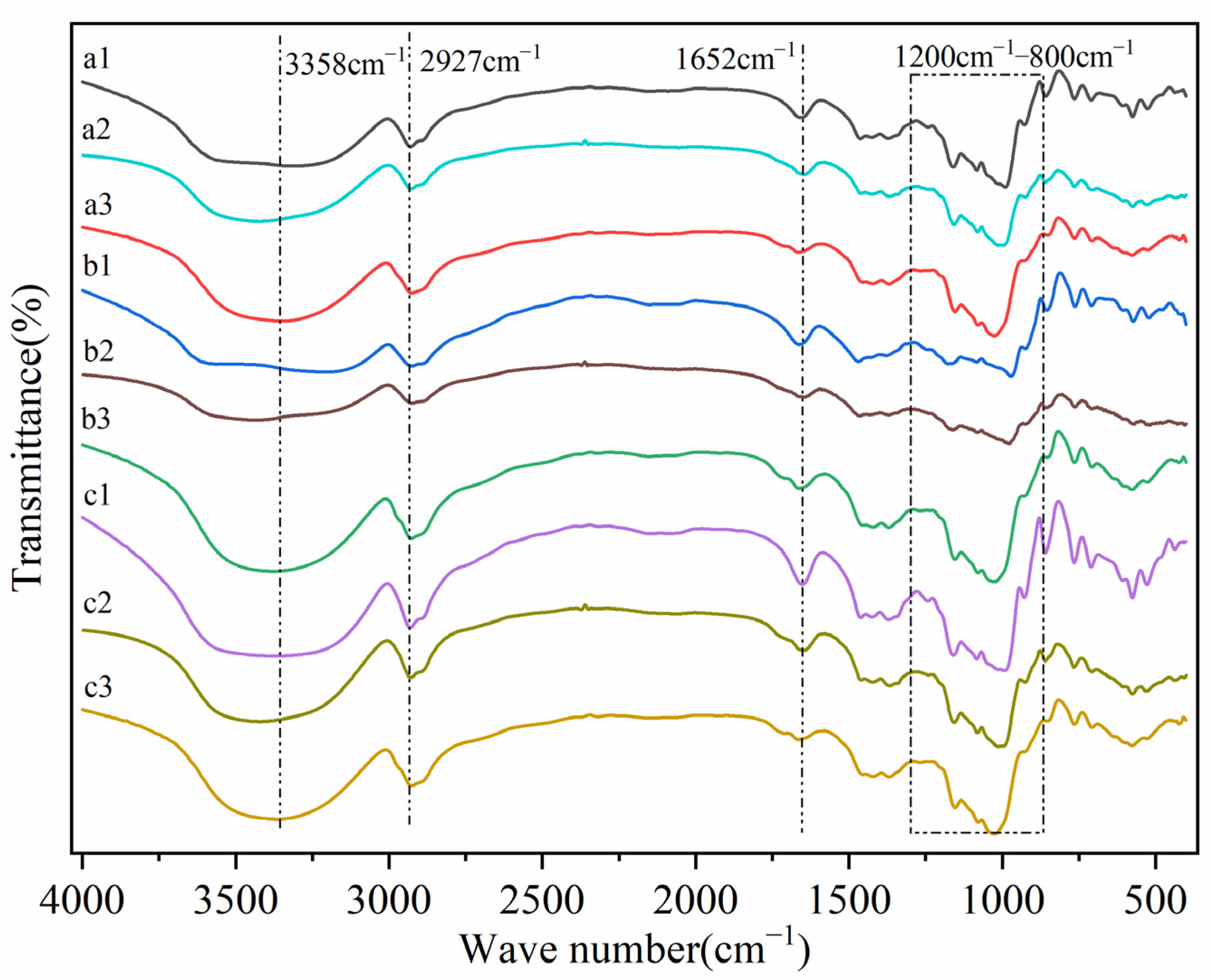
2.3.2. Fourier Transform Infrared Spectra (FTIR)
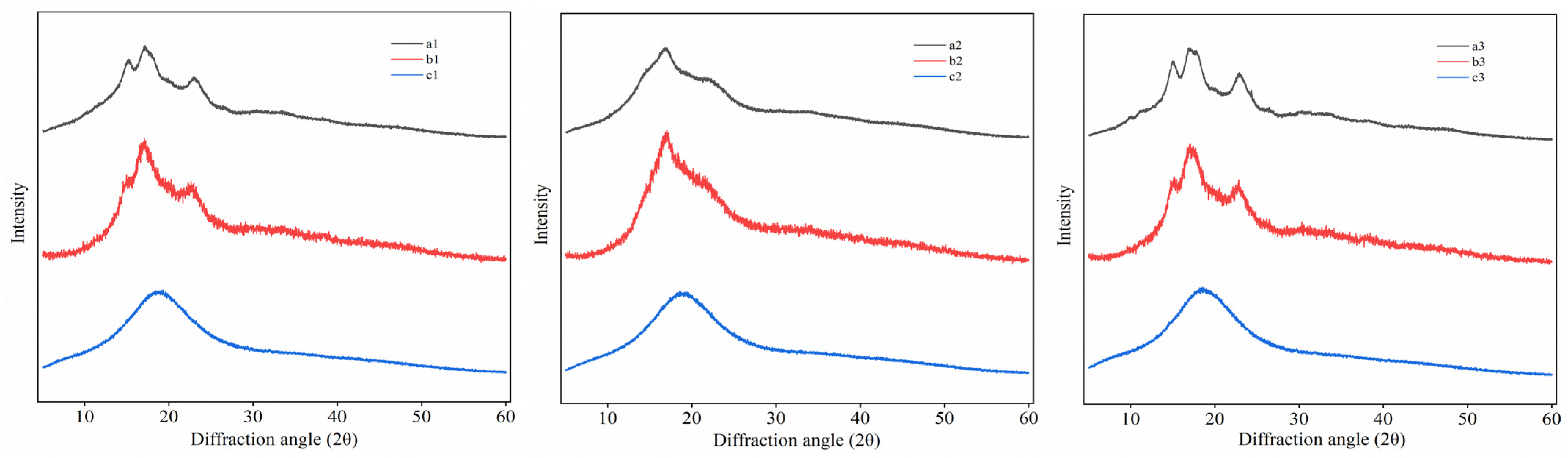
2.3.3. X-ray Diffraction (XRD)
2.3.4. Nuclear Magnetic Resonance Spectra (NMR)
2.4. Physicochemical Properties
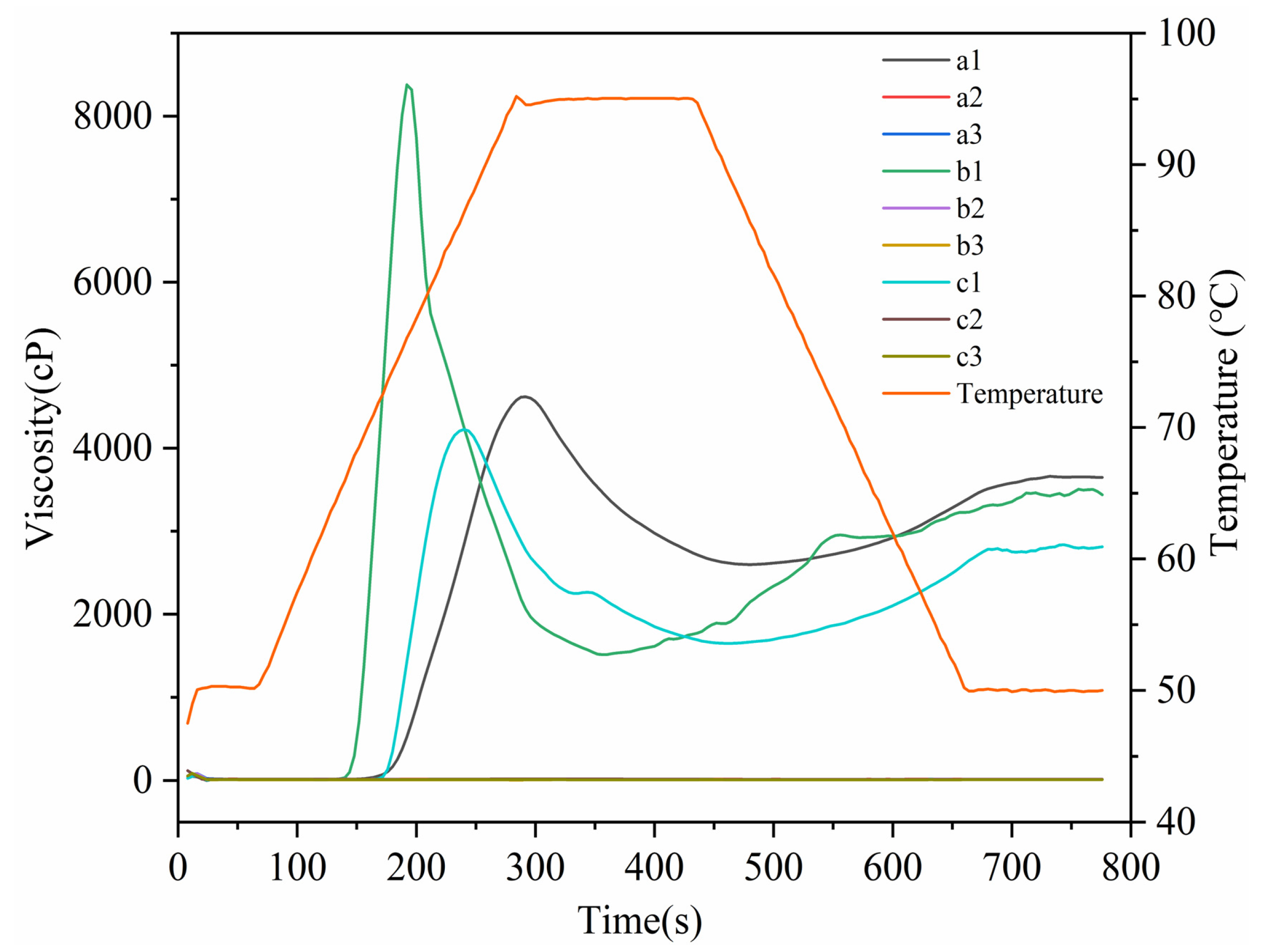
2.4.1. Pasting Property
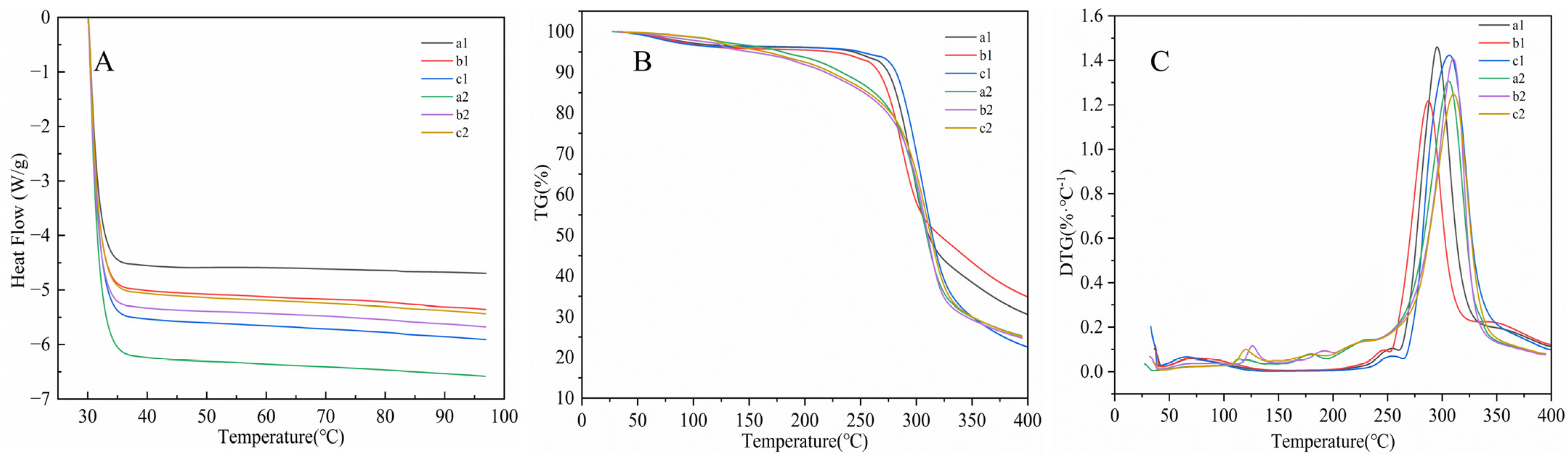
2.4.2. Thermal Properties
2.4.3. Solubility
2.4.4. Indigestible Ingredient Content
2.5. In Vitro Digestibility
2.6. Statistical Analysis
3. Results and Discussion
3.1. Morphological Analysis
3.2. FTIR Spectra Analysis
3.3. XRD Analysis
3.4. NMR Spectra Analysis
3.5. Pasting Properties
3.6. Thermal Properties Analysis
3.7. Solubility and Indigestible Ingredient Content Analyses
3.8. In Vitro Digestibility
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef]
- Waddell, I.S.; Orfila, C. Dietary fiber in the prevention of obesity and obesity-related chronic diseases: From epidemiological evidence to potential molecular mechanisms. Crit. Rev. Food Sci. Nutr. 2023, 63, 8752–8767. [Google Scholar] [CrossRef]
- Mysonhimer, A.R.; Holscher, H.D. Gastrointestinal Effects and Tolerance of Nondigestible Carbohydrate Consumption. Adv. Nutr. 2022, 13, 2237–2276. [Google Scholar] [CrossRef]
- Jochym, K.; Kapusniak, J.; Barczynska, R.; Sliżewska, K. New starch preparations resistant to enzymatic digestion. J. Sci. Food Agric. 2012, 92, 886–891. [Google Scholar] [CrossRef]
- Kapusniak, K.; Lubas, K.; Wojcik, M.; Rosicka-Kaczmarek, J.; Pavlyuk, V.; Kluziak, K.; Gonçalves, I.; Lopes, J.; Coimbra, M.A.; Kapusniak, J. Effect of Continuous and Discontinuous Microwave-Assisted Heating on Starch-Derived Dietary Fiber Production. Molecules 2021, 26, 5619. [Google Scholar] [CrossRef]
