Enhanced Solubilization and Biodegradation of HMW-PAHs in Water with a Pseudomonas mosselii-Released Biosurfactant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Screening of Biosurfactant-Producing Strains
2.2. Identification of Bacterial Strains with 16S rDNA Sequencing
2.3. SEM Observation of the Bacterial Strains
2.4. Optimal Growth Conditions of the Strains
2.4.1. Growth Curve of the Strains
2.4.2. Tolerance to Extreme Environmental Conditions
2.5. Experimental Study on Biosurfactant Production with Pseudomonas mosselii MP-6
2.5.1. Optimal Fermentation Conditions for Biosurfactant Production
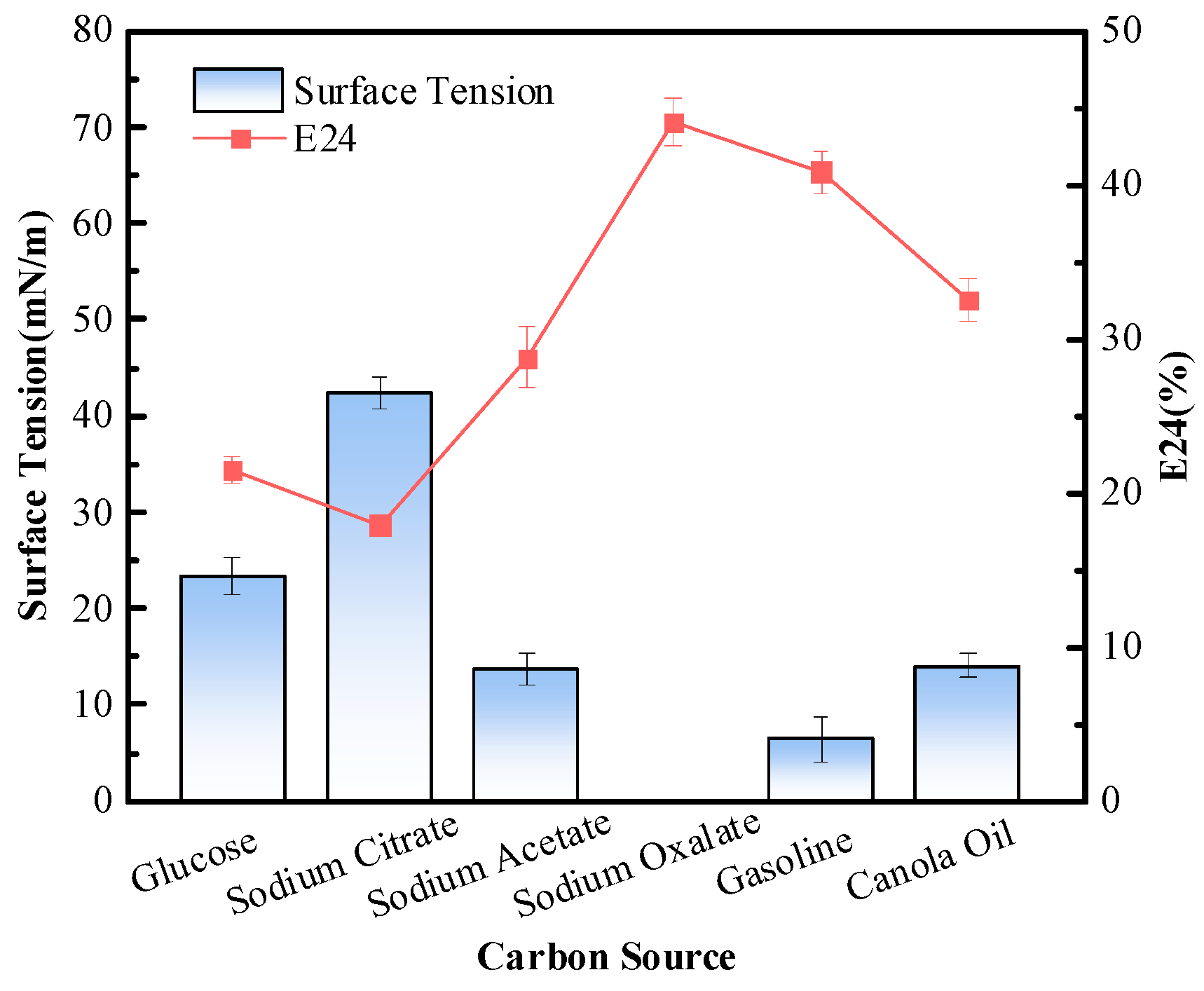
Carbon Source
Cultivation Time
2.5.2. Extraction of the Biosurfactant
2.5.3. Physicochemical Properties of the Biosurfactant
Chemical Structure of the Biosurfactant
Determination of the Critical Micelle Concentration (CMC) of the Biosurfactant
2.5.4. Tolerance of the Biosurfactant to Extreme Environmental Conditions
2.6. Solubilization and Biodegradation Promotion Effects of the Biosurfactant on PAHs
2.6.1. Promotion of the Solubilization of PAHs with the Biosurfactant
2.6.2. Promotion of the Biodegradation of PAHs with the Biosurfactant
3. Results and Discussion
3.1. Screening of Biosurfactant-Producing Strains
3.2. Physiological Characteristics of P. mosselii MP-6
3.2.1. Identification of the Strain
3.2.2. SEM Observation of the Strain
3.2.3. P. mosselii MP-6 Growth under Different Environmental Conditions
3.3. Optimal Fermentation Conditions for Biosurfactant Production
3.3.1. Carbon Source
3.3.2. Cultivation Time
