CuNi Alloy NPs Anchored on Electrospun PVDF-HFP NFs Catalyst for H2 Production from Sodium Borohydride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Work
2.3. Chemical Reduction in CuAc and NiAc Supported on PVDF-HFP NFs
2.4. Characterization
2.5. SHB Catalytic Hydrolysis Reaction
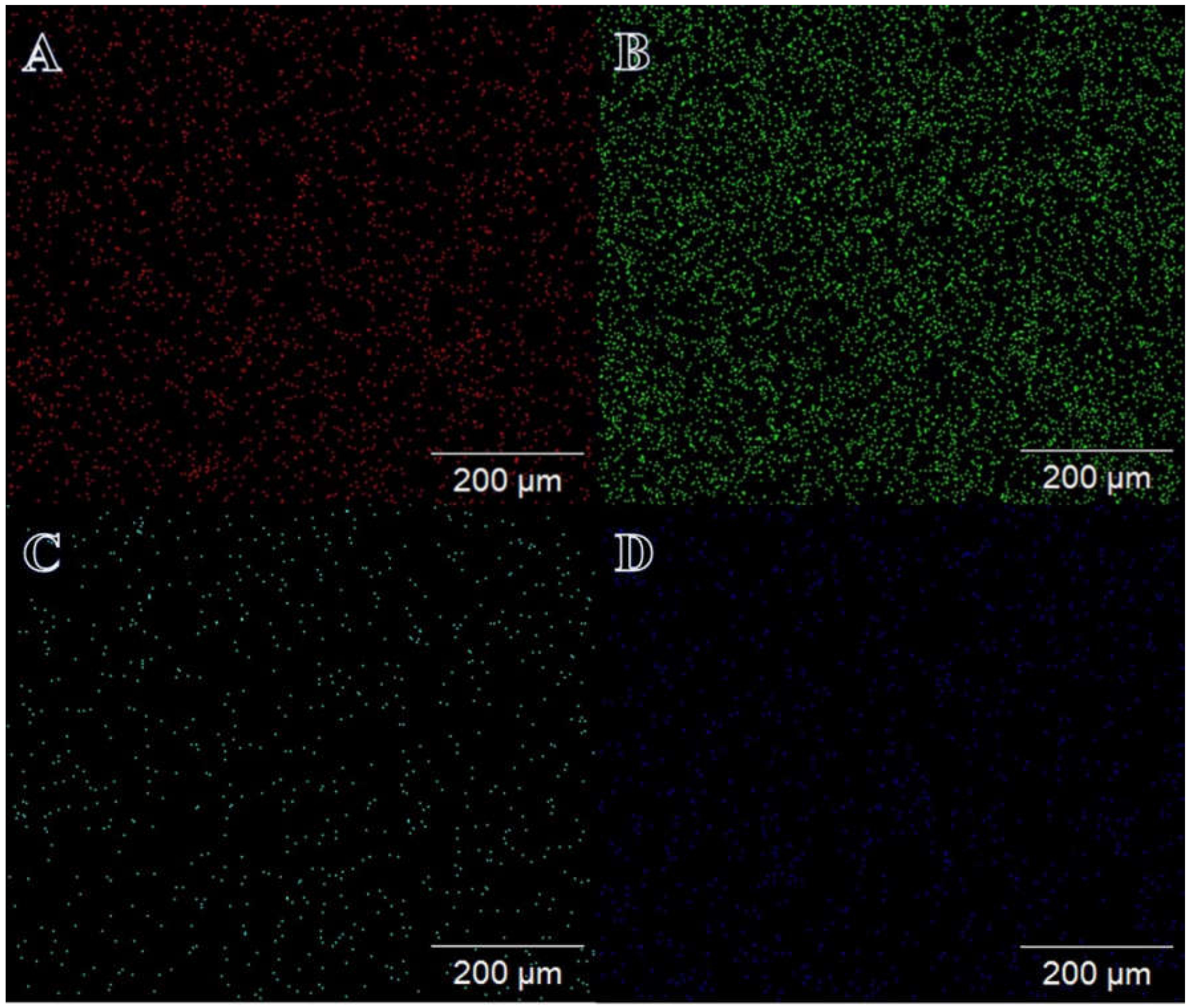
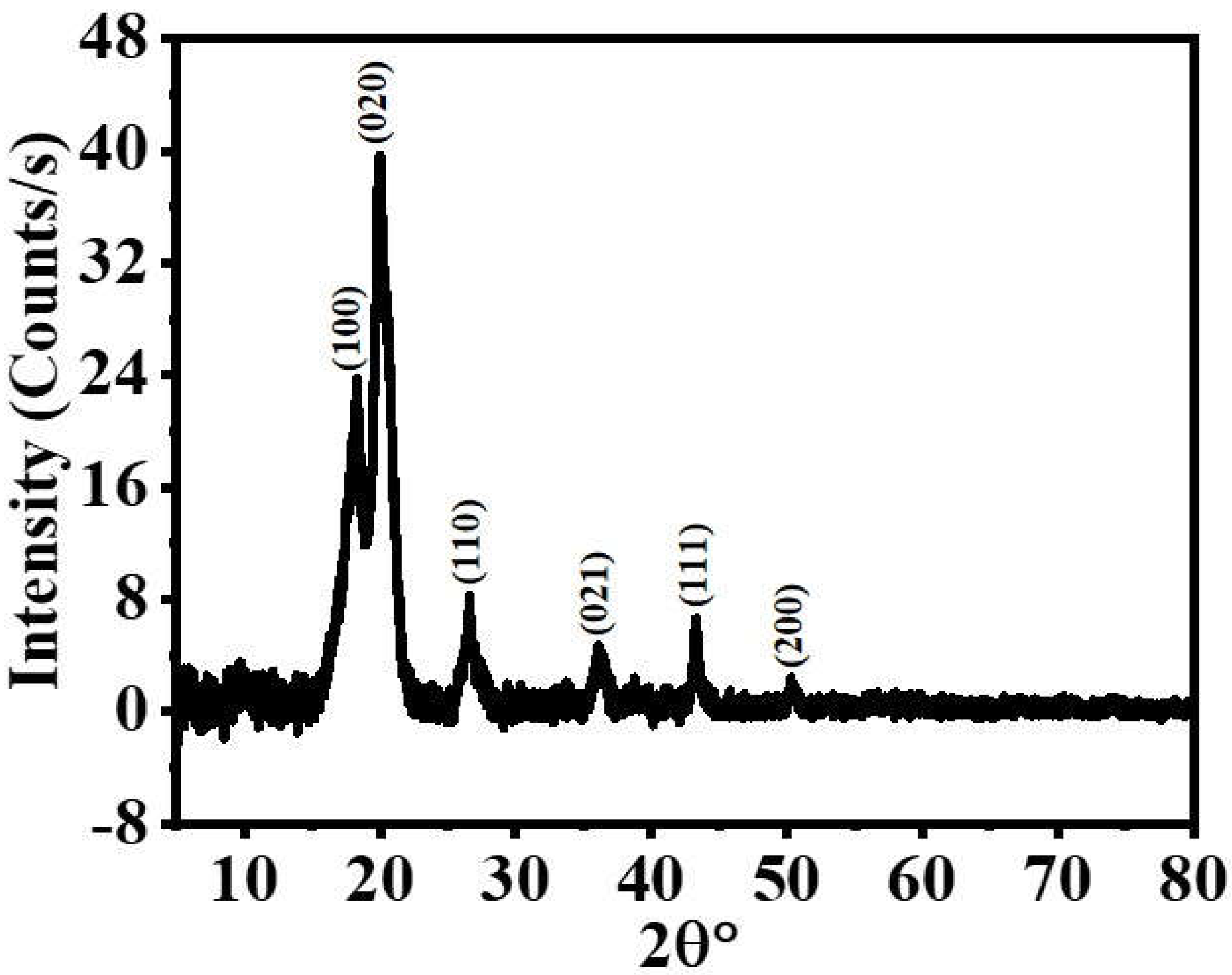
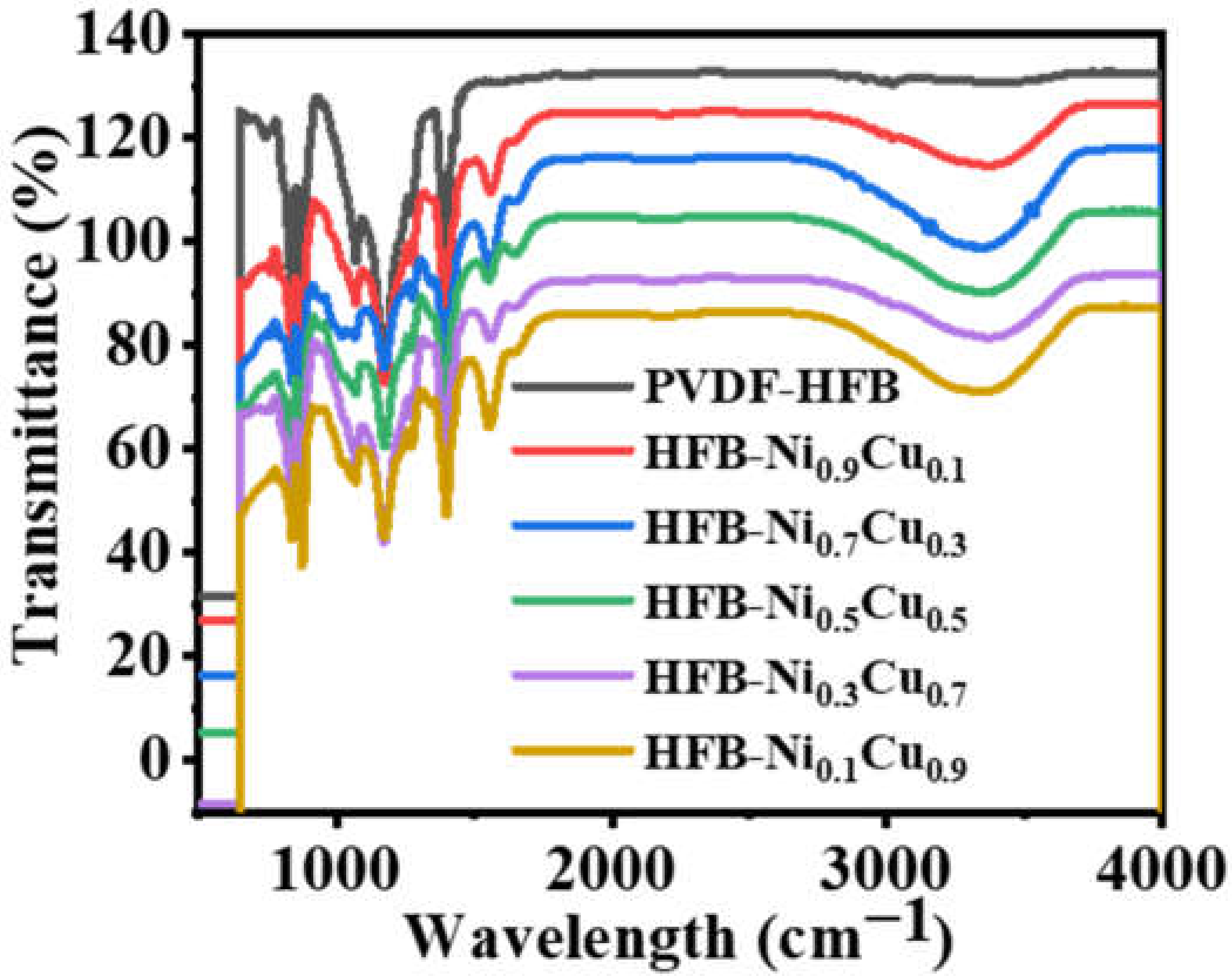
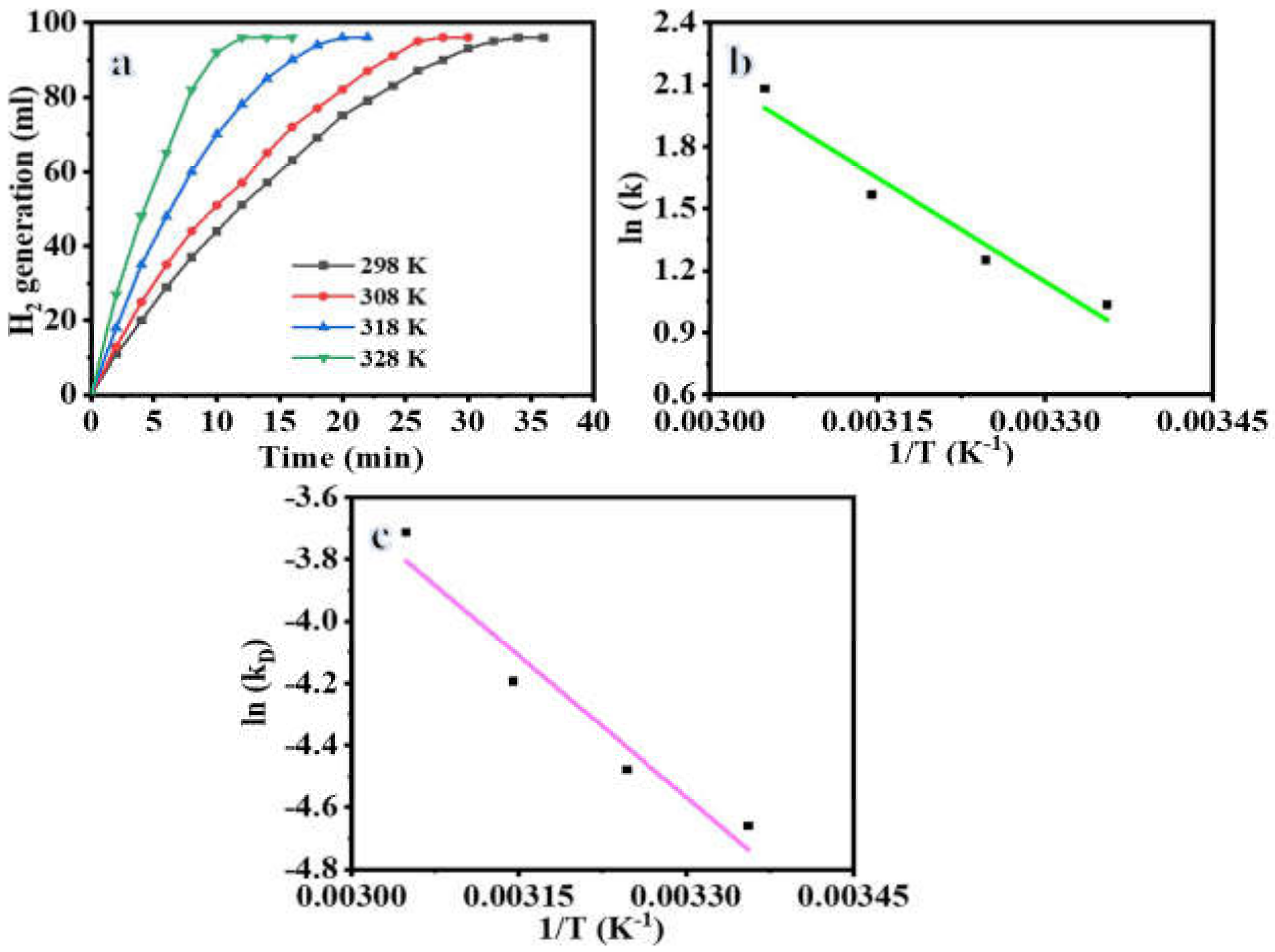
3. Results and Discussion
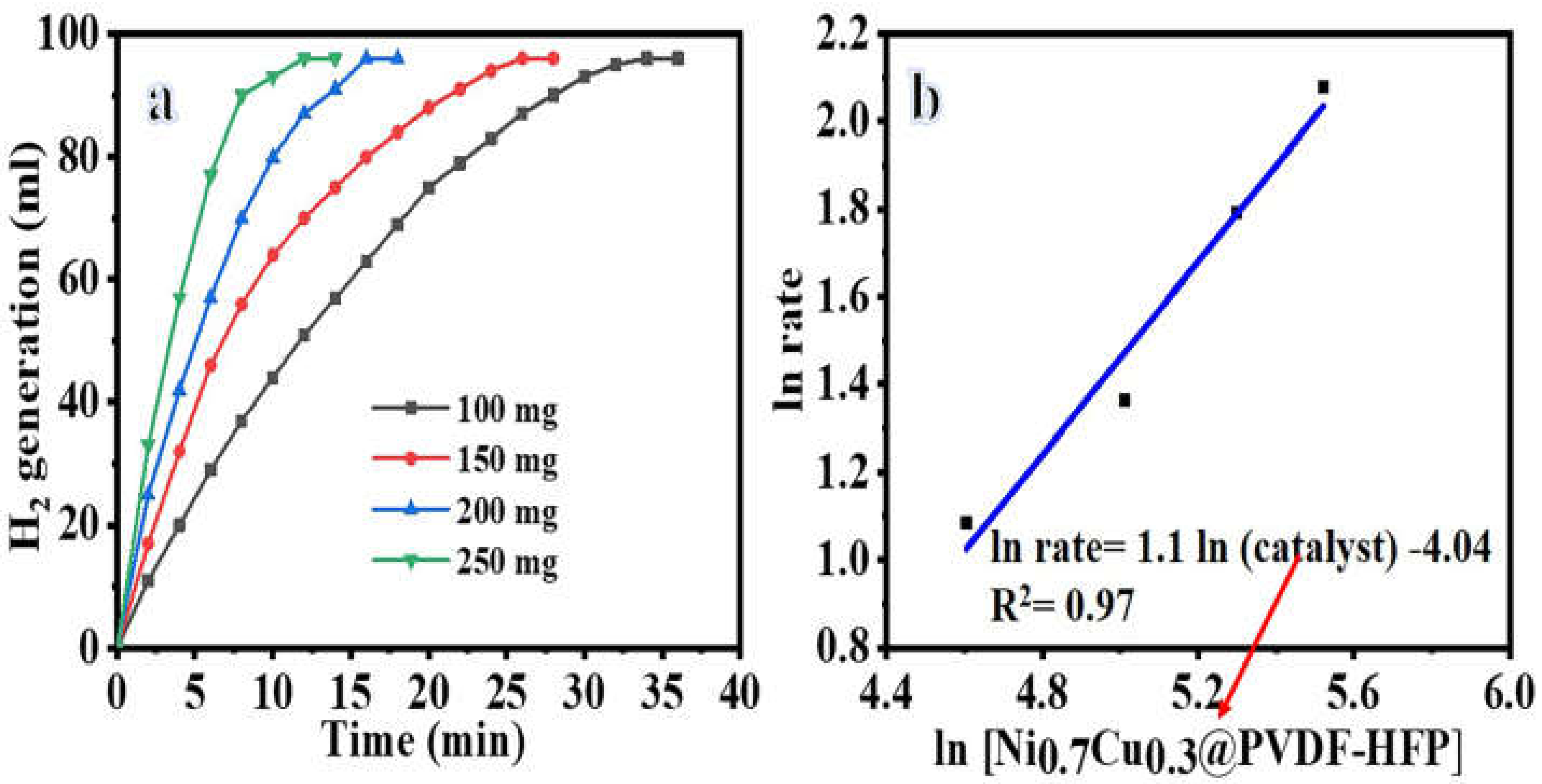
Hydrogen Release from SBH
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guimarães, L.N.D.M.R. Future Energy and the Use of Renewables. In Affordable and Clean Energy; Springer: Cham, Switzerland, 2021; Volume 6330, pp. 678–688. [Google Scholar]
- Kim, G.J.; Hwang, H.T. Thermal hydrolysis of solid-state sodium borohydride for noncatalytic hydrogen generation. Chem. Eng. J. 2021, 424, 130445. [Google Scholar] [CrossRef]
- Demirci, S.; Suner, S.S.; Yildiz, M.; Sahiner, N. Polymeric ionic liquid forms of PEI microgels as catalysts for hydrogen production via sodium borohydride methanolysis. J. Mol. Liq. 2022, 360, 119562. [Google Scholar] [CrossRef]
