Metal and Metal Oxide Nanoparticle Incorporation in Polyurethane Foams: A Solution for Future Antimicrobial Materials?
Abstract
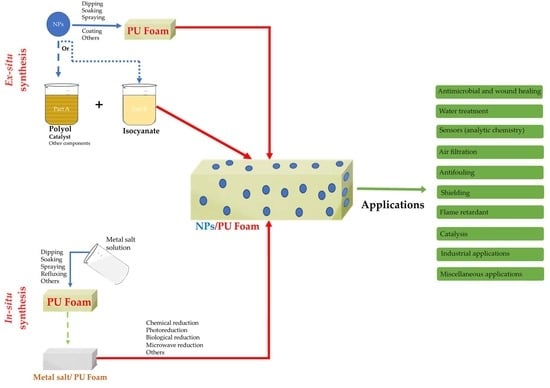
:1. Introduction
2. PU Foams—A Versatile and Widely Encountered Material
3. Methodology
3.1. Inclusion Criteria:
- -
- Research articles published in the time interval 2012–present, full text;
- -
- Articles published or available in English;
- -
- Incorporation of nanomaterials—for automatic screening, only the term “nano*” was used;
- -
- Development of materials with antimicrobial properties—for automatic screening, the automatic search “antimicrob* OR antibact* OR antifung*” was used;
- -
- Relevance of the review topic (new information provided).
3.2. Exclusion Criteria:
- -
- Articles published before 2012;
- -
- Book chapters or books;
- -
- Review or systematic review articles;
- -
- Conference papers, notes, letters, short surveys, errata, editorial or conference reviews;
- -
- Retracted papers;
- -
- Articles published in languages other than English;
- -
- Articles not presenting the incorporation of metal/metal oxides nanoparticles.
4. Results
5. Incorporation of Metal-Based Nanomaterials into PU Foams
| NP Type | NP Synthesis Method and Characteristics | PU Foams | Synthesis Process | Application | Main Findings | Ref. |
|---|---|---|---|---|---|---|
| Ag | Commercial NPs | PU foams containing natural polyols (hydroxypropyl methylcellulose, chitosan and sodium alginate) | Active ingredients (AgNPs at 0.4, 0.6, 0.8 and 1.0 mg/cm2 and asiaticoside powder at 5%) adsorbed | Dermal wound dressing | Average pore size: 228–262 μm, viscosity slightly increased; higher concentration of polyols led to higher AgNP-releasing profiles. Optimal formulation: 6% natural polyols and 1 mg/cm2 AgNPs | [9] |
| Ag | Commercial AgNPs, PVP-coated, 100 nm diameter, surface area 5.0 m2/g. | Commercial flexible foam | Incorporation via mechanical stirring at different concentrations (0.1, 0.5, 1%) | Antimicrobial applications | Homogeneous dispersion of AgNPs in a polymeric matrix at low concentrations, cluster formation at higher loadings. Optimum concentration by step compression stress relaxation was 0.1% AgNP; resilience, hardness and compression unaltered by NPs. No change in thermal stability induced by NPs | [10] |
| Ag | Phytosynthesis using phenolated lignin and sonication, 13.29 nm (TEM) | PU formulation: PEG, DC 5179 additive, MDI, DABCO | Impregnation via NP dispersion in a polyol mixture at 0.12, 0.2, 0.25% relative to final composition | Chronic wound treatment | Open cell structure, cell diameter decreased with an NP concentration increase, 40% increase in compression modulus, swelling ratios varied from 585% (0.25% NPs) to 1145% (0.12% NPs), density increased with NP content | [13] |
| Ag | Commercial, 100 nm | Polypropylene glycol-based PU foam | Incorporation of NPs into a polyol mixture, foams converted to negative Poisson’s ratio or auxetic polyurethane | Medical cushioning | Foams made using a higher compression ratio exhibited an increase in compression strength at higher strains and a higher density compared to PU foam. | [27] |
| Ag/Ag2O | Mixture of NPs obtained by chitosan treatment, spherical, 44–75 nm (SEM) | Commercial PU foams | Impregnated with nanochitosan and nanosilver/silver oxide | Coliform removal from water sources | Increased surface area (2.17 m2/g) | [28] |
| Ag | NPs obtained by the borohydride technique—average diameter 19 nm | Polyether type polyurethane foam (commercial) | Immersion in NP solution | Potential applications in analytical chemistry | Materials stable for at least four days; uniform color, indicating satisfactory dispersion of NPs. | [44] |
| Ag | NPs obtained by reduction with chitosan, spherical shape and a size range of~50 nm | Commercially available | Dipping in AgNP solution | In vivo antibacterial study | Coating thickness 3–5 µm; PU coating average pore size 400–600 µm | [45] |
| Ag | NPs obtained by reduction with sodium citrate, 25 nm crystallite (XRD), | Obtained from commercial polyisocyanate and polyol reactants | Dipping in AgNPs for 2, 4, 6, 8 h | Antimicrobial applications (water disinfection) | No detectable agglomeration of AgNPs; total size and porosity of foam unaltered; AgNPs unwashed after repeated cycles | [46] |
| Ag | Synthesis by electric explosion of wire in liquid, 90 nm diameter (SEM) | Obtained from commercial polyisocyanate and polyol (ethylene oxide/propylene oxide random copolymer (polyethylene glycol)) reactants | Incorporation of AgNP and recombinant human epidermal growth factor in foams | Dressing material for biomedical applications | No influence of incorporation on PU foam surface pore size (200–400 µm), AgNPs embedded inside the pores | [47] |
| Ag | Fungi extracellular synthesis of NPs, 4.24–23.2 nm diameter for Scopulaiopsos brumptii Salvanet-Duval particles, 6–26 nm for Peniciillium Citreonigum Dierck particles, spherical morphology (TEM) | Commercially available | Incorporated by soaking over night | Removal of pathogenic bacteria from wastewater | No evaluation of the material’s characteristics | [48] |
| Ag | Commercial NPs, 15–40 nm | Waterborne PU foams (commercially available emulsion) | Incorporation by mechanical foaming, AgNPs—0–4% | Bacteriostatic agent | Open cell structure; NPs uniformly dispersed; aggregation at higher NP concentration a rougher surface; up to 25.8 nm (AFM), pore size increased up to 34.24 µm; improved thermal properties; increase in air permeability, water vapor transmission, tensile strength (up to 1.26 Mpa, 412.39% increase at 2% NPs); elongation at break decreased with AgNP addition | [40] |
| Ag | Polymer-template-assisted assembly using glucose, PVP and NaCl, 180 °C for 18 h | Obtained from commercial isocyanate and polyethylene polyol reactants | Impregnation with graphene oxide (7 mg/mL), carbon nanotubes (7 mg/mL), AgNPs (15 mg/mL) and dopamine (0.1 mg/mL) | Industrial applications (such as sensors and electromagnetic shielding) | Final composites reached 12.28 N/mm (tensile strength), improved thermal stability, electric conductivity properties (2 × 10−4 S/cm−1) | [49] |
| Ag | Phytosynthesized using hibiscus leaf extract, spherical, 50–70 nm (TEM), compared with commercial NPs | Commercially available (modified by chemical treatment—hydrophilic) | Surface-coated on polyurethane foam, or fused on polyurethane foam | Pesticide adsorption in column studies | Highest pesticide removal (96% at 20 mL/h) for fused polyurethane foam with commercial NPs, surface-coated polyurethane foam (CPU) and fused polyurethane foam | [50] |
| Ag | Commercial | Commercial formulations | Incorporation in PU foams, comparison with other biocidal additives | Biocidal applications | AgNPs had the least effect on the technological parameters | [41] |
| Ag | Commercial nanowires, 70 nm diameter, 100–200 µm length | Commercially available | NW solution sprayed over PU foams | Clinical wound healing | Composites revealed excellent elasticity without plastic deformation, hydrophobic character | [42] |
| Ag | Reduction with ethanol on the surface of natural zeolite, diameter 4.61 nm (TEM) | Open-cell soft polyurethane foam | NPs/zeolite mixed during PU production | Biocidal application | Open cell foam structure, mean cell size distribution 121.68 μm | [51] |
| Ag | Commercial NPs, 30 nm | Disocyanate and polyol PU foam | AgNP and AgNP/GO nanocomposites prepared by pepsin reduction mixed in the polyol solution | Antibacterial applications | Compared with AgNP loading, the use of AgNPs/GO led to a more homogenous dispersion, 1.85% resilience improvement, 7.9% tensile strength improvement, 6.52% tensile elongation at break improvement | [43] |
| Ag | Phytosynthesized using a Verbena officinalis leaf extract, 42.57 nm (SEM) | Obtained from commercial polyisocyanate and polyol reactants | Incorporation by mixing in polyol solution | Antimicrobial nano-biofilter | The number of foam cavities increased with addition of NPs | [52] |
| Ag/Ag2O | Mixture of NPs obtained by chitosan treatment, spherical, 44–75 nm (SEM) | Commercially available | Impregnation by dipping with nanochitosan, nanosilver/silver oxide and nanochitosan-nanosilver/silver oxide | Phosphate removal from water sources | Increase in surface area, superior sorption capacity compared to individual nano-components | [74] |
| Ag/TiO2 | Produced by sintering at 600 °C, particle size 958.3 nm, by adding Ag to TiO2 NPs produced by sol–gel | Produced by the group, no recipe disclosed | Ag/TiO2/chitosan powder coated on bendable double mattress with added HAP powder | Bending mattress for bedridden patients | Bed mattress tested using a patient survey with good feedback | [21] |
| Cu | Sacrificial-anode electrochemical synthesis and TOAC stabilization, 2.6 nm diameter (TEM) | Commercially available. Two types of industrial foams, a filling material for mattresses (large and irregular pores, density: 25 kg/m3) and an automotive industry foam (small and regular pores, density: 21 kg/m3) | Dipping in diluted CuNP solutions (1:100, 1:1000) | Antimicrobial applications | Pore characteristics are not affected by NP uptake; higher and faster Cu release for higher initial CuNP solution and PU foams with larger pores | [12] |
| Cu | Nanosheets obtained via a CBD process | Commercially available | Dipping | Adsorption and antimicrobial properties | Pore size 150–500 μm, adsorption capacity 76.5 mg/g for Cr (VI), 714 mg/g for Congo Red dye | [54] |
| CuO | Direct thermal decomposition method, spherical shaped, 47.5 nm diameter (TEM) | Foams obtained via the one-shot method using a toluene diisocyanate and polyol system | CuONPs, starch and silicone surfactant mixed with polyol components | Antiseptic polyurethane foam dressings | Optimal NP synthesis at 600 °C, with optimal open cells of the corresponding foams | [55] |
| Ag3PO4 | Precipitation | Obtained from commercial toluene diisocyanate, polyols and polyvinyl alcohol | Dispersed in a flexible open-cell polyurethane mixture, followed by graphene oxide coating | Antimicrobial properties and acid red 87 dye adsorption | Open cell structure, adsorption efficiency of 97% for 0.05 g of nanocomposite | [56] |