3.4. Physiochemical Properties of the Biosurfactant
3.4.1. Chemical Structure of the Biosurfactant
3.4.2. Critical Micelle Concentrations (CMCs) of Biosurfactants
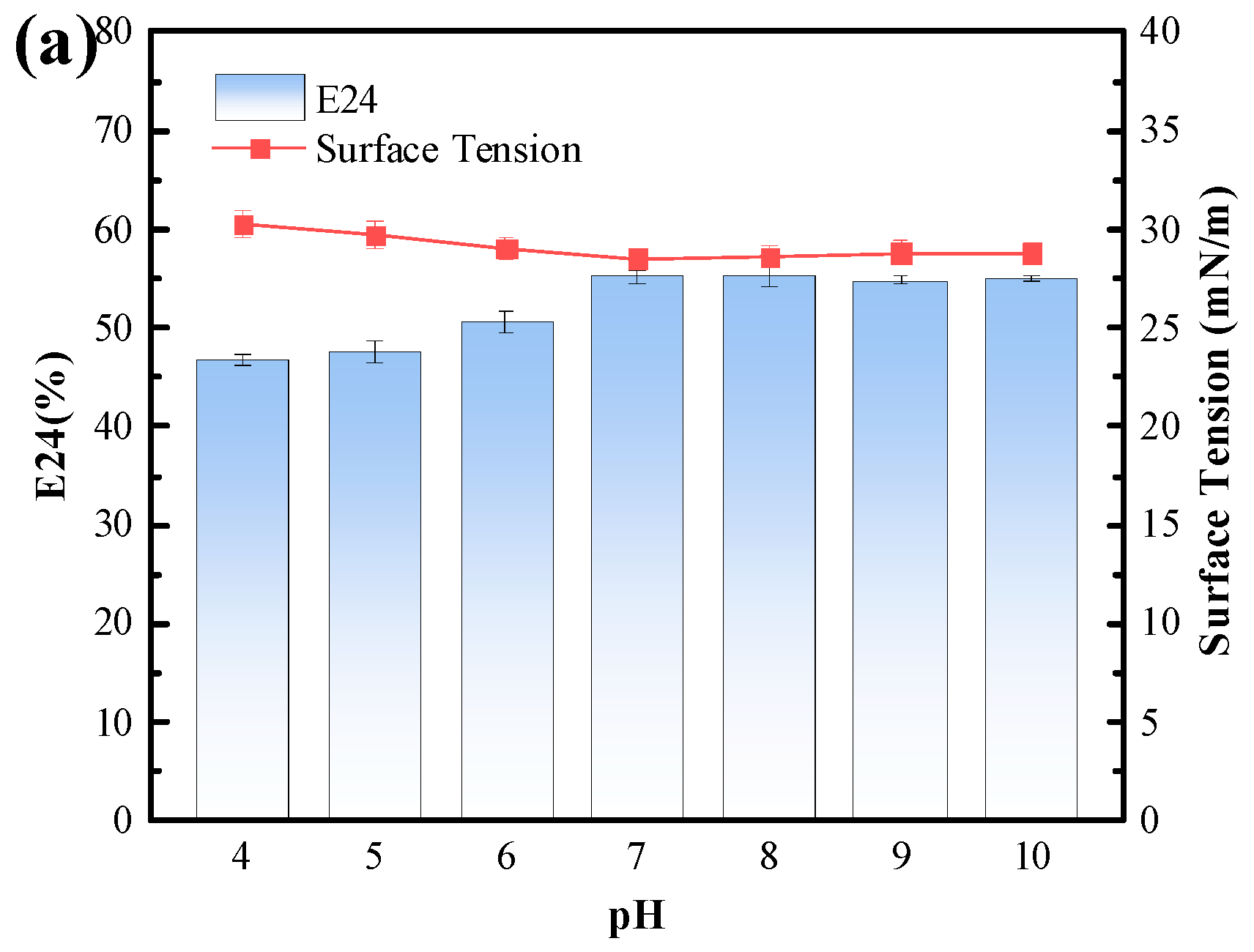
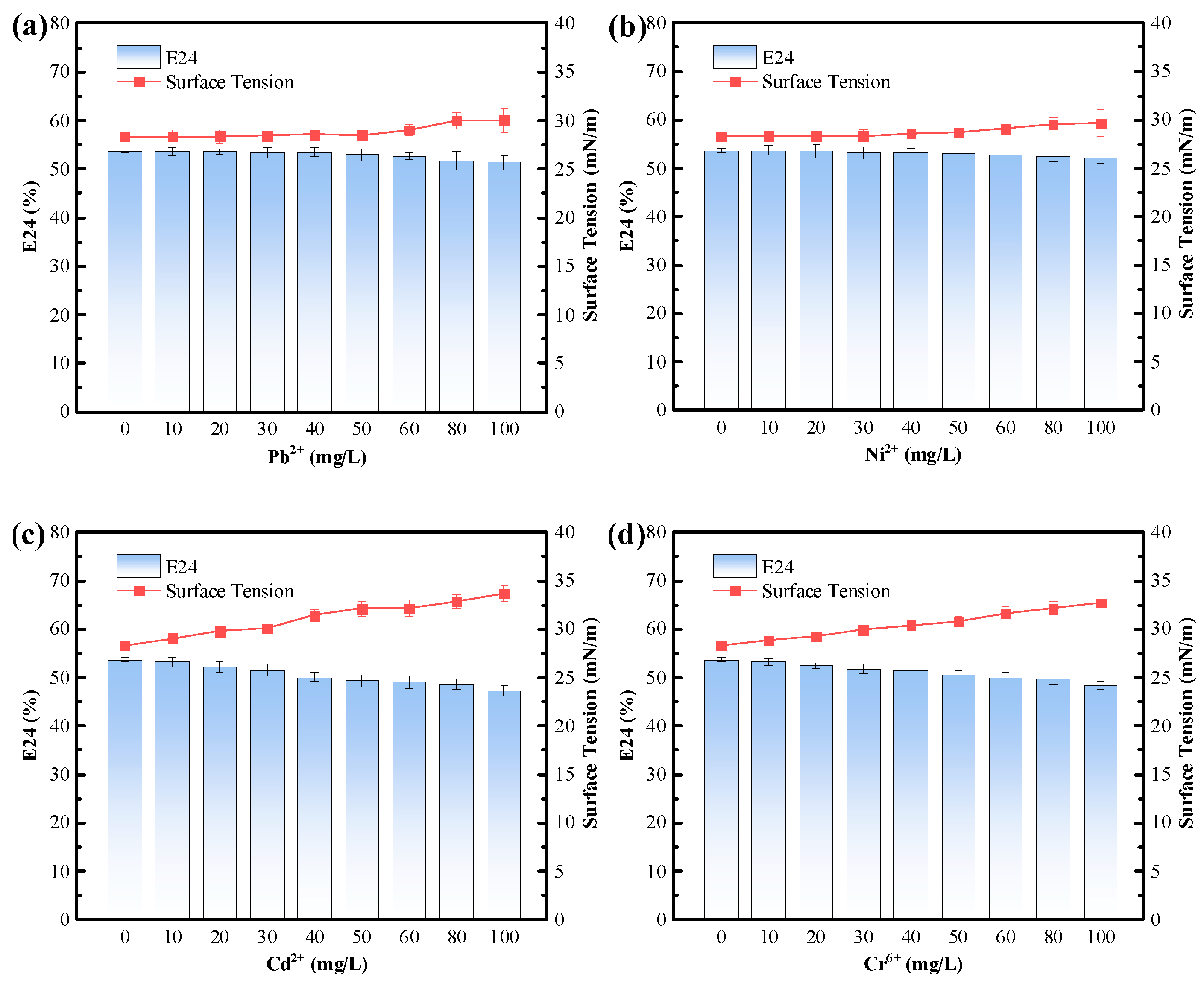
3.4.3. Tolerance of the Biosurfactant to Environmental Stresses
3.5. Biosurfactant Effects on the Solubilization and Biodegradation of PAHs
3.5.1. Biosurfactant Effects on the Solubilization of PAHs
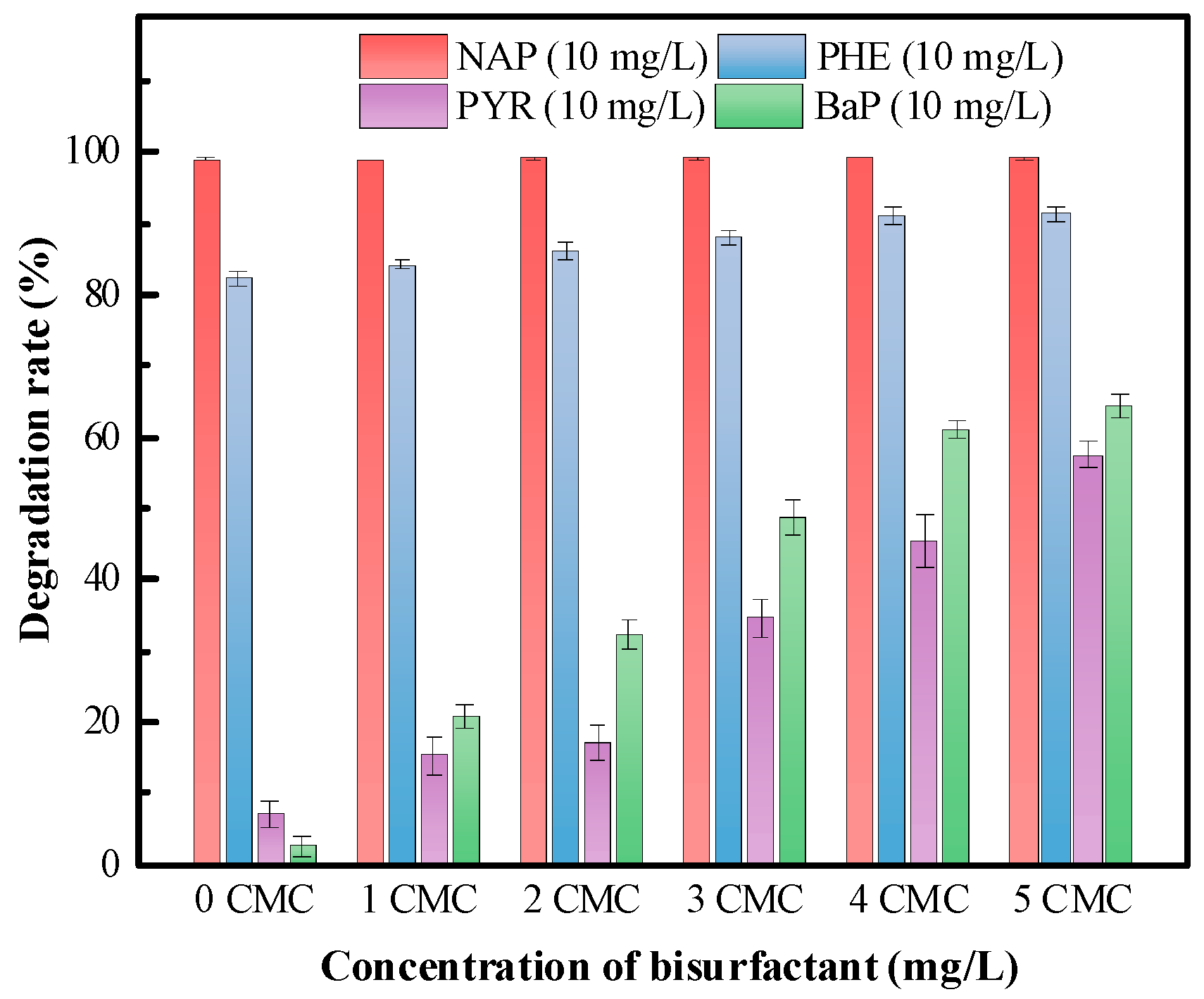
3.5.2. Biosurfactant Effects on the Biodegradation of PAHs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Z.H.; Chen, W.; Cheng, Y.; Li, J.B.; Chen, Z.L. Burkholderia cepacia immobilized onto rGO as a biomaterial for the removal of naphthalene from wastewater. Environ. Res. 2023, 235, 116663. [Google Scholar] [CrossRef]
- Xia, M.Q.; Fu, D.F.; Chakraborty, R.; Singh, R.P.; Terry, N. Enhanced crude oil depletion by constructed bacterial consortium comprising bioemulsifier producer and petroleum hydrocarbon degraders. Bioresour. Technol. 2019, 282, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Parab, V.; Phadke, M. Co-biodegradation studies of naphthalene and phenanthrene using bacterial consortium. J. Environ. Sci. Health Part A-Toxic/Hazard. Subst. Environ. Eng. 2020, 55, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Hu, H.Y.; Zanaroli, G.; Xu, P.; Tang, H.Z. A Pseudomonas sp. strain uniquely degrades PAHs and heterocyclic derivatives via lateral dioxygenation pathways. J. Hazard. Mater. 2021, 403, 123956. [Google Scholar] [CrossRef] [PubMed]