- Dutta, S. A review on production, storage of hydrogen and its utilization as an energy resource. J. Ind. Eng. Chem. 2014, 20, 1148–1156. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, C.-G.; Zhou, H.; Ye, E.; Xu, J.; Li, Z.; Loh, X.J. Current research trends and perspectives on solid-state nanomaterials in hydrogen storage. Research 2021, 2021, 3750689. [Google Scholar] [CrossRef] [PubMed]
- Van Hoecke, L.; Laffineur, L.; Campe, R.; Perreault, P.; Verbruggen, S.W.; Lenaerts, S. Challenges in the use of hydrogen for maritime applications. Energy Environ. Sci. 2021, 14, 815–843. [Google Scholar] [CrossRef]
- Yousef, A.; Barakat, N.A.; El-Newehy, M.H.; Ahmed, M.; Kim, H.Y. Catalytic hydrolysis of ammonia borane for hydrogen generation using Cu (0) nanoparticles supported on TiO2 nanofibers. Colloids Surf. A Physicochem. Eng. Asp. 2015, 470, 194–201. [Google Scholar] [CrossRef]
- Yousef, A.; Barakat, N.A.; Khalil, K.A.; Unnithan, A.R.; Panthi, G.; Pant, B.; Kim, H.Y. Photocatalytic release of hydrogen from ammonia borane-complex using Ni (0)-doped TiO2/C electrospun nanofibers. Colloids Surf. A Physicochem. Eng. Asp. 2012, 410, 59–65. [Google Scholar] [CrossRef]