| Ag | Ions from AgNO3 | Commercially available (30 kg/m3 density) | Successively dipping in poly(acrylic) acid, chitosan, Ti3C2 and metal solution | Flame retardancy and antibacterial applications | No visible damage; reduced thermal degradation rate, burning rate (156 mm/min, control 237 mm/min), PHRR, heat production speed; smoke suppression ability. Increased compression strength by 79.6% | [57] |
| Cu | Ions from CuSO4 | Discontinuous coating: micro-cracking; no influence on thermal stability; reduced burning rate (208 mm/min, control 237 mm/min), PHRR, heat production speed; smoke suppression ability; increased compression strength by 38.4% | [57] | |||
| Cu | Electroless deposition | Thermoplastic polyurethane (TPU) granules, commercially available | Deposition on TPU/ANF/Ti3C2Tx Mxene | Detection of human motion and electromagnetic interference shielding | Board compressive interval (0–344.5 kPa, 50% strain), good sensitivity at 0.46 kPa−1, rapid response/recovery time (100 ms), electromagnetic interference shielding at 79.09 dB in X band | [58] |
| CuO | Arc discharge in a controlled atmosphere synthesis, spherical, average size 34 nm (TEM) | Rigid polyurethane foams obtained using high-molecular-weight tannins (from Pinus radiata bark), polymeric diphenylmethane diisocyanate, dimethyl sulfoxide, SoudaFoam FR, polyol | Mixing in polyol solution, final concentration 2% | Miscellaneous applications | Decreased pore size, strengthened cell walls, improved mechanical properties, elastic modulus (3.7 MPa) and stress (max. 1.13 MPa), apparent density | [59] |
| Zn | Chemical precipitation from commercial ZnO NPs | Commercially available | Dipping | Oil–water separation | Superhydrophilic/superoleophobic features (oil contact angle 158°, water contact angle 0°), oil separation efficiency up to 99.5% | [60] |
| ZnO | Sol–gel method, spherical, 40 nm diameter (XRD, TEM) | Obtained from commercial isocyanate and polyol reactants | Incorporation by mixing in polyol solution | Photocatalytic degradation of textile dye methylene blue | Increased density with NP content, reduction in cell diameter, increased exposed surface area, open cell structure, superior MB degradation under solar irradiation | [14] |
| ZnO | NPs obtained by the sonochemical method in a biopolymer (starch, gelatin, chitosan, and agar) matrix; crystallite sizes: 15, 26, 19, and 12 nm (XRD), average diameter 80, 41, 38, and 60 nm (TEM); morphology: microspherical/rice-like/nanospherical/egg-shaped | Furniture-grade polyurethane foam, commercially available | Coating with ZnO—biopolymer | Antifungal pillow materials for automobile and hospital industries | ZnO starch and ZnO chitosan—homogeneous adhesion spread through the foam walls, maintaining the softness of the foam; ZnO gelatin and ZnO agar—continuous film-like growth; all samples revealed UV photoactivity | [61] |
| ZnO | Precipitation method, spherical, 50 nm | Obtained from commercial isocyanate and polyol reactants | ZnO added in the polyol, followed by mechanical stirring, foams obtained by a two-step method | Antibacterial applications | Foams presented polygon closed-cell structures with energetically stable hexagonal and pentagonal faces, cell size comparable to unloaded foams, maximum tensile strength (193.5 kPa) and suitable compressive strength at 1.5% ZnONPs | [62] |
| ZnO | Commercial NPs, 50–250 nm | Obtained from diisocyanate and bio-based polyester polyol reactants | Incorporation of NPs by thermally induced phase separation at 1, 2, 5, 10% | Potential wound dressing | Flexible membranes, thickness 150–230 μm, similar porous structures, pore size 10–60 μm, small negative influence on thermal properties, increased hydrophobicity with NP content, lower absorptivity and acceptable WVTR (up to 8.9 mg/cm2·h) | [63] |
| ZnO | Chemical reduction, calcination, spherical, crystallite size 18.4 nm | Commercially available | PU foams refluxed with ZnO NPs for 6 h. | Antibacterial activity, detection and removal of basic dyes from wastewater | Detection limits of 2.5 and 2.9 μg/L for brilliant green and toluidine blue dyes, removal percentages of 92.4–98.2%, increased surface area, average pore radius of 3.4 nm | [64] |
| ZnO | Chemical synthesis using KOH, 20–80 nm (TEM), crystal size 27 nm (XRD) | Rigid polyurethane foam obtained from commercial isocyanate and polyol | NPs added to the foam mixture at 5% relative to polyol content | Flame-retardant rigid PU foam | Increased cell size, decreased density, pore diameter of 481 µm, lower burning velocity (346 mm/min, compared with blank—275 mm/min) | [65] |
| ZnO | Low-temperature chemical synthesis method, nanorods, 0.3 µm thickness, 1.2 µm length (SEM) | Commercially available | Multi-step dip-coating and seed-growth procedure | Photocatalytic treatment of aqueous acid red 88 dye | Highly porous, maximum color removal of 97% reached in 180 min under UVA | [66] |
| ZnO | Commercial NPs | Obtained from commercial isocyanate and polyol (castor oil derivative) reactants | NPs (6%) and sheath palm residues added during the polyol and isocyanate mixture | Miscellaneous applications | ZnO acted as a cell nucleation agent—homogeneous and isotropic cell structures. Increased resistance to heat absorption, thermal stability, foam crystallinity and stiffness | [67] |
| ZnO | Co-precipitation, crystallite size 15 nm (XRD), semi-regular spherical and rod-shaped structure. | Commercially available, apparent density 12–15 kg/m3 (97%) | Deposition on foam containing reduced GO by two successive impregnation and hydrothermal processes | Photocatalysts for methylene blue degradation | Good dispersion and embedment of NPs into the foam structure, ZnO NPs reduced the photodegradation capacity of PU foams containing reduced GO | [68] |
| MgO | Chemical synthesis using NaOH, 10–75 nm (TEM), crystal size 12 nm (XRD) | Rigid polyurethane foam obtained from commercial isocyanate and polyol | NPs added to the foam mixture at 5% relative to polyol content | Flame-retardant rigid PU foam | Increased cell size, decreased density, pore diameter of 514 µm, lower burning velocity (333 mm/min, compared with blank—275 mm/min) | [65] |
| Au | Multi-branched AuNPs, synthesized using hydroquinone as a reducing agent and chitosan as a stabilizer under ultrasound, 45 nm branches, 40 nm average size of core (TEM) | Obtained from commercial isocyanate and polyol | Dipping for 24 h | Antibacterial dressing | High water absorption, small average pore size (smallest dimensions 98 nm), 500% absorptivity | [31] |
| W | Commercial, 40–60 nm | Shape memory polymer foam obtained from isocyanate (NCO) pre-polymer and alcohols | WNPs dispersed in the NCO pre-polymer, prior to foam blowing at 4% to 11%. | Radiopaque agent for neurovascular occlusion applications | Density increased with W incorporation (up to 0.060 g cm−3); pore density and volume changed with loading, constant overall porosity; increased viscosity (with W addition), Young modulus and tensile strength (up to 4%W); longer actuation times with W increase | [69] |
| TiO2 | Atanase form, hydrothermal treatment from tetrabutyl titanate and fluoric acid, 20–30 nm × 3 nm (TEM) | Commercially available | Dipping | Photocatalytic inactivation of airborne bacteria | Photoluminescence intensity decreases after loading with Mxene compared with pure TiO2 | [26] |
| TiO2 | Commercial spherical TiO2 (anatase), density 3.9 g/cm3, average diameter 25 nm | Commercially available flexible PU foams | Injection of NPs into the polyol followed by ultrasonic treatment | Industrial applications (sandwich panels) | Good dispersion of NPs in the matrix, decrease in cell size with NP content (up to 1%). 1% TiO2NPs foams—best thermal stability. Increased decomposition temperature, storage modulus, loss modulus and glass transition with NP addition | [70] |
| Pd | Hydrothermal synthesis using PVP, 2−6.5 nm (TEM) | Commercially available | Dipping | Recyclable catalyst for Suzuki–Miyaura cross-coupling reactions | NPs penetrated the foam up to 0.1 cm; foams contained 3D interconnected 100−500 μm pores; catalysts can be reused for 50 catalytic cycles | [71] |
| Fe3O4 | Vacuum coprecipitation | Obtained from Sapiumse biferum kernel oil polyol and diphenylmethane diisocyanate | Incorporation in foam mixture | Lightweight renewable microwave-absorbing material | Porous structure; at 9% Fe3O4 content, foam exhibited microwave absorbency (effective bandwidth of 4.62/4.72 GHz at 1.789 mm/2.0 mm thickness in paraffin/bio-based polyurethane matrix). Effective absorbing frequency of 13.84 GHz at 5 mm thickness; saturation magnetization of 15.18 emu/g (superparamagnetism) | [72] |
| Fe3O4 | Coprecipitation, 74 nm (TEM) | Obtained from commercial isocyanate and polyol | Incorporation of NPs and reduced GO by mixing in polyol solution | Electro-magnetic interference shielding material | Cylindrical cells with spherical shapes; average cell size of the composite decreases with filler concentration; cell density increased with the filler concentration; addition of the filler enhanced the compressive modulus and strength; maximum shielding efficiency 33 dB at 35% Fe3O4/rGO | [73] |
| Fe3O4 | Commercial, 50–100 nm | Obtained from commercial isocyanate and polyol | Incorporation of Fe3O4@APTES (developed via sol–gel) by mixing in the polyol solution | Arsenic and heavy metal removal from water | Homogenous cell structure; higher surface area and lower pore size compared to PU (9.225 m2/g, 8.4 nm); removal efficiency of 95%, 86%, 79% for As/Cd/Zn. | [74] |
| Fe3O4 | Phytosynthesis using Simmondsia chinensis (jojoba) defatted meal extract, rectangular shape 51.48 nm (XRD) | Commercially available | Impregnation through the dip adsorption method. | Drinking water defluorination | Increased thermal stability, superior adsorption capacity for Al2O3-modified foams (43.47 mg/g) compared to Fe3O4 (34.48 mg/g) | [75] |
| Al2O3 | Phytosynthesis using Simmondsia chinensis (jojoba) defatted meal extract, irregular shapes 11.64 nm (XRD) | Commercially available | Impregnation through the dip adsorption method. | Drinking water defluorination | [75] | |