- Mao, H.; Li, J.; Chen, Z.; Yan, S.; Li, H.; Wen, Y.; Wang, J. Molecular structure of different prepared pyrodextrins and the inhibitory effects on starch retrogradation. Food Res. Int. 2021, 143, 110305. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, J.; Ren, L.; Wu, J.; Chen, S. Preparation of high-quality resistant dextrin through pyrodextrin by a multienzyme complex. Food Biosci. 2022, 47, 101701. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, J.; Wang, Z.; Cheng, H.; Zhang, Y.; Lin, B.; Qin, L.; Bai, Y. Effects of reaction condition on glycosidic linkage structure, physical-chemical properties and in vitro digestibility of pyrodextrins prepared from native waxy maize starch. Food Chem. 2020, 320, 126491. [Google Scholar] [CrossRef]
- Mao, H.; Chen, Z.; Li, J.; Zhai, X.; Li, H.; Wen, Y.; Wang, J.; Sun, B. Structural comparisons of pyrodextrins during thermal degradation process: The role of hydrochloric acid. Food Chem. 2021, 349, 129174. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ji, J.; Yang, L.; Lei, N.; Wang, J.; Sun, B. Structural and physicochemical property changes during pyroconversion of native maize starch. Carbohydr. Polym. 2020, 245, 116560. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Muhmood, A.; Akhter, M.; Gao, X.; Sun, J.; Du, Z.; Wei, Y.; Zhang, T.; Wei, Y. Characterization, health benefits, and food applications of enzymatic digestion- resistant dextrin: A review. Int. J. Biol. Macromol. 2023, 253, 126970. [Google Scholar] [CrossRef]
- Hu, F.; Niu, Y.; Xu, X.; Hu, Q.; Su, Q.; Zhang, H. Resistant dextrin improves high-fat-high-fructose diet induced insulin resistance. Nutr. Metab. 2020, 17, 36. [Google Scholar] [CrossRef]