- Yousef, A.; Barakat, N.A.; Kim, H.Y. Electrospun Cu-doped titania nanofibers for photocatalytic hydrolysis of ammonia borane. Appl. Catal. A Gen. 2013, 467, 98–106. [Google Scholar] [CrossRef]
- Bell, C. Hydrogen Fuel Cell Cold Operation. Master’s Thesis, University of Minnesota, Minneapolis, MN, USA, 2019. [Google Scholar]
- Bogdanović, B.; Felderhoff, M.; Streukens, G. Hydrogen storage in complex metal hydrides. J. Serb. Chem. Soc. 2009, 74, 183–196. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Yao, Q.; Ding, Y.; Lu, Z.-H. Noble-metal-free nanocatalysts for hydrogen generation from boron-and nitrogen-based hydrides. Inorg. Chem. Front. 2020, 7, 3837–3874. [Google Scholar] [CrossRef]
- Patel, N.; Miotello, A. Progress in Co–B related catalyst for hydrogen production by hydrolysis of boron-hydrides: A review and the perspectives to substitute noble metals. Int. J. Hydrogen Energy 2015, 40, 1429–1464. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Nafady, A.; El-Halwany, M.; Brooks, R.M.; Abutaleb, A.; Yousef, A. Electrospun carbon nanofiber-encapsulated NiS nanoparticles as an efficient catalyst for hydrogen production from hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2019, 44, 21716–21725. [Google Scholar] [CrossRef]