| Al2O3 | Commercial NPs, 40 nm (SEM) | Obtained from commercial isocyanate and polyol | Incorporation of Al2O3 at 1, 2, 3, 5, 10% by mixing in the polyol | Sandwich composites for industrial applications | Damage to cellular structure increased with NP content, lower glass transition temperature with NP increase, highest damping ratio and buckling peak for 2% NPs, decrease in stiffness and strength with addition of NPs | [76] |
| SiO2 | Commercial, 20 nm | Commercially available (density of 30.3 kg/m2, tensile strength of 1.25 kg/m2, elongation of 130%) | Dip-coating | Bacterial anti-adhesion and antifouling applications | The foam demonstrated mechanical durability and stability; a high repellency to liquids such as water and oil; a high antifouling effect against polar and nonpolar liquid pollutants | [77] |
| MgO/Ni | Commercial nanoparticles, 40–60 nm/30–50 nm | Obtained from commercial isocyanate and bio-based polyol | Dipping | Electromagnetic interference shielding | Open cellular porous honeycomb morphology, average pore size: 300 μm, pore wall thickness: 15 μm, composites with 10% MgO and 1% Ni presented maximum shielding of 27.56 dB. | [78] |
| NP Type | NP Synthesis Method and Characteristics | PU Foams | Synthesis Process | Application | Main Findings | Ref. |
|---|---|---|---|---|---|---|
| Ag | In situ formation, quasispheric, 20–30 nm (TEM) | PU constructed using lignin-based polyols | Dipping in metal salt solution | Wound healing applications | Porous morphology, average pore diameter: 100 μm, pore size decreased with NP concentration increase, improved residual ash content, initial degradation temperature and mechanical strength, best results obtained for the highest Ag concentration | [7] |
| Ag | Spherical nanoparticles, 6–10 nm (TEM), smaller dimensions for inner particles | Commercial open-cell PU foam (average density 18.5 g dm−3) | Intermatrix synthesis inside foam via the NaBH4 method | Catalytic and bactericidal water treatment | Up to ten times higher metal content uptake compared with ex situ formation; stable final composites (<1% Ag leaching); significant catalytic activity, not diminished after 3 cycles | [8] |
| Ag | Average diameter 52 nm (SEM) | Polyether-type polyurethane foam (commercial) | In situ reduction of silver to NPs using ascorbic acid | Potential applications in analytical chemistry | In situ optimal synthesis—0.05 M sulfuric acid, 40 min. | [44] |
| Ag | Photoreduction (UV) synthesis of NPs, spheroidal NPs, grouped in 150–200 nm clusters | Industrial PU foams | Direct synthesis on the foam | Filters for air filtration | AgNPs penetrated the foam up to 5 mm with good homogeneity, no altering of the porous structure or polymeric surface chemical composition, fast release of antibacterial ions in physiological solution | [79] |
| Ag | Chemical synthesis (using NaBH4 method) and phytosynthesis using neem leaf extract | Obtained from commercial isocyanate and polyols (castor oil) reactants | Inter matrix synthesis approach, reduction performed directly on silver impregnated PU foams | Biomedical applications | NPs enhanced PU thermoxidative degradation (lower degradation temperature) | [80] |
| Ag | In situ reduction | Commercially available (76 par per inch, density 30.4 kg/m3) | Reduction of Ag+ ions to form AgNPs with glycerol in calcium alginate (CA)/PU foam composite | Antibacterial agent for point-of-use water disinfection | CA/PUF@Ag composites prepared with 0.25% w/v CA present a higher swelling ratio (8.0 g/g), larger initial AgNP loading (8.48 mg/g), a lower Ag release concentration (44.35 μg/L) and a lower Ag release rate (0.27%) after 14 days | [81] |
| Cu | In situ generated, at different copper concentrations | Neat flexible PU foams obtained by a one-step process | Dipping in CuSO4, maintained at 80 °C | Antimicrobial applications | Cell structure not significantly influenced; open cell content decreased from 97.42 to 96.64%; tensile and compressive strength improved, respectively, from 78.1 to 94.2 kPa and from 3.80 to 5.63 kPa | [82] |
| ZnO | Hydrothermal synthesis by seeding on the surface of PU foams | Commercially available | Dipping | Photodegradation of acid black 1 dye under UV and solar light | 85%/65% dye degradation achieved under UV/solar light irradiation. | [83] |
| SiO2 | Sol–gel synthesis in PU matrix, 5–60 nm | Obtained from commercial isocyanate and polyols reactants | Direct synthesis of the hybrid foams | Biomedical applications (dressing foams) | Low structural integrity of foams at >10% Si; increased stiffness with the silica contents; significant increase in durability, strength and elongation; no significant change in water vapor transmission rate | [84] |
| FeOOH | Chemical precipitation | Commercially available (polyether-based polyurethane foam, density, 30.00 kg/m3) | In situ growth on the surface of PU foam containing oxidized sodium alginate and dopamine | Flame-retardant coating, antimicrobial applications | LOI reached 25.5%, peak heat release rate reduced by 45.0%, smoke density decreased by 69.1%; good underwater superoleophobicity (oil contact angle under water 155.2°) | [85] |
| MnO2 | In situ formation | White and color industrial polyurethane foam waste from various sources: scrap from households, upholstery stores, furniture factories | Refluxed with KMnO4 in acidic medium | Antibacterial applications and removal of anionic and cationic dyes | MnO2 randomly distributed inside the spaces of the matrix, paramagnetic behavior (2.5 × 10−5 erg/G2 g), superior surface area (14.3 m2/g), 97.5–100% removal of methylene blue dye, 85–87% removal of Trypan blue | [86] |
| Au | Microwave irradiation, hydrothermal synthesis, 30 nm, seeds of 3 nm (TEM) | Commercial PU foam | In situ synthesis, foam inserted into the reaction medium | Catalytic sponge for semiautomated synthesis | Open cell structure, smooth surfaces of the cell walls, nanosized particles homogeneously distributed on cell walls, reaction rate comparable with state-of-the-art catalysts | [87] |
| Ag | Microwave irradiation, hydrothermal synthesis, 16 nm (TEM) | |||||
| Pd | Microwave irradiation, hydrothermal synthesis, 5 nm (TEM) | |||||
| AuPd | Microwave irradiation, hydrothermal synthesis, Au—130 nm, Pd—6 nm (TEM) | Open cell structure, smooth surfaces of the cell walls, nanosized inhomogeneous particles, inconsistent reaction rates for 4-nitrophenol reduction |
6. Antimicrobial Properties and Biocompatibility of Metal-Nanoparticle-Modified PU Foams
7. Other Types of Modified PU Foams with Antimicrobial Properties
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Joseph, J.; Patel, R.M.; Wenham, A.; Smith, J.R. Biomedical applications of polyurethane materials and coatings. Trans. IMF 2018, 96, 121–129. [Google Scholar] [CrossRef]
- Dutta, A.S. 2-Polyurethane Foam Chemistry. In Recycling of Polyurethane Foams; Thomas, S., Rane, A.V., Kanny, K., Abita, V.K., Thomas, M.G., Eds.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 17–27. [Google Scholar]
- Gama, N.; Ferreira, A.; Barros-Timmons, A. Polyurethane Foams: Past, Present, and Future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef]
- Udabe, E.; Isik, M.; Sardon, H.; Irusta, L.; Salsamendi, M.; Sun, Z.; Zheng, Z.; Yan, F.; Mecerreyes, D. Antimicrobial polyurethane foams having cationic ammonium groups. J. Appl. Polym. Sci. 2017, 134, 45473. [Google Scholar] [CrossRef]
- Dixit, A.; Sabnis, A.; Balgude, D.; Kale, S.; Gada, A.; Kudu, B.; Mehta, K.; Kasar, S.; Handa, D.; Mehta, R.; et al. Synthesis and characterization of citric acid and itaconic acid-based two-pack polyurethane antimicrobial coatings. Polymer Bull. 2023, 80, 2187–2216. [Google Scholar] [CrossRef]
- Paladini, F.; Cooper, I.R.; Pollini, M. Development of antibacterial and antifungal silver-coated polyurethane foams as air filtration units for the prevention of respiratory diseases. J. Appl. Microbiol. 2014, 116, 710–717. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Ma, X.; Qiu, S.; Chen, J.; Lu, G.; Jia, Z.; Zhu, J.; Yang, Q.; Chen, J.; et al. Antimicrobial Lignin-Based Polyurethane/Ag Composite Foams for Improving Wound Healing. Biomacromolecules 2022, 23, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Domènech, B.; Ziegler, K.; Vigués, N.; Olszewski, W.; Marini, C.; Mas, J.; Muñoz, M.; Muraviev, D.N.; Macanás, J. Polyurethane foams doped with stable silver nanoparticles as bactericidal and catalytic materials for the effective treatment of water. New J. Chem. 2016, 40, 3716–3725. [Google Scholar] [CrossRef]
- Namviriyachote, N.; Lipipun, V.; Akkhawattanangkul, Y.; Charoonrut, P.; Ritthidej, G.C. Development of polyurethane foam dressing containing silver and asiaticoside for healing of dermal wound. Asian J. Pharm. Sci. 2019, 14, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Madkour, T.M.; Abdelazeem, E.A.; Tayel, A.; Mustafa, G.; Siam, R. In situ polymerization of polyurethane-silver nanocomposite foams with intact thermal stability, improved mechanical performance, and induced antimicrobial properties. J. Appl. Polym. Sci. 2016, 133, 43125. [Google Scholar] [CrossRef]
- Saleemi, M.A.; Lim, V. Overview of antimicrobial polyurethane-based nanocomposite materials and associated signalling pathways. Europ. Polym. J. 2022, 167, 111087. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Picca, R.A.; Ronco, R.; Bonerba, E.; Tantillo, G.; Pollini, M.; Sannino, A.; Valentini, A.; Cataldi, T.R.I.; Cioffi, N. Investigation of Industrial Polyurethane Foams Modified with Antimicrobial Copper Nanoparticles. Materials 2016, 9, 544. [Google Scholar] [CrossRef] [PubMed]
- Morena, A.G.; Stefanov, I.; Ivanova, K.; Pérez-Rafael, S.; Sánchez-Soto, M.; Tzanov, T. Antibacterial Polyurethane Foams with Incorporated Lignin-Capped Silver Nanoparticles for Chronic Wound Treatment. Ind. Eng. Chem. Res. 2020, 59, 4504–4514. [Google Scholar] [CrossRef]
- Chandan, M.R.; Radhakrishnan, K.; Bal, D.K.; Rizwan, M.; Shaik, A.H. Flexible Polyurethane Foam-ZnO Nanocomposite for Photocatalytic Degradation of Textile Dye. Fibers Polym. 2020, 21, 2314–2320. [Google Scholar] [CrossRef]
- Mordor Intelligence—Mordor Intelligence, Polyurethane Market Size & Share Analysis—Growth Trends & Forecasts (2023–2028). Available online: https://www.mordorintelligence.com/industry-reports/polyurethane-market (accessed on 12 November 2023).