- Włodarczyk, M.; Śliżewska, K.; Barczyńska, R.; Kapuśniak, J. Effects of Resistant Dextrin from Potato Starch on the Growth Dynamics of Selected Co-Cultured Strains of Gastrointestinal Bacteria and the Activity of Fecal Enzymes. Nutrients 2022, 14, 2158. [Google Scholar] [CrossRef] [PubMed]
- Lefranc-Millot, C.; Guérin-Deremaux, L.; Wils, D.; Neut, C.; Miller, L.E.; Saniez-Degrave, M.H. Impact of a resistant dextrin on intestinal ecology: How altering the digestive ecosystem with NUTRIOSE®, a soluble fibre with prebiotic properties, may be beneficial for health. J. Int. Med. Res. 2012, 40, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Rakmai, J.; Haruthaithanasan, V.; Chompreeda, P.; Chatakanonda, P.; Yonkoksung, U. Development of gluten-free and low glycemic index rice pancake: Impact of dietary fiber and low-calorie sweeteners on texture profile, sensory properties, and glycemic index. Food Hydrocoll. Health 2021, 1, 100034. [Google Scholar] [CrossRef]
- Himat, A.S.; Gautam, S.; Garcia, J.P.C.; Vidrio-Sahagún, A.X.; Liu, Z.; Bressler, D.; Vasanthan, T. Starch-based novel ingredients for low glycemic food formulation. Bioact. Carbohydr. Diet. Fibre. 2021, 26, 100275. [Google Scholar] [CrossRef]
- Xia, C.; Zhong, L.; Wang, J.; Zhang, L.; Chen, X.; Ji, H.; Ma, S.; Dong, W.; Ye, X.; Huang, Y.; et al. Structural and digestion properties of potato starch modified using an efficient starch branching enzyme AqGBE. Int. J. Biol. Macromol. 2021, 184, 551–557. [Google Scholar] [CrossRef]
- Trithavisup, K.; Krusong, K.; Tananuwong, K. In-depth study of the changes in properties and molecular structure of cassava starch during resistant dextrin preparation. Food Chem. 2019, 297, 124996. [Google Scholar] [CrossRef]
- Trithavisup, K.; Shi, Y.C.; Krusong, K.; Tananuwong, K. Molecular structure and properties of cassava-based resistant maltodextrins. Food Chem. 2022, 369, 130876. [Google Scholar] [CrossRef]