- Ding, X.-L.; Yuan, X.; Jia, C.; Ma, Z.-F. Hydrogen generation from catalytic hydrolysis of sodium borohydride solution using cobalt–copper–boride (Co–Cu–B) catalysts. Int. J. Hydrogen Energy 2010, 35, 11077–11084. [Google Scholar] [CrossRef]
- Deonikar, V.G.; Rathod, P.V.; Pornea, A.M.; Puguan, J.M.C.; Park, K.; Kim, H. Hydrogen generation from catalytic hydrolysis of sodium borohydride by a Cu and Mo promoted Co catalyst. J. Ind. Eng. Chem. 2020, 86, 167–177. [Google Scholar] [CrossRef]
- Huang, C.-C.; Lo, S.-L.; Lien, H.-L. Zero-valent copper nanoparticles for effective dechlorination of dichloromethane using sodium borohydride as a reductant. Chem. Eng. J. 2012, 203, 95–100. [Google Scholar] [CrossRef]
- Didehban, A.; Zabihi, M.; Shahrouzi, J.R. Experimental studies on the catalytic behavior of alloy and core-shell supported Co-Ni bimetallic nano-catalysts for hydrogen generation by hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2018, 43, 20645–20660. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Brooks, R.M.; Abutaleb, A.; El-Halwany, M.; El-Newehy, M.H.; Yousef, A. Electrospun carbon nanofibers containing Co-TiC nanoparticles-like superficial protrusions as a catalyst for H2 gas production from ammonia borane complex. Ceram. Int. 2017, 43, 15735–15742. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Brooks, R.M.; Ahmad, M.; El-Halwany, M.; El-Newehy, M.H.; Yousef, A. In-situ synthesis of amorphous co nanoparticles supported onto TiO2 nanofibers as a catalyst for hydrogen generation from the hydrolysis of ammonia borane. J. Nanosci. Nanotechnol. 2018, 18, 4714–4719. [Google Scholar] [CrossRef]
- Brooks, R.M.; Maafa, I.M.; Al-Enizi, A.M.; El-Halwany, M.M.; Ubaidullah, M.; Yousef, A. Electrospun bimetallic nicr nanoparticles@ carbon nanofibers as an efficient catalyst for hydrogen generation from ammonia borane. Nanomaterials 2019, 9, 1082. [Google Scholar] [CrossRef]
- Saka, C.; Eygi, M.S.; Balbay, A. CoB doped acid modified zeolite catalyst for enhanced hydrogen release from sodium borohydride hydrolysis. Int. J. Hydrogen Energy 2020, 45, 15086–15099. [Google Scholar] [CrossRef]
- Akti, F. Hydrogen generation from hydrolysis of sodium borohydride by silica xerogel supported cobalt catalysts: Positive roles of amine modification and calcination treatment. Fuel 2021, 303, 121326. [Google Scholar] [CrossRef]
- Diban, N.; Pacuła, A.; Kumakiri, I.; Barquín, C.; Rivero, M.J.; Urtiaga, A.; Ortiz, I. TiO2–Zeolite Metal Composites for Photocatalytic Degradation of Organic Pollutants in Water. Catalysts 2021, 11, 1367. [Google Scholar] [CrossRef]
- Daneshyar, A.; Ghaedi, M.; Sabzehmeidani, M.M. H2S adsorption onto Cu-Zn–Ni nanoparticles loaded activated carbon and Ni-Co nanoparticles loaded γ-Al2O3: Optimization and adsorption isotherms. J. Colloid Interface Sci. 2017, 490, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Yousef, A.; Barakat, N.A.; El-Newehy, M.; Kim, H.Y. Chemically stable electrospun NiCu nanorods@ carbon nanofibers for highly efficient dehydrogenation of ammonia borane. Int. J. Hydrogen Energy 2012, 37, 17715–17723. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Zhang, H.; Zhu, Y.; Mi, G. Porously hierarchical Cu@ Ni cubic-cage microstructure: Very active and durable catalyst for hydrolytically liberating H2 gas from ammonia borane. Renew. Energy 2016, 99, 1038–1045. [Google Scholar] [CrossRef]
- Patel, N.; Patton, B.; Zanchetta, C.; Fernandes, R.; Guella, G.; Kale, A.; Miotello, A. Pd-C powder and thin film catalysts for hydrogen production by hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2008, 33, 287–292. [Google Scholar] [CrossRef]