- Cui, M.; Chai, Z.; Lu, Y.; Zhu, J.; Chen, J. Developments of polyurethane in biomedical applications: A review. Res. Chem. Mater. 2023, 2, 262–276. [Google Scholar] [CrossRef]
- Miri, Z.; Farè, S.; Ma, Q.; Haugen, H.J. Updates on polyurethane and its multifunctional applications in biomedical engineering. Progr. Biomed. Eng. 2023, 5, 04200. [Google Scholar] [CrossRef]
- Imran, M.; Kanti Bandyopadhyay, A.; Karan Singh Gandhi, T.; Rahaman, A.; Rehaan Chandan, M. Mechanical property enhancement of flexible polyurethane foam using alumina particles. Mater. Today Proc. 2021, 45, 4040–4044. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.H. Performance evaluations of flexible polyurethane foams manufactured with castor oil-based bio-polyol. Polym. Test. 2023, 124, 108069. [Google Scholar] [CrossRef]
- Dupuis, R.; Duboeuf, O.; Kirtz, B.; Aubry, E. Characterization of vibrational mechanical properties of polyurethane foam. In Proceedings of the 2015 Annual Conference on Experimental and Applied Mechanics; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 123–128. [Google Scholar]
- Cheng, C.W.; Yang, C.I.; Ou, S.L.; Chi, H.W.; Lui, P.W.; Chen, W.Y.; Ao, W.; Lai, F.M. Bending Mattress and Antibacterial Effect of TiO2/nAg/Chitosan-nanoparticle-applied Intelligent Patient Bed. Sens. Mater. 2020, 32, 1757–1766. [Google Scholar] [CrossRef]
- Nguyen-Ha, T.M.; Nguyen, T.B.; Nguyen, T.A.; Pham, L.H.; Nguyen, D.H.; Nguyen, D.M.; Hoang, D.; Oh, E.; Suhr, J. Novel high-performance sustainable polyurethane nanocomposite foams: Fire resistance, thermal stability, thermal conductivity, and mechanical properties. Chem. Eng. J. 2023, 474, 145585. [Google Scholar] [CrossRef]
- Yin, S.; Du, Y.; Liang, X.; Xie, Y.; Xie, D.; Mei, Y. Surface coating of biomass-modified black phosphorus enhances flame retardancy of rigid polyurethane foam and its synergistic mechanism. Appl. Surf. Sci. 2023, 637, 157961. [Google Scholar] [CrossRef]
- Zeng, F.; Men, X.; Chen, M.; Liu, B.; Han, Q.; Huang, S.; Zhao, H.; Wang, Y. Molecular-micron multiscale toughening and flame retarding for polyurethane foams. Chem. Eng. J. 2023, 454, 140023. [Google Scholar] [CrossRef]
- Yan, R.; Wang, R.; Lou, C.W.; Lin, J.H. Comparison of tensile and compressive characteristics of intra/interply hybrid laminates reinforced high-density flexible foam composites. J. Appl. Polym. Sci. 2015, 132, 41438. [Google Scholar] [CrossRef]
- Lu, S.Y.; Meng, G.; Wang, C.; Chen, H. Photocatalytic inactivation of airborne bacteria in a polyurethane foam reactor loaded with a hybrid of MXene and anatase TiO2 exposing {001} facets. Chem. Eng. J. 2021, 404, 126526. [Google Scholar] [CrossRef]
- Vinay, V.C.; Varma, D.S.M.; Chandan, M.R.; Sivabalan, P.; Jaiswal, A.K.; Swetha, S.; Kaczmarek, B.; Sionkowska, A. Study of silver nanoparticle-loaded auxetic polyurethane foams for medical cushioning applications. Polym. Bull. 2022, 79, 4233–4250. [Google Scholar] [CrossRef]
- Sasidharan, A.P.; Meera, V.; Raphael, V.P. Coliform removal efficacy of polyurethane foam impregnated with chitosan nanoparticles and silver/silver oxide nanoparticles. Water Supply 2022, 22, 5675–5687. [Google Scholar] [CrossRef]
- Binxia, Y.; Weiguang, H.; Jianben, L.; Bing, Z.; Rui, Z.; Lan, C. Theoretical and experimental study on sound absorption performance of Al2O3-polyurethane foam and microperforated plate composite structure. J. Low. Freq. Noise Vibr. Active Control 2023, 42, 890–897. [Google Scholar] [CrossRef]
- Yuan, B.; Fang, X.; Liu, J.; Liu, Y.; Zhu, R. Improved Sound Absorption Properties in Polyurethane Foams by the Inclusion of Al2O3 Nanoparticles. Shock. Vib. 2021, 2021, 8010391. [Google Scholar] [CrossRef]
- Huynh, P.T.; Nguyen, G.D.; Tran, K.T.L.; Ho, T.M.; Lam, V.Q.; Bown, M.; Ngo, T.V.K. Study on green preparation of multi-branched gold nanoparticles loaded flexible polyurethane foam for antibacterial dressing. Nanocomposites 2022, 8, 167–174. [Google Scholar] [CrossRef]
- Nandy, A.; Houl, Y.; Zhao, W.; D’Souza, N.A. Thermal heat transfer and energy modeling through incorporation of phase change materials (PCMs) into polyurethane foam. Renew. Sustain. Energy Rev. 2023, 182, 113410. [Google Scholar] [CrossRef]
- Kiddell, S.; Kazemi, Y.; Sorken, J.; Naguib, H. Influence of Flash Graphene on the acoustic, thermal, and mechanical performance of flexible polyurethane foam. Polym. Test. 2023, 119, 107919. [Google Scholar] [CrossRef]
- Kausar, A. Polyurethane Composite Foams in High-Performance Applications: A Review. Polym. Plast. Technol. Eng. 2018, 57, 346–369. [Google Scholar] [CrossRef]
- Hasan, S.M.; Fletcher, G.K.; Monroe, M.B.B.; Wierzbicki, M.A.; Nash, L.D.; Maitland, D.J. Shape memory polymer foams synthesized using glycerol and hexanetriol for enhanced degradation resistance. Polymers 2020, 12, 2290. [Google Scholar] [CrossRef] [PubMed]
- Gurgel, D.; Bresolin, D.; Sayer, C.; Cardozo Filho, L.; Hermes de Araújo, P.H. Flexible polyurethane foams produced from industrial residues and castor oil. Ind. Crop Prod. 2021, 164, 113377. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The Prisma 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Sim, W.; Barnard, R.T.; Blaskovich, M.A.T.; Ziora, Z.M. Antimicrobial Silver in Medicinal and Consumer Applications: A Patent Review of the Past Decade (2007–2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef]
- Paladini, F.; Pollini, M. Antimicrobial Silver Nanoparticles for Wound Healing Application: Progress and Future Trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef]
- Zhao, B.; Qian, Y.; Qian, X.; Fan, J.; Feng, Y. Fabrication and characterization of waterborne polyurethane/silver nanocomposite foams. Polymer Compos. 2019, 40, 1492–1498. [Google Scholar] [CrossRef]
- Kraev, I.D.; Pykhtin, A.A.; Lonskii, S.L.; Kurshev, E.V.; Terekhov, I.V. Effect of Biocidal Additives on Technological Parameters in the Manufacture of Polyurethane Foams. Inorg. Mater. Appl. Res. 2021, 12, 125–132. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, Y.; Liu, J.; Yang, J.; Jia, N.; Zhu, C.; Zhang, J. Optimizing microenvironment by integrating negative pressure and exogenous electric fields via a flexible porous conductive dressing to accelerate wound healing. Biomater. Sci. 2021, 9, 238–251. [Google Scholar] [CrossRef]
- Yang, Z.; Li, K.R.; Zhang, Y.Y.; Hu, J.L.; Li, T.Y.; Weng, Z.X.; Wu, L.X. Antibiotic Silver Particles Coated Graphene Oxide/polyurethane Nanocomposites Foams and Its Mechanical Properties. Chin. J. Struct. Chem. 2022, 41, 2203125–2203131. [Google Scholar]
- Apyari, V.V.; Volkov, P.A.; Dmitrienkoet, S.G. Synthesis and optical properties of polyurethane foam modified with silver nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 015001. [Google Scholar]
- Park, J.K.; Lee, J.H.; Kwak, J.J.; Shin, H.B.; Jung, H.W.; Bae, S.W.; Yeo, E.D.; Lee, Y.K.; Yang, S.S. Evaluation of an antimicrobial silver foam dressing. Wounds 2013, 25, 153–191. [Google Scholar] [PubMed]
- Nadafan, M.; Malekfar, R.; Izadi-Darbandi, A.; Dehghani, Z. Microstructural and antibacterial properties of silver nanoparticle-decorated porous polyurethane surface for water purification. Desal. Water Treat. 2016, 57, 21286–21293. [Google Scholar] [CrossRef]
- Choi, H.J.; Thambi, T.; Yang, Y.H.; Bang, S.I.; Kim, B.S.; Pyun, D.G.; Lee, D.S. AgNP and rhEGF-incorporating synergistic polyurethane foam as a dressing material for scar-free healing of diabetic wounds. RSC Adv. 2017, 7, 13714–13725. [Google Scholar] [CrossRef]
- Moustafa, M.T. Removal of pathogenic bacteria from wastewater using silver nanoparticles synthesized by two fungal species. Water Sci. 2017, 31, 164–176. [Google Scholar] [CrossRef]
- Gedam, S.S.; Chaudhary, A.K.; Vijayakumar, R.P.; Goswami, A.K.; Bajad, G.S.; Pal, D. Thermal, mechanical and morphological study of carbon nanotubes-graphene oxide and silver nanoparticles based polyurethane composites. Mater. Res. Express 2019, 6, 085308. [Google Scholar] [CrossRef]
- Varghese, J.; Chandan, M.R.; Shanthakumar, S. Fixed bed column study for pesticide removal using silver nanoparticles-embedded polyurethane foam and glass beads. Chem. Eng. Commun. 2020, 207, 1337–1346. [Google Scholar] [CrossRef]