- Weil, W.; Weil, R.C.; Keawsompong, S.; Sriroth, K.; Seib, P.A.; Shi, Y.-C. Pyrodextrin from waxy and normal tapioca starches: Physicochemical properties. Food Hydrocoll. 2020, 104, 105745. [Google Scholar] [CrossRef]
- Bai, Y.; Shi, Y.C. Chemical structures in pyrodextrin determined by nuclear magnetic resonance spectroscopy. Carbohydr. Polym. 2016, 151, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.; Zhang, T.; Jiang, B.; Chen, J. Purification and Characterization of Resistant Dextrin. Foods 2021, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ren, F.; Yu, X.; Zhang, Z.; Xu, D.; Tong, Q. Pasting investigation, SEM observation and the possible interaction study on rice starch-pullulan combination. Int. J. Biol. Macromol. 2015, 73, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Le Thanh-Blicharz, J.; Błaszczak, W.; Szwengiel, A.; Paukszta, D.; Lewandowicz, G. Molecular and Supermolecular Structure of Commercial Pyrodextrins. J. Food Sci. 2016, 81, C2135–C2142. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Borowska, M.; Wegrzynowska-Drzymalska, K.; Chelminiak-Dudkiewicz, D.; Kowalonek, J.; Kaczmarek, H. Photochemical Reactions in Dialdehyde Starch. Molecules 2018, 23, 3358. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, T.; Ma, Q.; Zhu, Y.; Shen, R. Structure Characterization and Potential Probiotic Effects of Sorghum and Oat Resistant Dextrins. Foods 2022, 11, 1877. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, I.; Nogami, Y.; Ohkuma, K. Indigestible dextrin. U.S. Patent 5,358,729 A, 25 October 1994. [Google Scholar]
- Edwards, C.H.; Warren, F.J.; Milligan, P.J.; Butterworth, P.J.; Ellis, P.R. A novel method for classifying starch digestion by modelling the amylolysis of plant foods using first-order enzyme kinetic principles. Food Funct. 2014, 5, 2751–2758. [Google Scholar] [CrossRef]