- Zang, T.T.; Wu, H.Z.; Zhang, Y.X.; Wei, C.H. The response of polycyclic aromatic hydrocarbon degradation in coking wastewater treatment after bioaugmentation with biosurfactant-producing bacteria Pseudomonas aeruginosa S5. Water Sci. Technol. 2021, 83, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Chirwa, E.M.N.; Lutsinge-Nembudani, T.B.; Fayemiwo, O.M.; Bezza, F.A. Biosurfactant assisted degradation of High Molecular Weight Polycyclic Aromatic Hydrocarbons by mixed cultures from a car service oil dump from Pretoria Central Business District (South Africa). J. Clean. Prod. 2021, 290, 125183. [Google Scholar] [CrossRef]
- Varjani, S.J.; Upasani, V.N. Carbon spectrum utilization by an indigenous strain of Pseudomonas aeruginosa NCIM 5514: Production, characterization and surface active properties of biosurfactant. Bioresour. Technol. 2016, 221, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.F.; Wang, H.Y.; Jing, Y.N.; Liu, M.; Wang, D.G.; Li, Y.M.; Bao, M.T. An efficient and environmental-friendly dispersant based on the synergy of amphiphilic surfactants for oil spill remediation. Chemosphere 2019, 215, 241–247. [Google Scholar] [CrossRef]
- Sharma, S.; Pandey, L.M. Production of biosurfactant by Bacillus subtilis RSL-2 isolated from sludge and biosurfactant mediated degradation of oil. Bioresour. Technol. 2020, 307, 123261. [Google Scholar] [CrossRef]
- Gharibzadeh, F.; Kalantary, R.R.; Nasseri, S.; Esrafili, A.; Azari, A. Reuse of polycyclic aromatic hydrocarbons (PAHs) contaminated soil washing effluent by bioaugmentation/biostimulation process. Sep. Purif. Technol. 2016, 168, 248–256. [Google Scholar] [CrossRef]
- Johnson, P.; Trybala, A.; Starov, V.; Pinfield, V.J. Effect of synthetic surfactants on the environment and the potential for substitution by biosurfactants. Adv. Colloid Interface Sci. 2021, 288, 123261. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, I.M.; Tsaousi, K.; Rudden, M.; Marchant, R.; Alameda, E.J.; Roman, M.G.; Banat, I.M. Rhamnolipid and surfactin production from olive oil mill waste as sole carbon source. Bioresour. Technol. 2015, 198, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Varjani, S.J.; Upasani, V.N. Critical review on biosurfactant analysis, purification and characterization using rhamnolipid as a model biosurfactant. Bioresour. Technol. 2017, 232, 389–397. [Google Scholar] [CrossRef]
- Souza, E.C.; Vessoni-Penna, T.C.; Oliveira, R.P.D. Biosurfactant-enhanced hydrocarbon bioremediation: An overview. Int. Biodeterior. Biodegrad. 2014, 89, 88–94. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Rodrigues, A.I.; de Freitas, V.; Azevedo, Z.; Teixeira, J.A.; Rodrigues, L.R. Valorization of agro-industrial wastes towards the production of rhamnolipids. Bioresour. Technol. 2016, 212, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Chen, B.H. Surfactant-mediated Biodegradation of Polycyclic Aromatic Hydrocarbons. Materials 2009, 2, 76–94. [Google Scholar] [CrossRef]
- Lamichhane, S.; Krishna, K.C.B.; Sarukkalige, R. Surfactant-enhanced remediation of polycyclic aromatic hydrocarbons: A review. J. Environ. Manag. 2017, 199, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.W.; Li, F.; Zhan, Y.; Zhu, L.Z. Shifts in microbial community structure during in situ surfactant-enhanced bioremediation of polycyclic aromatic hydrocarbon-contaminated soil. Environ. Sci. Pollut. Res. 2016, 23, 14451–14461. [Google Scholar] [CrossRef] [PubMed]
- Nalvothula, R.; Challa, S.; Peddireddy, V.; Merugu, R.; Rudra, M.P.P.; Alataway, A.; Dewidar, A.Z.; Elansary, H.O. Isolation, Molecular Identification and Amino Acid Profiling of Single-Cell-Protein-Producing Phototrophic Bacteria Isolated from Oil-Contaminated Soil Samples. Molecules 2022, 27, 6265. [Google Scholar] [CrossRef] [PubMed]
- Satpute, S.K.; Banat, I.M.; Dhakephalkar, P.K.; Banpurkar, A.G.; Chopade, B.A. Biosurfactants, bioemulsifiers and exopolysaccharides from marine microorganisms. Biotechnol. Adv. 2010, 28, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Cazals, F.; Huguenot, D.; Crampon, M.; Colombano, S.; Betelu, S.; Galopin, N.; Perrault, A.; Simonnot, M.-O.; Ignatiadis, I.; Rossano, S. Production of biosurfactant using the endemic bacterial community of a PAHs contaminated soil, and its potential use for PAHs remobilization. Sci. Total Environ. 2020, 709, 136143. [Google Scholar] [CrossRef]
- Jadhav, V.V.; Yadav, A.; Shouche, Y.S.; Aphale, S.; Moghe, A.; Pillai, S.; Arora, A.; Bhadekar, R.K. Studies on biosurfactant from Oceanobacillus sp BRI 10 isolated from Antarctic sea water. Desalination 2013, 318, 64–71. [Google Scholar] [CrossRef]
- Nie, M.Q.; Yin, X.H.; Ren, C.Y.; Wang, Y.; Xu, F.; Shen, Q.R. Novel rhamnolipid biosurfactants produced by a polycyclic aromatic hydrocarbon-degrading bacterium Pseudomonas aeruginosa strain NY3. Biotechnol. Adv. 2010, 28, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Shi, R.J.; Ma, F.; Han, S.Q.; Zhang, Y. Oxygen effects on rhamnolipids production by Pseudomonas aeruginosa. Microb. Cell Factories 2018, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.L.; Wang, Y.X.; Zang, T.T.; Wei, J.Y.; Wu, H.Z.; Wei, C.H.; Qiu, G.; Li, F. A biosurfactant-producing Pseudomonas aeruginosa S5 isolated from coking wastewater and its application for bioremediation of polycyclic aromatic hydrocarbons. Bioresour. Technol. 2019, 281, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Patowary, K.; Patowary, R.; Kalita, M.C.; Deka, S. Characterization of Biosurfactant Produced during Degradation of Hydrocarbons Using Crude Oil As Sole Source of Carbon. Front. Microbiol. 2017, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Bharali, P.; Singh, S.P.; Dutta, N.; Gogoi, S.; Bora, L.C.; Debnath, P.; Konwar, B.K. Biodiesel derived waste glycerol as an economic substrate for biosurfactant production using indigenous Pseudomonas aeruginosa. Rsc Adv. 2014, 4, 38698–38706. [Google Scholar] [CrossRef]
- Zang, T.T.; Wu, H.Z.; Yan, B.; Zhang, Y.X.; Wei, C.H. Enhancement of PAHs biodegradation in biosurfactant/phenol system by increasing the bioavailability of PAHs. Chemosphere 2021, 266, 128941. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.J.; Guo, C.L.; Wei, Y.F.; Lin, W.J.; Yi, X.Y.; Lu, G.N.; Dang, Z. Cosolubilization synergism occurrence in codesorption of PAH mixtures during surfactant-enhanced remediation of contaminated soil. Chemosphere 2016, 144, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Parthipan, P.; Cheng, L.; Dhandapani, P.; Elumalai, P.; Huang, M.Z.; Rajasekar, A. Impact of biosurfactant and iron nanoparticles on biodegradation of polyaromatic hydrocarbons (PAHs). Environ. Pollut. 2022, 306, 119384. [Google Scholar] [CrossRef]
- Innemanová, P.; Filipová, A.; Michalíková, K.; Wimmerová, L.; Cajthaml, T. Bioaugmentation of PAH-contaminated soils: A novel procedure for introduction of bacterial degraders into contaminated soil. Ecol. Eng. 2018, 118, 93–96. [Google Scholar] [CrossRef]
- Bezza, F.A.; Chirwa, E.M.N. The role of lipopeptide biosurfactant on microbial remediation of aged polycyclic aromatic hydrocarbons (PAHs)-contaminated soil. Chem. Eng. J. 2017, 309, 563–576. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, M.; Wang, S.; Chen, B.; Qiu, R.; Fan, G. Enhanced Solubilization and Biodegradation of HMW-PAHs in Water with a Pseudomonas mosselii-Released Biosurfactant. Polymers 2023, 15, 4571. https://doi.org/10.3390/polym15234571
Xia M, Wang S, Chen B, Qiu R, Fan G. Enhanced Solubilization and Biodegradation of HMW-PAHs in Water with a Pseudomonas mosselii-Released Biosurfactant. Polymers. 2023; 15(23):4571. https://doi.org/10.3390/polym15234571
Chicago/Turabian StyleXia, Mingqian, Shibin Wang, Bo Chen, Rongpeng Qiu, and Gongduan Fan. 2023. "Enhanced Solubilization and Biodegradation of HMW-PAHs in Water with a Pseudomonas mosselii-Released Biosurfactant" Polymers 15, no. 23: 4571. https://doi.org/10.3390/polym15234571