- Panthi, G.; Barakat, N.A.; Khalil, K.A.; Yousef, A.; Jeon, K.-S.; Kim, H.Y. Encapsulation of CoS nanoparticles in PAN electrospun nanofibers: Effective and reusable catalyst for ammonia borane hydrolysis and dyes photodegradation. Ceram. Int. 2013, 39, 1469–1476. [Google Scholar] [CrossRef]
- Panthi, G.; Barakat, N.A.; Risal, P.; Yousef, A.; Pant, B.; Unnithan, A.R.; Kim, H.Y. Preparation and characterization of nylon-6/gelatin composite nanofibers via electrospinning for biomedical applications. Fibers Polym. 2013, 14, 718–723. [Google Scholar] [CrossRef]
- Yousef, A.; Barakat, N.A.; Amna, T.; Unnithan, A.R.; Al-Deyab, S.S.; Kim, H.Y. Influence of CdO-doping on the photoluminescence properties of ZnO nanofibers: Effective visible light photocatalyst for waste water treatment. J. Lumin. 2012, 132, 1668–1677. [Google Scholar] [CrossRef]
- Afeesh, R.; Barakat, N.A.; Al-Deyab, S.S.; Yousef, A.; Kim, H.Y. Nematic shaped cadmium sulfide doped electrospun nanofiber mat: Highly efficient, reusable, solar light photocatalyst. Colloids Surf. A Physicochem. Eng. Asp. 2012, 409, 21–29. [Google Scholar] [CrossRef]