- Rahaman, A.; Kumari, A.; Zeng, X.A.; Adil Farooq, M.; Siddique, R.; Khalifa, I.; Siddeeg, A.; Ali, M.; Faisal Manzoor, M. Ultrasound based modification and structural-functional analysis of corn and cassava starch. Ultrason. Sonochem. 2021, 80, 105795. [Google Scholar] [CrossRef]
- Chorfa, N.; Nlandu, H.; Belkacemi, K.; Hamoudi, S. Physical and Enzymatic Hydrolysis Modifications of Potato Starch Granules. Polymers 2022, 14, 2027. [Google Scholar] [CrossRef]
- Surendra Babu, A.; Parimalavalli, R.; Rudra, S.G. Effect of citric acid concentration and hydrolysis time on physicochemical properties of sweet potato starches. Int. J. Biol. Macromol. 2015, 80, 557–565. [Google Scholar] [CrossRef]
- Valodkar, M.; Thakore, S. Isocyanate crosslinked reactive starch nanoparticles for thermo-responsive conducting applications. Carbohydr. Res. 2010, 345, 2354–2360. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Y.; Hu, W.; Qian, J.Y.; Ding, X.L.; Guan, C.R.; Lu, Y.Q.; Cao, Y. Multi-scale structures of cassava and potato starch fractions varying in granule size. Carbohydr. Polym. 2018, 200, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, L.; Ma, C.; Zhang, Y. Thermal Behavior of Sweet Potato Starch by Non-Isothermal Thermogravimetric Analysis. Materials 2019, 12, 699. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Cai, L.; Doutch, J.; Gilbert, E.P.; Shi, Y.C. Structural changes from native waxy maize starch granules to cold-water-soluble pyrodextrin during thermal treatment. J. Agric. Food Chem. 2014, 62, 4186–4194. [Google Scholar] [CrossRef]
- Han, X.; Kang, J.; Bai, Y.; Xue, M.; Shi, Y.C. Structure of pyrodextrin in relation to its retrogradation properties. Food Chem. 2018, 242, 169–173. [Google Scholar] [CrossRef]
- Laurentin, A.; Cárdenas, M.; Ruales, J.; Pérez, E.; Tovar, J. Preparation of indigestible pyrodextrins from different starch sources. J. Agric. Food Chem. 2003, 51, 5510–5515. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Kang, J.; Shi, Y.C. Changes in molecular size and shape of waxy maize starch during dextrinization. Food Chem. 2021, 348, 128983. [Google Scholar] [CrossRef]
- Toraya-Aviles, R.; Segura-Campos, M.; Chel-Guerrero, L.; Betancur-Ancona, D. Some Nutritional Characteristics of Enzymatically Resistant Maltodextrin from Cassava (Manihot esculenta Crantz) Starch. Plant Food. Hum. Nutr. 2017, 72, 149–155. [Google Scholar] [CrossRef]
- Jochym, K.K.; Nebesny, E. Enzyme-resistant dextrins from potato starch for potential application in the beverage industry. Carbohydr. Polym. 2017, 172, 152–158. [Google Scholar]
- Mateo-Gallego, R.; Moreno-Indias, I.; Bea, A.M.; Sánchez-Alcoholado, L.; Fumanal, A.J.; Quesada-Molina, M.; Prieto-Martín, A.; Gutiérrez-Repiso, C.; Civeira, F.; Tinahones, F.J. An alcohol-free beer enriched with isomaltulose and a resistant dextrin modulates gut microbiome in subjects with type 2 diabetes mellitus and overweight or obesity: A pilot study. Food Funct. 2021, 12, 3635–3646. [Google Scholar] [CrossRef]
- Van Hung, P.; Huong, N.T.; Phi, N.T.; Tien, N.N. Physicochemical characteristics and in vitro digestibility of potato and cassava starches under organic acid and heat-moisture treatments. Int. J. Biol. Macromol. 2017, 95, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, S.; Huang, Y.; Huang, J.; Li, Y. Effect of in vitro gastrointestinal digestion on phenolic compounds and antioxidant properties of soluble and insoluble dietary fibers derived from hulless barley. J. Food Sci. 2021, 86, 628–634. [Google Scholar] [CrossRef] [PubMed]



| Measured Parameter | Solubility (%) | Indigestible Ingredient Content (%) |
|---|---|---|
| a1 | 64.73 ± 0.35 d | 34.32 ± 0.15 e |
| b1 | 64.55 ± 0.40 d | 36.28 ± 0.98 d |
| c1 | 61.27 ± 0.28 e | 36.16 ± 1.97 d |
| a2 | 97.30 ± 0.17 c | 80.60 ± 0.30 c |
| b2 | 98.21 ± 0.20 b | 84.96 ± 0.52 a |
| c2 | 99.82 ± 0.10 a | 82.57 ± 0.43 b |
| Measured Parameter | C∞ (%) | k (10−2, min−1) | R2 |
|---|---|---|---|
| a1 | 90.29 ± 2.45 | 2.11 | 0.993 |
| a2 | 37.35 ± 0.47 | 12.6 | 0.994 |
| a3 | 7.14 ± 0.65 | 1.32 | 0.973 |
| b1 | 73.92 ± 0.92 | 3.74 | 0.997 |
| b2 | 41.03 ± 0.64 | 8.5 | 0.992 |
| b3 | N/A | N/A | N/A |
| c1 | 77.40 ± 1.50 | 3.25 | 0.993 |
| c2 | 37.09 ± 0.23 | 16 | 0.998 |
| c3 | 7.60 ± 0.53 | 2.02 | 0.957 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Hou, Y.; Wang, Z.; Liao, A.; Pan, L.; Zhang, M.; Xue, Y.; Wang, J.; Liu, Y.; Huang, J. A Comparative Study of Resistant Dextrins and Resistant Maltodextrins from Different Tuber Crop Starches. Polymers 2023, 15, 4545. https://doi.org/10.3390/polym15234545
Chen X, Hou Y, Wang Z, Liao A, Pan L, Zhang M, Xue Y, Wang J, Liu Y, Huang J. A Comparative Study of Resistant Dextrins and Resistant Maltodextrins from Different Tuber Crop Starches. Polymers. 2023; 15(23):4545. https://doi.org/10.3390/polym15234545
Chicago/Turabian StyleChen, Xinyang, Yinchen Hou, Zhen Wang, Aimei Liao, Long Pan, Mingyi Zhang, Yingchun Xue, Jingjing Wang, Yingying Liu, and Jihong Huang. 2023. "A Comparative Study of Resistant Dextrins and Resistant Maltodextrins from Different Tuber Crop Starches" Polymers 15, no. 23: 4545. https://doi.org/10.3390/polym15234545