- Czél, G.; Vanyorek, L.; Sycheva, A.; Kerekes, F.; Szőri-Dorogházi, E.; Janovszky, D. Antimicrobial effect of silver nanoparticles plated natural zeolite in polyurethane foam. Express Polym. Lett. 2021, 15, 853–864. [Google Scholar] [CrossRef]
- Sanchooli, N.; Sanchooli, E.; Khandan Barani, H. Investigating the effect of water filter made using polyurethane foam containing silver nanoparticles on controlling Yersinia ruckeri in Oncorhynchus mykiss water tanks. Iran. J. Fish. Sci. 2022, 21, 500–515. [Google Scholar]
- Sasidharan, A.P.; Meera, V.; Raphael, V.P. Novel polyurethane foams loaded with nanoparticles—Synthesis, characterisation, and evaluation of phosphate removal efficacies. Int. J. Environ. Sci. Technol. 2022, 19, 7483–7502. [Google Scholar] [CrossRef]
- Sasidharan, V.; Sachan, D.; Chauhan, D.; Talreja, N.; Ashfaq, M. Three-dimensional (3D) polymer—Metal–carbon framework for efficient removal of chemical and biological contaminants. Sci. Rep. 2021, 11, 7708. [Google Scholar] [CrossRef]
- Ashjari, H.R.; Dorraji, M.S.S.; Fakhrzadeh, V.; Eslami, H.; Rasoulifard, M.H.; Rastgouy-Houjaghan, M.; Gholizadeh, P.; Kafil, H.S. Starch-based polyurethane/CuO nanocomposite foam: Antibacterial effects for infection control. Int. J. Biol. Macromol. 2018, 111, 1076–1082. [Google Scholar] [CrossRef]
- Gheydari, M.; Seyed Dorraji, M.S.; Fazli, M.; Rasoulifard, M.H.; Almaie, S.; Daneshvar, H.; Ashjari, H.R. Preparation of open-cell polyurethane nanocomposite foam with Ag3PO4 and GO: Antibacterial and adsorption characteristics. J. Polym. Res. 2021, 28, 69. [Google Scholar] [CrossRef]
- Lin, B.; Yin Yuen, A.C.; Oliver, S.; Liu, J.; Yu, B.; Yang, W.; Wu, S.; Yeoh, G.H.; Wang, C.H. Dual functionalisation of polyurethane foam for unprecedented flame retardancy and antibacterial properties using layer-by-layer assembly of MXene chitosan with antibacterial metal particles. Compos. Part B Eng. 2022, 244, 110147. [Google Scholar] [CrossRef]
- Qian, K.; Zhou, J.; Miao, M.; Wu, H.; Thaiboonrod, S.; Fang, J.; Feng, X. Highly Ordered Thermoplastic Polyurethane/Aramid Nanofiber Conductive Foams Modulated by Kevlar Polyanion for Piezoresistive Sensing and Electromagnetic Interference Shielding. Nano-Micro Lett. 2023, 15, 88. [Google Scholar] [CrossRef]
- Díaz-Gomez, A.; Godoy, M.; Berrio, M.E.; Ramirez, J.; Jaramillo, A.F.; Medina, C.; Montaño, M.; Meléndrez, M.F. Evaluation of the Mechanical and Fire Resistance Properties of Rigid Tannin Polyurethane Foams with Copper Oxide Nanoparticles. Fibers Polym. 2022, 23, 1797–1806. [Google Scholar] [CrossRef]
- Satria, M.; Saleh, T.A. Facile approach of eco-friendly superhydrophilic/underwater superoleophobic zinc-functionalized polyurethane foams for continuous oil-water separation. J. Mol. Liq. 2022, 367, 120341. [Google Scholar] [CrossRef]
- Sanoop, P.K.; Mahesh, K.V.; Nampoothiri, K.M.; Mangalaraja, R.V.; Ananthakumar, S. Multifunctional ZnO-biopolymer nanocomposite coatings for health-care polymer foams and fabrics. J. Appl. Polym. Sci. 2012, 126, E233–E244. [Google Scholar] [CrossRef]
- Dorraji, S.M.; Rasoulifard, M.H.; Shajeri, M.; Ashjari, H.R.; Azizi, M.; Rastgouy-Houjaghan, M. The role of prepared ZnO nanoparticles on improvement of mechanical and antibacterial properties of flexible polyurethane foams: Experimental modeling. Polym. Bull. 2018, 75, 1519–1533. [Google Scholar] [CrossRef]
- Bužarovska, A.; Dinescu, S.; Lazar, A.D.; Serban, M.; Pircalabioru, G.G.; Costache, M.; Gualandi, C.; Averous, L. Nanocomposite foams based on flexible biobased thermoplastic polyurethane and ZnO nanoparticles as potential wound dressing materials. Mater. Sci. Eng. C 2019, 104, 109893. [Google Scholar] [CrossRef]
- Moawed, E.A.; Eissa, M.S.; Al-Tantawy, S.A. Application of polyurethane foam/zinc oxide nanocomposite for antibacterial activity, detection, and removal of basic dyes from wastewater. Int. J. Environ. Sci. Technol. 2023, 20, 7767–7774. [Google Scholar] [CrossRef]
- Vo, D.K.; Do, T.D.; Nguyen, B.T.; Tran, C.K.; Nguyen, T.A.; Nguyen, D.M.; Pham, L.H.; Nguyen, T.D.; Nguyen, T.-D.; Hoang, D. Effect of metal oxide nanoparticles and aluminum hydroxide on the physicochemical properties and flame-retardant behavior of rigid polyurethane foam. Constr. Build. Mater. 2022, 356, 129268. [Google Scholar] [CrossRef]
- Kardeş, M.; Yatmaz, H.C.; Öztürk, K. ZnO Nanorods Grown on Flexible Polyurethane Foam Surfaces for Photocatalytic Azo Dye Treatment. ACS Appl. Nano Mater. 2023, 6, 6605–6613. [Google Scholar] [CrossRef]
- Zanini, N.C.; de Souza, A.G.; Barbosa, R.F.S.; Rosa, D.S.; Mulinari, D.R. A novel hybrid polyurethane composites with ZnO particles and sheath palm residues: Synergistic effect. Polym. Compos. 2021, 42, 532–542. [Google Scholar] [CrossRef]
- Morsy, M.; Abdel-Salam, A.I.; Gomaa, I.; Moustafa, H.; Kalil, H.; Helal, A. Highly Efficient Photocatalysts for Methylene Blue Degradation Based on a Platform of Deposited GO-ZnO Nanoparticles on Polyurethane Foam. Molecules 2023, 28, 108. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.M.; Harmon, G.; Zhou, F.; Raymond, J.E.; Gustafson, T.P.; Wilson, T.S.; Maitland, D.J. Tungsten-loaded SMP foam nanocomposites with inherent radiopacity and tunable thermo-mechanical properties. Polym. Adv. Technol. 2016, 27, 195–203. [Google Scholar] [CrossRef]
- Keshavarz, M.; Zebarjad, S.M.; Daneshmanesh, H.; Moghim, M.H. On the role of TiO2 nanoparticles on thermal behavior of flexible polyurethane foam sandwich panels. J. Therm. Anal. Calorim. 2017, 127, 2037–2048. [Google Scholar] [CrossRef]
- Sahoo, L.; Mondal, S.; Beena, N.C.; Gloskovskii, A.; Manju, U.; Topwal, D.; Gautam, U.K. 3D Porous Polymeric-Foam-Supported Pd Nanocrystal as a Highly Efficient and Recyclable Catalyst for Organic Transformations. ACS Appl. Mater. Interfaces 2021, 13, 10120–10130. [Google Scholar] [CrossRef]
- Xu, X.; Tian, X.; Bo, G.; Su, X.; Yan, J.; Yan, Y. Synthesis of Lightweight Renewable Microwave-Absorbing Bio-Polyurethane/Fe3O4 Composite Foam: Structure Analysis and Absorption Mechanism. Int. J. Mol. Sci. 2022, 23, 12301. [Google Scholar] [CrossRef]
- Oraby, H.; Tantawy, H.R.; Correa-Duarte, M.A.; Darwish, M.; Elsaidy, A.; Naeem, I.; Senna, M.H. Tuning Electro-Magnetic Interference Shielding Efficiency of Customized Polyurethane Composite Foams Taking Advantage of rGO/Fe3O4 Hybrid Nanocomposites. Nanomaterials 2022, 12, 2805. [Google Scholar] [CrossRef]
- Moghaddam, S.T.; Ghasemi, T.; Hussein, F.B. Effect of reaction parameters on Arsenic removal capacity from aqueous solutions using modified magnetic PU foam nanocomposite. Results Mater. 2023, 17, 100373. [Google Scholar] [CrossRef]
- Kumari, S.; Khan, S. Defluoridation technology for drinking water and tea by green synthesized Fe3O4/Al2O3 nanoparticles coated polyurethane foams for rural communities. Sci. Rep. 2017, 7, 8070. [Google Scholar] [CrossRef]
- Khan, T.; Aydın, O.A.; Acar, V.; Aydın, M.R.; Hülagü, B.; Bayrakçeken, H.; Seydibeyoğlu, M.Ö.; Akbulut, H. Experimental Investigation of Mechanical and Modal Properties of Al2O3 Nanoparticle Reinforced Polyurethane Core Sandwich Structures. Mater. Today Commun. 2020, 24, 101233. [Google Scholar] [CrossRef]
- Cho, D.; Oh, J.K. Silica Nanoparticle-Infused Omniphobic Polyurethane Foam with Bacterial Anti-Adhesion and Antifouling Properties for Hygiene Purposes. Nanomaterials 2023, 13, 2035. [Google Scholar] [CrossRef]
- Selvaraj, V.K.; Subramanian, J.; Jeyamani, E.; Azeez, A.; Keerthivasan, R. A study to determine electromagnetic interference-shielding effectiveness on bio-based polyurethane foam reinforced with PVDF/MgO/Ni for emerging applications. J. Appl. Polym. Sci. 2023, 140, e53386. [Google Scholar] [CrossRef]
- Picca, R.A.; Paladini, F.; Sportelli, M.C.; Pollini, M.; Giannossa, L.C.; Di Franco, C.; Panico, A.; Mangone, A.; Valentini, A.; Cioffi, N. Combined approach for the development of efficient and safe nanoantimicrobials: The case of nanosilver-modified polyurethane foams. ACS Biomater. Sci. Eng. 2017, 3, 1417–1425. [Google Scholar] [CrossRef]
- Subramanian, K.; Somachandran, R. Synthesis of Castor Oil based Pristine and Silver Nanoparticle Embedded Polyurethanes and their Characterization by Thermal and Antibacterial Activity Analysis for Biomedical Applications. Asian J. Chem. 2022, 34, 967–974. [Google Scholar] [CrossRef]
- Xu, M.Q.; Luo, H.Y.; Rong, H.W.; Wu, S.H.; Zheng, Z.X.; Chen, B.Y. Calcium alginate gels-functionalized polyurethane foam decorated with silver nanoparticles as an antibacterial agent for point-of-use water disinfection. Int. J. Biol. Macromol. 2023, 231, 123289. [Google Scholar] [CrossRef]
- Li, C.; Ye, H.; Ge, S.; Yao, Y.; Ashok, B.; Hariram, N.; Liu, H.; Tian, H.; He, Y.; Guo, G. Fabrication and properties of antimicrobial flexible nanocomposite polyurethane foams with in situ generated copper nanoparticles. J. Mater. Res. Technol. 2022, 19, 3603–3615. [Google Scholar] [CrossRef]
- Inderyas, A.; Bhatti, I.A.; Ashar, A.; Ashraf, M.; Ghani, A.; Yousaf, M.; Mohsin, M.; Ahmad, M.; Rafique, S.; Masood, N.; et al. Synthesis of immobilized ZnO over polyurethane and photocatalytic activity evaluation for the degradation of azo dye under UV and solar light irardiation. Mater. Res. Express 2020, 7, 025033. [Google Scholar] [CrossRef]
- Song, E.H.; Jeong, S.H.; Park, J.U.; Kim, S.; Kim, H.E.; Song, J. Polyurethane-silica hybrid foams from a one-step foaming reaction, coupled with a sol-gel process, for enhanced wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 866–874. [Google Scholar] [CrossRef]
- Meng, D.; Wang, K.; Wang, W.; Sun, J.; Wang, H.; Gu, X.; Zhang, S. A biomimetic structured bio-based flame retardant coating on flexible polyurethane foam with low smoke release and antibacterial ability. Chemosphere 2023, 312, 137060. [Google Scholar] [CrossRef]
- Moawed, E.A.; Kiwaan, H.A.; El-Zakzouk, S.K.; El-Sonbati, M.A.; El-Zahed, M.M. Chemical Recycling of Polyurethane Foam Waste and Application for Antibacterial and Removal of Anionic and Cationic Dyes. Braz. J. Chem. Eng. 2023, 40, 389–401. [Google Scholar] [CrossRef]
- Gazil, O.; Bernardi, J.; Lassus, A.; Virgilio, N.; Unterlass, M.M. Urethane functions can reduce metal salts under hydrothermal conditions: Synthesis of noble metal nanoparticles on flexible sponges applied in semi-automated organic reduction. J. Mater. Chem. A 2023, 11, 12703. [Google Scholar] [CrossRef]
- Sachsenmaier, S.; Peschel, A.; Ipach, I.; Kluba, T. Antibacterial potency of V.A.C. GranuFoam Silver(®) dressing. Injury 2013, 44, 1363–1367. [Google Scholar] [CrossRef]
- Rembe, J.D.; Fromm-Dornieden, C.; Böhm, J.; Stuermer, E.K. Influence of human acute wound fluid on the antibacterial efficacy of different antiseptic polyurethane foam dressings: An in vitro analysis. Wound Repair. Regen. 2018, 26, 27–35. [Google Scholar] [CrossRef]
- Lee, D.N.; Gwon, K.; Nam, Y.; Lee, S.J.; Tran, N.M.; Yoo, H. Polyurethane Foam Incorporated with Nanosized Copper-Based Metal-Organic Framework: Its Antibacterial Properties and Biocompatibility. Int. J. Mol. Sci. 2021, 22, 13622. [Google Scholar] [CrossRef]
- Rawson, K.B.; Neuberger, T.; Smith, T.; Reddy, H.R.K.; Haussener, T.J.; Sebahar, P.R.; Looper, R.E.; Isaacson, B.M.; Shero, J.; Pasquina, P.F.; et al. Antibiofilm potential of a negative pressure wound therapy foam loaded with a first-in-class tri-alkyl norspermidine-biaryl antibiotic. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1780–1788. [Google Scholar] [CrossRef]
- Regulski, M.; Myntti, M.F.; James, G.A. Anti-biofilm efficacy of commonly used wound care products in in vitro settings. Antibiotics 2023, 12, 536. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, E.; Garren, M.; Manuel, J.; Douglass, M.; Devine, R.; Mondal, A.; Kumar, A.; Ashcraft, M.; Pandey, R.; Handa, H. Superhydrophobic and conductive foams with antifouling and oil-water separation properties. ACS Appl. Mater. Interfaces 2023, 15, 7610–7626. [Google Scholar] [CrossRef] [PubMed]
- El-Zahed, M.; Kiwaan, H.; Farhat, A.; Moawed, E.; El-Sonbati, M. Application of thiourea polyurethane@copper sulfide composite for antibacterial potential. Egypt. J. Chem. 2023, 66, 31–36. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver nanoparticles: Mechanism of action and probable bio-application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Panyala, N.D.; Peña-Méndez, E.M.; Havel, J. Silver or silver nanoparticles: A hazardous threat to the environment and human health? J. Appl. Biomed. 2008, 6, 117–129. [Google Scholar] [CrossRef]
- Wiemann, M.; Vennemann, A.; Blaske, F.; Sperling, M.; Karst, U. Silver Nanoparticles in the Lung: Toxic Effects and Focal Accumulation of Silver in Remote Organs. Nanomaterials 2017, 7, 441. [Google Scholar] [CrossRef] [PubMed]
- Velicogna, J.R.; Schwertfeger, D.M.; Jesmer, A.H.; Scroggins, R.P.; Princz, J.I. The bioaccumulation of silver in Eisenia andrei exposed to silver nanoparticles and silver nitrate in soil. NanoImpact 2017, 6, 11–18. [Google Scholar] [CrossRef]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of Metal and Metal Oxide Nanoparticles on Plant: A Critical Review. Front. Chem. 2017, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, B.; Fang, T. Chemical transformation of silver nanoparticles in aquatic environments: Mechanism, morphology and toxicity. Chemosphere 2018, 191, 324–334. [Google Scholar] [CrossRef]
- Boonkaew, B.; Kempf, M.; Kimble, R.; Cuttle, L. Cytotoxicity testing of silver-containing burn treatments using primary and immortal skin cells. Burns 2014, 40, 1562–1569. [Google Scholar] [CrossRef]
- Norozi, S.; Ghollasi, M.; Salimi, A.; Halabian, R.; Shahrousvad, M. Mesenchymal stem cells osteogenic differentiation by ZnO nanoparticles and polyurethane bimodal foam nanocomposites. Cell Tissue Bank. 2023, 1–19. [Google Scholar] [CrossRef]
- Pahlevanneshan, Z.; Deypour, M.; Kefayat, A.; Rafienia, M.; Sajkiewicz, P.; Esmaeely Neisiany, R.; Enayati, M.S. Polyurethane-Nanolignin Composite Foam Coated with Propolis as a Platform for Wound Dressing: Synthesis and Characterization. Polymers 2021, 13, 3191. [Google Scholar] [CrossRef]
- Yoon, S.; Chen, B. Modulating the Properties of Poly(glycerol sebacate)-Based Polyurethane Hydrogels Using an Organoclay. ACS Biomater. Sci. Eng. 2022, 8, 786–800. [Google Scholar] [CrossRef]
- McFerran, A.; McIvor, M.J.; Lemoine, P.; Meenan, B.J.; Acheson, J.G. Biocompatible Nanocomposite Coatings Deposited via Layer-by-Layer Assembly for the Mechanical Reinforcement of Highly Porous Interconnected Tissue-Engineered Scaffolds. Bioengineering 2022, 9, 585. [Google Scholar] [CrossRef]
- Karizmenh, M.S.; Poursamar, S.A.; Kefayat, A.; Farahbakhsh, Z.; Rafienia, M. An in vitro and in vivo study of PCL/chitosan electrospun mat on polyurethane/propolis foam as a bilayer wound dressing. Biomater. Adv. 2022, 135, 112667. [Google Scholar]
- Sasidharan, A.P.; Meera, V.; Raphael, V.P. Nanochitosan impregnated polyurethane foam in the removal of phosphate and coliforms from greywater. Nanotechnol. Environ. Eng. 2023, 8, 131–142. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, X.; Gong, Q.; Xie, Y.; Fei, F.; Fan, L. Preparation and modification of antibacterial polyurethane foam for oil–water separation. J. Mater. Res. 2023, 38, 2701–2712. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications–A review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.; Lungulescu, E.M.; Nicula, N.; Somoghi, R.; Ditu, L.M.; Ungureanu, C.; Sutan, A.N.; Draghiceanu, O.A.; Paunescu, A.; et al. Phytosynthesis and radiation-assisted methods for obtaining metal nanoparticles. J. Mater. Sci. 2020, 55, 1915–1932. [Google Scholar] [CrossRef]
- ISO 5999:2013; International Organization for Standardization, Flexible Cellular Polymeric Materials—Polyurethane Foam for Load-Bearing Applications Excluding Carpet Underlay—Specification. Available online: https://www.iso.org/standard/59740.html (accessed on 24 September 2023).
- ISO 6915:2019; International Organization for Standardization, Flexible Cellular Polymeric Materials—Polyurethane Foam for Laminate Use—Specification. Available online: https://www.iso.org/standard/77358.html (accessed on 24 September 2023).
- ISO 8873-3:2007; International Organization for Standardization. Rigid Cellular Plastics—Spray-Applied Polyurethane Foam for Thermal Insulation—Part 3: Test methods. Available online: https://www.iso.org/standard/39407.html (accessed on 24 September 2023).
- ISO 8873-2:2007; International Organization for Standardization. Rigid Cellular Plastics—Spray-Applied Polyurethane Foam for Thermal Insulation—Part 2: Application. Available online: https://www.iso.org/standard/39406.html (accessed on 24 September 2023).
- ISO 8873-1:2006; International Organization for Standardization. Rigid Cellular Plastics—Spray-Applied Polyurethane Foam for Thermal Insulation—Part 1: Material specifications. Available online: https://www.iso.org/standard/39378.html (accessed on 24 September 2023).
- ISO 1856:2018; International Organization for Standardization. Flexible Cellular Polymeric Materials—Determination of Compression Set. Available online: https://www.iso.org/standard/70213.html (accessed on 24 September 2023).
- ISO 2440:2019; International Organization for Standardization. Flexible and Rigid Cellular Polymeric Materials—Accelerated Ageing Tests. Available online: https://www.iso.org/standard/77359.html (accessed on 24 September 2023).
- D3574-17(2017); ASTM International. Standard Test Methods for Flexible Cellular Materials—Slab, Bonded, and Molded Urethane Foams. Available online: https://www.astm.org/d3574-17.html (accessed on 24 September 2023).
- ASTM C1620-16(2023); ASTM International. Standard Specification for Aerosol Polyurethane and Aerosol Latex Foam Sealants. Available online: https://www.astm.org/d1620-16.html (accessed on 24 September 2023).
- ASTM D7487-18; ASTM International. Standard Practice for Polyurethane Raw Materials: Polyurethane Foam Cup Test. Available online: https://www.astm.org/d7487-18.html (accessed on 24 September 2023).
- ASTM F1839-08(2021); ASTM International. Standard Specification for Rigid Polyurethane Foam for Use as a Standard Material for Testing Orthopaedic Devices and Instruments. Available online: https://www.astm.org/f1839-08r21.html (accessed on 24 September 2023).
- ASTM C1806-21(2021); ASTM International. Standard Test Method for Measuring the Flow Rate of Aerosol Foam Sealants. Available online: https://www.astm.org/c1806-21.html (accessed on 24 September 2023).
- ASTM C1536-19 (2019); ASTM International. Standard Test Method for Measuring the Yield for Aerosol Foam Sealants. Available online: https://www.astm.org/c1536-19.html (accessed on 24 September 2023).
- ASTM E1730-19 (2019); ASTM International. Standard Specification for Rigid Foam for Use in Structural Sandwich Panel Cores. Available online: https://www.astm.org/e1730-19.html (accessed on 24 September 2023).
- ASTM C1303/C1303M-22 (2022); ASTM International. Standard Test Method for Predicting Long-Term Thermal Resistance of Closed-Cell Foam Insulation. Available online: https://www.astm.org/c1303_c1303m-22.html (accessed on 24 September 2023).
- ASTM D4273-23 (2023); ASTM International. Standard Test Method for Polyurethane Raw Materials: Determination of Primary Hydroxyl Content of Polyether Polyols. Available online: https://www.astm.org/d4273-23.html (accessed on 24 September 2023).
- ASTM D4274-23 (2023); ASTM International. Standard Test Methods for Testing Polyurethane Raw Materials: Determination of Hydroxyl Numbers of Polyols. Available online: https://www.astm.org/d4274-23.html (accessed on 24 September 2023).




| P (Problem) | Insufficient Antimicrobial Properties of PU Foams |
|---|---|
| I (Intervention) | Development of nanomaterial-containing PU foams for biomedical applications |
| C (Comparison) | PU foams, other antimicrobial materials |
| O (Outcome) | Development of antimicrobial PU foams containing metallic nanoparticles |
| NP Type | Metal Content, as Described by Each Study | PU Matrix | Microorganisms | Main Findings on Antibacterial Activity | Ref. |
|---|---|---|---|---|---|
| ZnO | 0.2% biopolymer-ZnO (ZnO final concentration—40 mg/g) | Commercial PU foams coated with ZnO—functional biopolymers: starch, gelatin, chitosan, and agar (1:20 solid to liquid ratio) | Aspergillus niger | Optical microscopic images showed that ZnO containing agar coatings has no significant fungus growth | [45] |
| Ag | 1.0% w/w | Medical-grade PU foam dipped in AgNPs | S. aureus E. coli | 99.9% reduction in viable cell numbers after 1 h, 6 h, 12 h, 24 h, and 48 h of exposure | [52] |
| Ag | - | Commercial V.A.C. GranuFoam Silver Dressing | S. aureus S. epidermidis | ZOI: 4.4 mm after 24 h and 4.7 mm after 39 h ZOI: 7.5 mm after 24 h and 8.1 mm after 39 h | [88] |
| Ag | 1.04 × 10−3 M (colloidal suspension) | PU foam soaked in a AgNP solution for 8 h | E. coli | Bacterial growth inhibition after 20 min exposure to polyurethane coated with AgNPs | [46] |
| Ag | 0.1, 0.5 and 1% | PU foam incorporating AgNPs by mechanical stirring | Klebsiella sp. Staphylococcus sp. E. coli | Bacterial growth inhibition by 0.1% AgNP foam | [10] |
| Ag | 11 to 21 mg Ag per g matrix | Commercial open-cell PU foams loaded with AgNPs obtained via intermatrix synthesis | E. coli | 100% of bacteria killed in less than 6.5 h; the bacterial mortality rate was ca. 1000 CFU mL−1 s −1 | [8] |
| Cu | 0.3–1.3% | Two types of industrial PU foams dipped in diluted CuNP solutions (1:100, 1:1000) | S. aureus E. coli K. marxianus | A higher CuNP loading was generally correlated to a higher concentration of released ions and an increased inhibition of colony growth after 24 h | [12] |
| Ag | - | PU foams incorporating AgNPs and recombinant human epidermal growth factor | S. aureus E. coli | ZOIs for AgNP-PUFs and AgNP/rhEGF-PUFs were significantly larger than that of the PUFs and at the same time higher against S. aureus after 24 h | [48] |
| Ag | 1118.6 mg/L | PU foams soaked in AgNP solution overnight | Fecal coliforms Fecal streptococci S. aureus | Effective removal of total coliforms (97.3%), fecal coliforms (99.9%), fecal streptococci (99.9%) and S. aureus (99.9%) from wastewater after 24 h | [46] |
| Ag | 100 g/m2 | Direct synthesis of AgNPs on an industrial PU foam surface | S. aureus E. coli | 96% and 97% bacterial reduction after 24 h; no bacterial growth was observed in the 24 h following the recultivation of surviving bacteria | [47] |
| Ag | 0.95 mg/cm2 1.20 mg/cm2 0.50 mg/cm2 0.90 mg/cm2 1.34 mg/cm2 1.30 mg/cm2 | Commercial PU foam dressings: Biatain Ag, Mepilex Ag, UrgoCell Silver, Allevyn Ag, Acticoat Moisture Control Ag, PolyMem Silver | E. coli S. aureus P. aeruginosa | Biatain Ag, Mepilex Ag, and Allevyn Ag showed the highest antibacterial activity under challenging conditions with human acute wound fluid | [89] |
| CuO | - | Foams obtained via the one-shot method incorporating CuO NPs, starch and silicone surfactant mixed with polyol components | E. coli S. aureus P. aeruginosa E. faecalis C. albicans | The highest antimicrobial activity against hospital infections was obtained for CuO NPs obtained at 600 °C after only 120 min of exposure | [55] |
| ZnO | 1.5 wt% | ZnO added to the polyol, followed by mechanical stirring, foams obtained by a two-step method | E. coli S. aureus | Bacterial growth reduction after 24 h with a more pronounced effect against E. coli | [62] |
| Ag | 0.4, 0.6, 0.8 and 1.0 mg/cm2 | AgNPs and asiaticoside powder at 5% adsorbed on PU foams containing natural polyols (hydroxypropyl methylcellulose, chitosan and sodium alginate) | P. aeruginosa S. aureus E. coli B. subtilis | Great antibacterial activity for PU formulations with 1 mg/cm2 silver (ZOI: ~ 2.5–3.5 mm) | [9] |
| Ag | 0–4 wt% | Waterborne PU foams incorporating AgNPs via mechanical foaming | E. coli S. aureus | PU matrix filled with 2 wt% AgNPs proved its antibacterial activity (bacteriostatic rates were 98.23% and 97.38%, respectively) | [40] |
| ZnO | 1, 2, 5, 10% | Thermoplastic PU foam incorporating ZnO NPs via the thermally induced phase separation method | S. aureus E. faecalis E. coli P. aeruginosa | The highest ZnO concentration (10%) led to a 103 fold reduction in CFUs; 55% reduction in biofilm formation on the surface of the composites with no significant differences between ZnO concentrations | [63] |
| Ag | 0.12, 0.2, 0.25% relative to final composition | Impregnation of NP dispersion in polyol mixture | S. aureu P. aeruginosa | An increase in the Ag NP content in the foams led to a higher antibacterial activity. PUF-0.25%NP showed over 4 and 5 logs bacterial growth reduction | [13] |
| Ag/TiO2 | - | Ag/TiO2/chitosan powder coated on a bendable double mattress with added HAP powder | S. aureus | 99% antibacterial efficiency | [21] |
| Ag | 1 wt% | PU foam incorporating Ag NP/zeolite during production | E. coli M. luteus | More pronounced antibacterial effect against Gram-positive bacteria (M. luteus) | [51] |
| Ag | 1 wt% | Foams converted to negative Poisson’s ratio or auxetic PU foam incorporating AgNPs | S. aureus S. epidermidis P. aeruginosa E. coli | A higher compression factor greatly enhanced the antibacterial activity | [27] |
| Ag3PO4 | - | Ag3PO4 NPs dispersed in a flexible open-cell polyurethane mixture, followed by graphene oxide coating | S. aureus E. coli | 0.1 g of GO/Ag3PO4 PU foam inhibited the colonies’ growth after 24 h | [56] |
| Cu | - | PU foam incorporated with Cu-BTC NPs | P. aeruginosa K. pneumoniae methicillin-resistant S. aureus | Selective and significant bactericidal effect; efficiency rates: 66.3% 99.3% 30.8% | [90] |
| Cu | 0.2 M (colloidal suspension) | PU foam dip coated with Cu NPs | E. coli S. aureus | 0.2 g of Cu PU foam effectively removed bacteria from wastewater in 3 h | [54] |
| TiO2 | - | PU foams coated with {001}TiO2/Ti3C2Tx (MXene) nanosheets | E. coli | Superior inactivation efficiency of airborne E. coli under UV photocatalysis; Different inactivation mechanisms between UV irradiation and UV photocatalysis (bacteria are not able to reactivate after photocatalytic oxidation) | [26] |
| Ag | 0.002, 0.021, and 0.088 wt% | PU foam obtained using lignin-based polyols dipped in metal salt solution | E. coli S. aureus | >99% antibacterial rate against E. coli within 1 h and S. aureus within 4 h | [7] |
| Ag | 10% w/w | Commercial (GF Silver) | methicillin-resistant S. aureus A. baumannii | ZOI: 1.52 mm ZOI: 2.04 mm | [91] |
| Ag | 50 and 100 mg | PU foams obtained by mixing “green” Ag NPs in polyol solution | Y. ruckeri | ZOI: 15.33 ± 1.6 for 50 mg Ag and 14.83 ±0.76 mm for 100 mg Ag | [52] |
| Ag | 0.002 M (silver nitrate solution) | PU foams impregnated with AgNPs incorporated by intermatrix synthesis | E. coli B. subtilis | AgNP increased the ZOI diameter, showing antibacterial action against both bacterial strains | [80] |
| Ag | 0.4% | AgNPs and AgNP/GO nanocomposites prepared by pepsin reduction mixed in the polyol solution | S. aureus | The foam containing AgNP/GO induced a larger ZOI, as it is a more effective antibacterial agent | [43] |
| Ag and Cu | 1 wt% | PU foams successively dipped in poly(acrylic) acid, chitosan, Ti3C2 and metal solution | P. aeruginosa S. aureus | Significant reduction in bacterial growth (Ag-coated PU: 99.97% for P. aeruginosa and 88.9% for S. aureus; Cu-coated PU: 58.7% for P. aeruginosa and 72.4% for S. aureus) | [57] |
| Ag/AgO | - | PU foams impregnated with nanochitosan and Ag/AgO NPs | E. coli | 100% removal efficiency | [28] |
| Cu | 1 mM, 5 mM, 25 mM, 125 mM, 250 mM (copper salt solution) | PU dipped in CuSO4 solution | E. coli P. aeroginosa B. licheniformis S. aureus | Good antibacterial activities were obtained even with low concentrations of CuSO4 (ZOI varied between 28 and 40 mm) | [82] |
| Au | 5–15 μg/mL | Au multi-branched NPs incorporated into PU foam by dipping for 24 h | S. aureus E. coli | >95% and ~ 85% removal efficiency | [31] |
| Ag | 0.5 mM, 2 mM, 5 mM (silver salt concentration) | Calcium alginate (CA)/PU foam composite decorated with Ag NPs | E. coli | ZOI: 1.8–4.5 mm; OD600 value of the bacterial suspension filtered through CA/PUF@Ag decreased to a very low level (<0.05) | [81] |
| Ag | - | Commercial PU Foam–Ag Salt | P. aeruginosa S. aureus | Unable to inhibit bacterial biofilm | [92] |
| Cu | - | Nanosized Cu and graphene were incorporated into PU mix | E. coli | Viability of planktonic and adhered E. coli reduced to 99.66% and 96% | [93] |
| CuS | 50, 100 and 150 μg/mL | Immobilization of CuS NPs on PU foam via the seeding method | B. cereus P. aeruginosa | Significant difference between bacterial strains (no antibacterial effect of the 50 μg/mL PU composite on P. aeruginosa growth); Excellent antibacterial activity for the highest content of CuS NPs (ZOI: 15 mm for B. cereus and 12 mm for P. aeruginosa) | [94] |
| FeOOH | - | In situ growth on the surface of a flexible PU foam containing oxidized sodium alginate and dopamine | E. coli S. aureus | Colonies of both bacteria did not grow on modified PU surfaces, and the number of both bacteria decreased significantly after 12 h | [85] |
| ZnO | 1 wt% | PU foams refluxed with ZnO NPs for 6 h | E. coli S. aureus S. typhimurium | A decrease in bacterial growth was observed after 4 h; the antibacterial effect was more pronounced for S. aureus and S. typhimurium | [64] |
| SiO2 | - | Fluorinated silica NP suspension deposited on PU foams via dip-coating | E. coli S. epidermidis | Reduction by >90% per unit area (1–2 log units) in bacterial adhesion | [77] |
| MnO2 | - | PU foam wastes refluxed with KMnO4 in acidic medium | B. cereus S. aureus E. coli | ZOI: 8.8 mm ZOI: 7.5 mm ZOI: 7.1 mm | [86] |
| NM | PU Foam Composition | Assays | Main Morphological Findings | Main Findings on Biological Activity | Ref. |
|---|---|---|---|---|---|
| NL, 45–80 nm | Polyethylene glycol, glycerol, NL, 1, 6-diisocyanato-hexane (NCO/OH ratio: 1.2) and water as a blowing agent, coated with propolis | Morphological investigations Antimicrobial—ZOI test against Staphylococcus aureus (ATCC 25923) and Escherichia coli (ATCC 25922) Biocompatibility (L929 fibroblasts) In vivo wound healing | Increased tensile strength, and elongation at break; average pore diameter 110 µm, apparent porosity 87.9%, density 0.28 g/cm3, water absorption 242%, contact angle 50.1 ± 2.1° ZOI: E. coli 7.2 mm, S. aureus 11.2 mm Cell viability > 90%, good fibroblast adhesion Significantly (p < 0.05) higher wound closure rate (~90% after 10 days) compared with the control (<60%) | Antibacterial activity (ZOI: E. coli 7.2 mm, S. aureus 11.2 mm); Good biocompatibility on L929 fibroblasts (cell viability > 90%, good cell adhesion); Significantly (p < 0.05) higher wound closure rate (~90% after 10 days) compared to control (<60%) in in vivo rat studies | [104] |
| NCl, Cloisite 30B | Hexamethylene diisocyanate, poly(ethylene glycol) reacted in tetrahydrofuran with tin(II) and added to a mixture of poly(glycerol sebacate) and Cloisite 30B; the resulting mixture was casted into polytetrafluoroethylene molds | Morphological drug loading and release tests. Biodegradation (lipase enzyme) Biocompatibility (L929 cell line) | Excellent transparency, pore size 94.3 µm, Young’s moduli 0.10 MPa, compressive stress at 75% strain values 0.29 MPa, contact angle 86.0°, water swelling ratio 212.3%; dye loading MB 41.8 mg/g, MO 15.6 mg/g, SG 6 mg/g, dye release 11.1/12.6/3.4 mg/g Mass loss with lipase 35.6% No evidence of cytotoxicity, increasing cell metabolic activity and good cell morphology | No evidence of cytotoxicity on L929 fibroblasts, increased cell metabolic activity and good cell morphology | [105] |
| Composite: poly(ethylenimine), poly(acrylic acid), Na+ montmorillonite, poly(diallydimethylammonium chloride), chitosan and sodium alginate | Composite deposited via the layer-by-layer technique on commercial PU foams | Morphological and mechanical assays Cell viability | Open cell structure, elastic modulus increased up to 6.01 MPa, similar porosity Significantly lower cytotoxicity for the chitosan and poly(diallydimethylammonium chloride) coatings | Significantly lower cytotoxicity for the chitosan and poly(diallydimethylammonium chloride) coatings on U-2 OS bone cells | [106] |
| poly-ε-caprolactone/chitosan nanofibres | Nanofiber mat as the sublayer, PU foam coated with ethanolic extract of propolis | Morphological and physico-mechanical properties Antibacterial activity (Staphylococcus aureus, Escherichia coli) Cytotoxicity assays (L929 Fibroblast) In vivo study | Bead-free, randomly oriented, continuous nanofibers, 207 nm; the layer composite porosity reduced to 68%, tensile strength to 6.21 MPa, elongation at break to 371%, contact angle to 58.6°; significant increase in swelling ratio; 17.7% degradation in 28 days ZOI 0.53 mm (S. aureus)/1.54 mm (E coli) Significantly enhances the cell viability Significantly and effectively accelerated the healing process | Antibacterial activity (ZOI: 0.53 mm for S. aureus, and 1.54 mm for E. coli); Significantly enhanced the viability of L292 fibroblasts; Significantly and effectively accelerated the healing process in in vivo murine models | [107] |
| Chitosan, 56–112 nm | Nanochitosan soaked in commercial PU foams | Morphological evaluation Phosphate removal Antimicrobial properties and coliform removal | Nanoparticles not agglomerated, no influence on morphological features Adsorption capacity ~ 17 mg/g Inhibition of coliform growth (>99%) Bacterial growth inhibition efficiency = 77.53% | Removed >99% of coliforms from the synthetic graywater Bacterial growth inhibition efficiency = 77.53% | [108] |
| MWCNTs | PU modified with amino acid (mixed in the polyol solution), soaked in dopamine solution, finally MWCNT solution added | Morphological evaluation Hydrophobicity and lipophilicity evaluation Oil sorption and oil water separation Antibacterial activity (S. aureus, E. coli) | MWCNT aggregates observed on surface, porous structure intact, water contact angle: 153°, lipophilic nature, over 97% efficiency in oil/water or organic solvent/water mixture separation, high antibacterial activity E. coli (>80%) and S. aureus (>75%); activity against Gram-negative bacteria was maintained at a high level after repeated use | High antibacterial activity against E. coli (>80%) and S. aureus (>75%); activity maintained at a high level against Gram-negative bacteria after repeated use | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fierascu, R.C.; Lungulescu, E.-M.; Fierascu, I.; Stan, M.S.; Voinea, I.C.; Dumitrescu, S.I. Metal and Metal Oxide Nanoparticle Incorporation in Polyurethane Foams: A Solution for Future Antimicrobial Materials? Polymers 2023, 15, 4570. https://doi.org/10.3390/polym15234570
Fierascu RC, Lungulescu E-M, Fierascu I, Stan MS, Voinea IC, Dumitrescu SI. Metal and Metal Oxide Nanoparticle Incorporation in Polyurethane Foams: A Solution for Future Antimicrobial Materials? Polymers. 2023; 15(23):4570. https://doi.org/10.3390/polym15234570
Chicago/Turabian StyleFierascu, Radu Claudiu, Eduard-Marius Lungulescu, Irina Fierascu, Miruna S. Stan, Ionela C. Voinea, and Silviu Ionel Dumitrescu. 2023. "Metal and Metal Oxide Nanoparticle Incorporation in Polyurethane Foams: A Solution for Future Antimicrobial Materials?" Polymers 15, no. 23: 4570. https://doi.org/10.3390/polym15234570