- Remanan, S.; Padmavathy, N.; Rabiya, R.; Ghosh, S.; Das, T.K.; Bose, S.; Sen, R.; Das, N.C. Converting polymer trash into treasure: An approach to prepare MoS2 nanosheets decorated PVDF sponge for oil/water separation and antibacterial applications. Ind. Eng. Chem. Res. 2020, 59, 20141–20154. [Google Scholar] [CrossRef]
- Das, T.K.; Remanan, S.; Ghosh, S.; Das, N.C. An environment friendly free-standing cellulose membrane derived for catalytic reduction of 4-nitrophenol: A sustainable approach. J. Environ. Chem. Eng. 2021, 9, 104596. [Google Scholar] [CrossRef]
- Chen, C.; Tang, Y.; Vlahovic, B.; Yan, F. Electrospun polymer nanofibers decorated with noble metal nanoparticles for chemical sensing. Nanoscale Res. Lett. 2017, 12, 451. [Google Scholar] [CrossRef] [Green Version]
- Jin, W.J.; Lee, H.K.; Jeong, E.H.; Park, W.H.; Youk, J.H. Preparation of polymer nanofibers containing silver nanoparticles by using poly (N-vinylpyrrolidone). Macromol. Rapid Commun. 2005, 26, 1903–1907. [Google Scholar] [CrossRef]
- Mallick, K.; Witcomb, M.J.; Dinsmore, A.; Scurrell, M.S. Fabrication of a metal nanoparticles and polymer nanofibers composite material by an in situ chemical synthetic route. Langmuir 2005, 21, 7964–7967. [Google Scholar] [CrossRef]
- Yousef, A.; Brooks, R.M.; El-Halwany, M.; Obaid, M.; El-Newehy, M.H.; Al-Deyab, S.S.; