AKD Emulsions Stabilized by Guar Gel: A Highly Efficient Agent to Improve the Hydrophobicity of Cellulose Paper
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Guar Gel
2.3. Preparation of the AKD Emulsions
2.4. Characterization of the AKD Emulsions
2.4.1. Rheological Properties Measurement
2.4.2. Stability Analysis
2.4.3. Microstructure of the AKD Emulsions
2.4.4. Emulsion Droplets Diameter
2.5. Preparation of the Packaging Paper and Evaluation of Hydrophobicity
3. Results and Discussion
3.1. Characterization of the Guar Gel
3.2. Effect of Guar Gel on Emulsion Properties
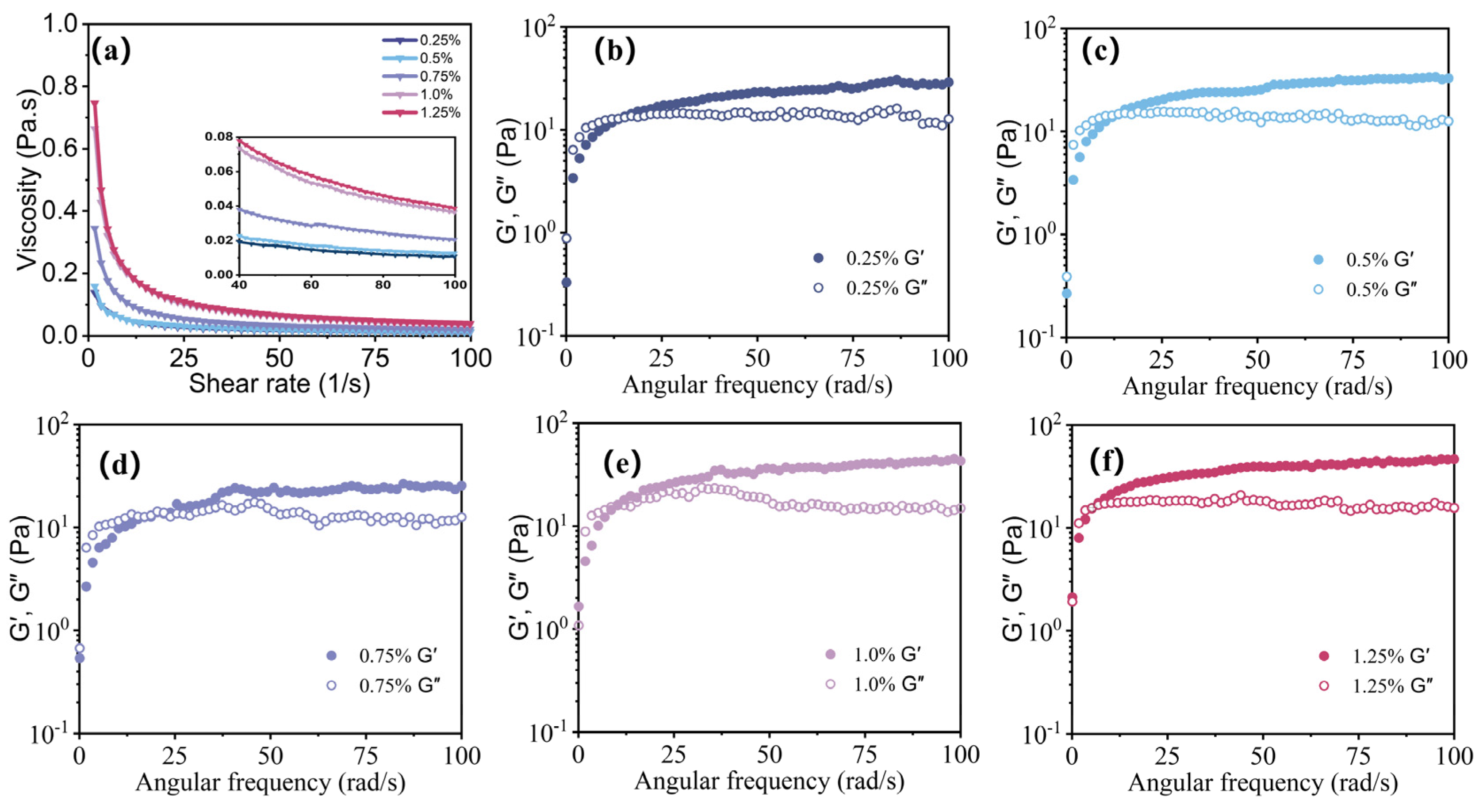
3.2.1. Effect of Guar Gel Concentration on the Rheological Properties of the AKD Emulsions
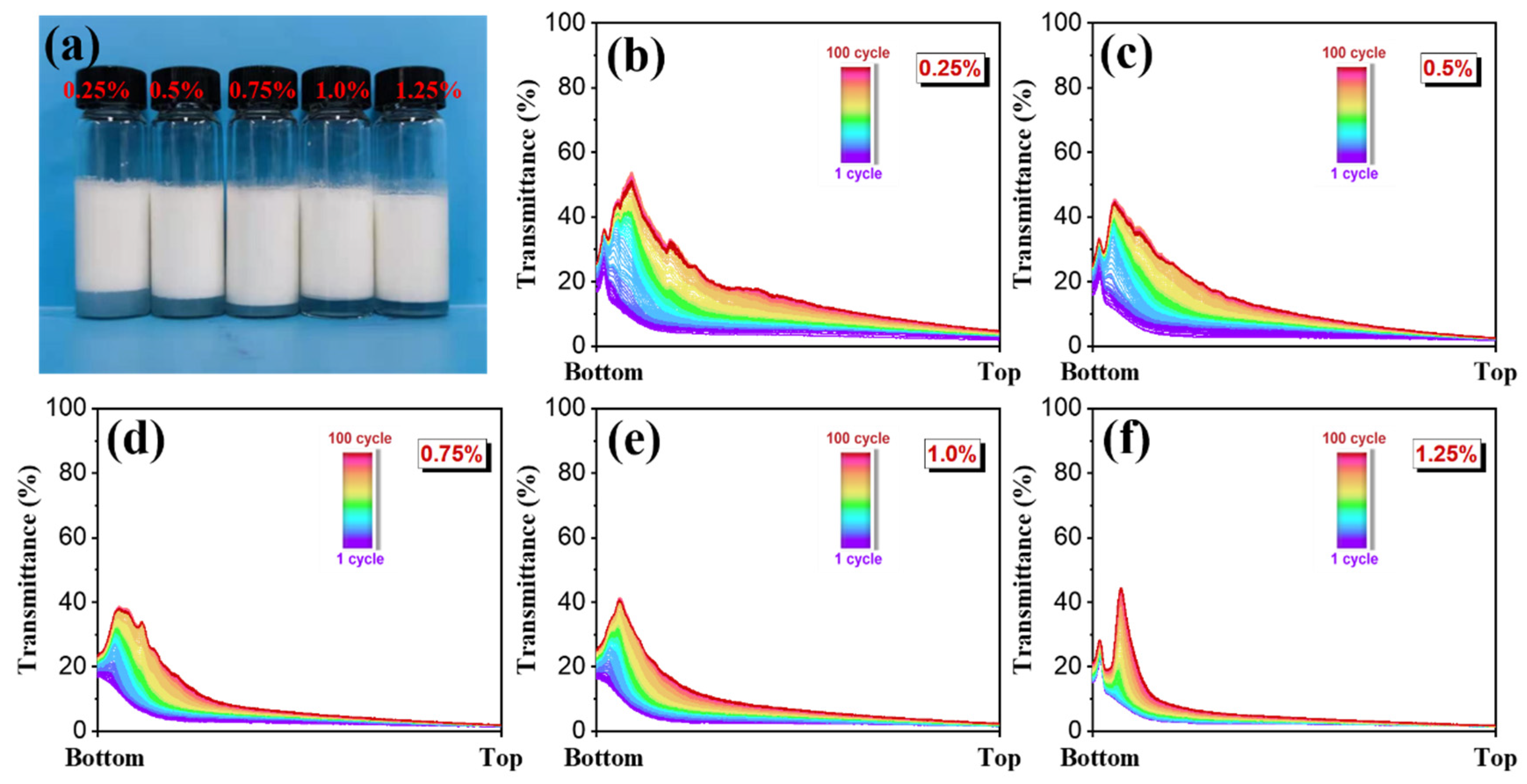
3.2.2. Effect of Guar Gel Concentration on Emulsion Stability and Droplet Diameter
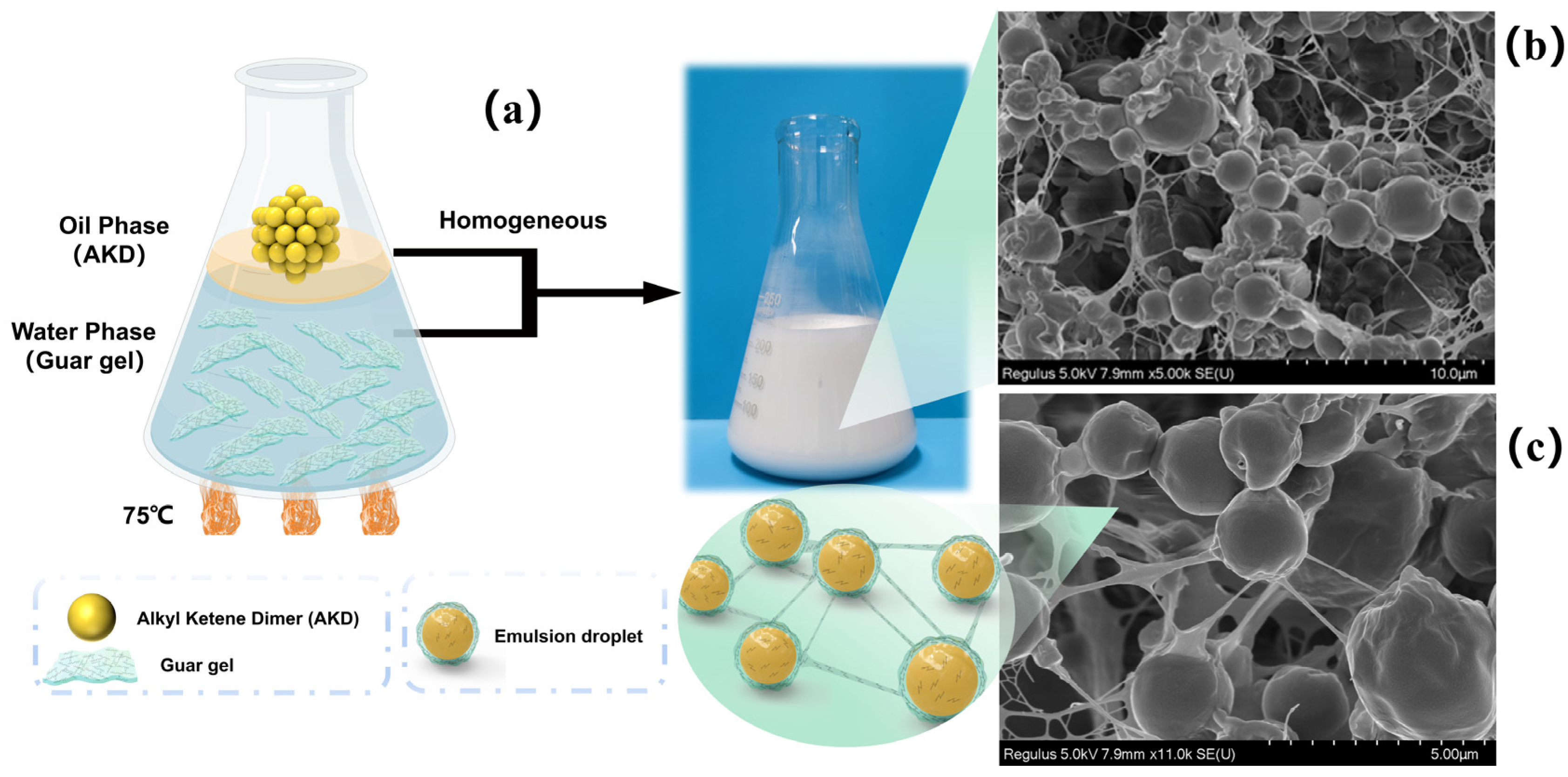
3.3. Structure of the AKD Emulsions
3.4. The Hydrophobicity of Paper Sized by AKD Emulsions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.; Chauhan, V.S.; Chakrabarti, S.K. Separation and analysis techniques for bound and unbound alkyl ketene dimer (AKD) in paper: A review. Arab. J. Chem. 2016, 9, 1636–1642. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, W.X.; Li, G.D.; Lucia, L.A.; Wang, H.L.; Yu, D.H. Unique alkyl ketene dimer Pickering-based dispersions: Preparation and application to paper sizing. J. Appl. Polym. Sci. 2017, 135, 45730. [Google Scholar] [CrossRef]
- Ho, T.M.; Razzaghi, A.; Ramachandran, A.; Mikkonen, K.S. Emulsion characterization via microfluidic devices: A review on interfacial tension and stability to coalescence. Adv. Colloid. Interface Sci. 2022, 299, 102541. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Wu, F.; Shen, Y.; Gao, D.; Li, H. Application of CS-CHO-g-PMMA emulsion in paper reinforcement and protection. Nord. Pulp Pap. Res. J. 2020, 35, 641–648. [Google Scholar] [CrossRef]
- Wang, X.C.; Liu, Y.; Liu, X.H.; You, X.Y.; Zhang, H.J. Degradable gelatin-based supramolecular coating for green paper sizing. ACS Appl. Mater. Interfaces 2021, 13, 1367–1376. [Google Scholar] [CrossRef]
- Li, G.D.; Song, Z.P.; Liu, W.X.; Yu, D.H.; Wang, H.L. Alkyl ketene dimer emulsions stabilized by layered double hydroxide particles modified with glutamic acid. Ind. Eng. Chem. Res. 2017, 56, 11435–11442. [Google Scholar] [CrossRef]
- Chen, X.S.; Wang, H.L.; Liu, W.X.; Li, G.D.; Yu, D.H.; Song, Z.P. A stable AKD-in-water emulsion: Stabilized by MSG-modified laponite nanoparticle. J. Dispers. Sci. Technol. 2016, 38, 1067–1072. [Google Scholar] [CrossRef]
- Arancibia, F.; Izquierdo, E.; Pereira, M. Stabilization of the emulsion of Alkenyl Succinic Anhydride (ASA) in water using cellulose nanofibrils. Chem. Eng. Sci. 2021, 233, 116407. [Google Scholar] [CrossRef]
- Yoon, D.H.; Jang, J.W.; Seo, S.S.; Jeong, S.H.; Cheong, I.W. Preparation of cationic poly(acrylamide)/bentonite nanocomposites from in situ inverse emulsion copolymerization and their performance in papermaking. Adv. Powder Technol. 2013, 21335, 1–9. [Google Scholar] [CrossRef]
- Lindstrom, T.; Larsson, P.T. Alkyl Ketene Dimer (AKD) sizing—A review. Nord. Pulp Pap. Res. J. 2008, 23, 202–209. [Google Scholar] [CrossRef]
- Lindstrom, T.; Glad-Nordmark, G. A study of AKD-size retention, reaction and sizing efficiency. Part 3: The effects of fibre charge density and electrolyte concentration on size retention. Nord. Pulp Pap. Res. J. 2007, 22, 161–166. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.R.; Fei, G.Q.; Wang, H.H. Study on preparation and sizing properties of soap-free styrene propylene polymer/AKD composite emulsion. Chin. Pap. J. 2009, 2, 60–63. [Google Scholar]
- Casas, J.A.; Mohedano, A.F.; García-Ochoa, F. Viscosity of guar gum and xanthan/guar gum mixture solutions. J. Sci. Food Agric. 2000, 80, 1722–1727. [Google Scholar] [CrossRef]
- Finley, J.W.; Soto-Vaca, A.; Heimbach, J.; Rao, T.P.; Juneja, L.R.; Slavin, J.; Fahey, G.C. Safety assessment and caloric value of partially hydrolyzed guar gum. J. Agric. Food Chem. 2013, 61, 1756–1771. [Google Scholar] [CrossRef]
- Rojas, O.J.; Neuman, R.D. Adsorption of polysaccharide wet-end additives in papermaking systems. Colloid. Surf. A 1999, 155, 419–432. [Google Scholar] [CrossRef]
- Li, N.; Liu, C.J.; Chen, W. Facile access to guar gum based supramolecular hydrogels with rapid self-Healing ability and multistimuli responsive gel–sol transitions. J. Agric. Food Chem. 2019, 67, 746–752. [Google Scholar] [CrossRef]
- Thombare, N.; Jha, U.; Mishra, S.; Siddiqui, M.Z. Borax cross-linked guar gum hydrogels as potential adsorbents for water purification. Carbohyd. Polym. 2017, 168, 274–281. [Google Scholar] [CrossRef]
- Deng, C.C.; Brooks, W.L.A.; Abboud, K.A.; Sumerlin, B.S. Boronic acid-based hydrogels undergo self-Healing at neutral and acidic pH. ACS Macro Lett. 2015, 4, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Grządka, E. Stability of manganese dioxide by guar gum in the absence or presence of surfactants. Cellulose 2014, 21, 1641–1654. [Google Scholar] [CrossRef]
- Sokhal, K.S.; Dasaroju, G.; Bulasara, V.K. Formation, stability and comparison of water/oil emulsion using gum arabic and guar gum and effect of aging of polymers on drag reduction percentage in water/oil flow. Vacuum 2019, 159, 247–253. [Google Scholar] [CrossRef]
- Dong, H.; Ding, Q.J.; Jiang, Y.F.; Li, X.; Han, W.J. Pickering emulsions stabilized by spherical cellulose nanocrystals. Carbohyd. Polym. 2021, 265, 118101. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Zhao, R.; Hu, F.H.; Lu, P.; Ji, D.D.; Luo, Q.; Li, G.D.; Yu, D.H.; Wang, H.L.; Song, Z.P.; et al. Laponite/lauric arginate stabilized AKD Pickering emulsions with shell-tunable hydrolytic resistance for use in sizing paper. Appl. Clay Sci. 2021, 206, 106085. [Google Scholar] [CrossRef]
- China National Standard GB/T 460-2008; Paper-Determination of the Sizing Value. China National Standard: Beijing, China, 2008.
- Dai, L.; Zhang, L.Q.; Wang, B.B.; Yang, B.; Khan, I.; Khan, A.; Ni, Y.H. Multifunctional self-assembling hydrogel from guar gum. Chem. Eng. J. 2017, 330, 1044–1051. [Google Scholar] [CrossRef]
- Qiu, L.W.; Chen, W.D.; Wang, C.; Yang, X.W. Scanning electron microscopy analysis of guar gum in the dissolution, gelation and gel-breaking process. Polym. Test. 2018, 65, 95–99. [Google Scholar] [CrossRef]
- Liu, W.; Gao, H.X.; McClements, D.J.; Zhou, L.; Wu, J.; Zou, L.Q. Stability, rheology, and β-carotene bioaccessibility of high internal phase emulsion gels. Food Hydrocolloid 2019, 88, 210–217. [Google Scholar] [CrossRef]
- Tzoumaki, M.V.; Moschakis, T.; Kiosseoglou, V.; Biliaderis, C.G. Oil-in-water emulsions stabilized by chitin nanocrystal particles. Food Hydrocolloid 2011, 25, 1521–1529. [Google Scholar] [CrossRef]
- Erçelebi, E.A.; Ibanoğlu, E. Rheological properties of whey protein isolate stabilized emulsions with pectin and guar gum. Eur. Food Res. Technol. 2009, 229, 281–286. [Google Scholar] [CrossRef]
- Cheng, C.; Wu, Z.H.; Wang, Y.; Chen, J.; Zhong, Y.J.; Liang, R.H.; Peng, S.F.; McClements, D.J.; Liu, W. Tunable high Internal phase emulsions (HIPEs) formulated using lactoferrin-gum arabic complexes. Food Hydrocolloid 2021, 113, 106445. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.G.; Dai, C.X.; Wang, Y.; Chen, W.Y.; Ju, X.R.; Yuan, J.; He, R. Physical stability and microstructure of rapeseed protein isolate/gum arabic stabilized emulsions at alkaline pH. Food Hydrocolloid 2019, 88, 50–57. [Google Scholar] [CrossRef]
- Martínez-Padilla, L.P.; Sosa-Herrera, M.G.; Osnaya-Becerril, M. Effect of the konjac glucomannan concentration on the rheological behaviour and stability of sodium caseinate oil-in-water emulsions. Int. Dairy J. 2021, 117, 104993. [Google Scholar] [CrossRef]
- Ouyang, W.; Qiu, Y.R. Rheological properties of polyvinyl butyral/Pluronic F127/PEG200 blend systems. J. Cent. S. Univ. Technol. 2011, 18, 1891–1896. [Google Scholar] [CrossRef]
- Bai, L.; Huan, S.Q.; Xiang, W.C.; Rojas, O.J. Pickering emulsions by combining cellulose nanofibrils and nanocrystals: Phase behavior and depletion stabilization. Green Chem. 2018, 20, 1571–1582. [Google Scholar] [CrossRef]
- Miao, M.; Huang, C.; Jia, X.; Cui, S.W.; Jiang, B.; Zhang, T. Physicochemical characteristics of a high molecular weight bioengineered α-D-glucan from Leuconostoc citreum SK24.002. Food Hydrocolloid 2015, 50, 37–43. [Google Scholar] [CrossRef]
- Sun, C.G.; Boluk, Y. Rheological behavior and particle suspension capability of guar gum: Sodium tetraborate decahydrate gels containing cellulose nanofibrils. Cellulose 2016, 5, 3013–3022. [Google Scholar] [CrossRef]
- Tan, H.; Liu, W.X.; Yu, D.H.; Li, H.D.; Hubbe, M.A.; Gong, B.; Zhang, W.; Wang, H.L.; Li, G.D. ASA-in-water emulsions stabilized by laponite nanoparticles modified with tetramethylammonium chloride. Chem. Eng. Sci. 2014, 116, 682–693. [Google Scholar] [CrossRef]
- Sobisch, T.; Lerche, D. Thickener performance traced by multisample analytical centrifugation. Colloid. Surf. A 2008, 331, 114–118. [Google Scholar] [CrossRef]
- Wang, P.P.; Luo, Z.G.; Chen, C.; Fu, X.; Tamer, T.M. Effects of octenyl succinic anhydride groups distribution on the storage and shear stability of Pickering emulsions formulated by modified rice starch. Carbohyd. Polym. 2020, 228, 115389. [Google Scholar] [CrossRef]
- Hagiopol, C.; Johnston, J.W. Chemistry of Modern Papermaking; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Yang, L.M.; Lu, S.; Li, J.J.; Zhang, F.S.; Cha, R.T. Nanocrystalline cellulose-dispersed AKD emulsion for enhancing the mechanical and multiple barrier properties of surface-sized paper. Carbohydr. Res. 2016, 136, 1035–1040. [Google Scholar] [CrossRef]
- Ren, Z.Y.; Chen, Z.Z.; Zhang, Y.Y.; Lin, X.R.; Li, B. Novel food-grade Pickering emulsions stabilized by tea water-insoluble protein nanoparticles from tea residues. Food Hydrocolloid 2019, 96, 322–330. [Google Scholar] [CrossRef]
- Li, X.M.; Zhu, J.; Pan, Y.; Meng, R.; Zhang, B.; Chen, H.Q. Fabrication and characterization of Pickering emulsions stabilized by octenyl succinic anhydride -modified gliadin nanoparticle. Food Hydrocolloid 2019, 90, 19–27. [Google Scholar] [CrossRef]
- Sun, B.; Hou, Q.X.; Liu, Z.H.; He, Z.B.; Ni, Y.H. Stability and efficiency improvement of ASA in internal sizing of cellulosic paper by using cationically modified cellulose nanocrystals. Cellulose 2014, 4, 2879–2887. [Google Scholar] [CrossRef]
- Shen, W.; Parker, I.H. A preliminary study of the spreading of AKD in the presence of capillary structures. J. Colloid. Interface Sci. 2001, 240, 172–181. [Google Scholar] [CrossRef]
- Bruel, C.; Queffeulou, S.; Darlow, T.; Virgilio, N.; Tavares, J.R.; Patience, G.S. Experimental methods in chemical engineering: Contact angles. Can. J. Chem. Eng. 2019, 97, 829–1032. [Google Scholar] [CrossRef]
- Chen, H.; Yang, J.; Hu, Z.J.; Zheng, B.B.; Sun, J.; Wo, Q.Z.; Zeng, X.Z.; Qiu, X.F.; Song, C.Y.; Zhu, R.F. Effects of AKD sizing on the morphology and pore distribution properties of OCC fibers. J. Nanomater. 2019, 2019, 9490602. [Google Scholar] [CrossRef]
- Holik, H. Handbook of Paper and Board; John Wiley and Sons: Hoboken, NJ, USA, 2013; p. 198. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Li, Y.; Wang, H.; Song, Z.; Tan, C.; Li, G.; Yu, D.; Liu, W. AKD Emulsions Stabilized by Guar Gel: A Highly Efficient Agent to Improve the Hydrophobicity of Cellulose Paper. Polymers 2023, 15, 4669. https://doi.org/10.3390/polym15244669
Liu X, Li Y, Wang H, Song Z, Tan C, Li G, Yu D, Liu W. AKD Emulsions Stabilized by Guar Gel: A Highly Efficient Agent to Improve the Hydrophobicity of Cellulose Paper. Polymers. 2023; 15(24):4669. https://doi.org/10.3390/polym15244669
Chicago/Turabian StyleLiu, Xiaona, Yingpu Li, Huili Wang, Zhaoping Song, Congping Tan, Guodong Li, Dehai Yu, and Wenxia Liu. 2023. "AKD Emulsions Stabilized by Guar Gel: A Highly Efficient Agent to Improve the Hydrophobicity of Cellulose Paper" Polymers 15, no. 24: 4669. https://doi.org/10.3390/polym15244669





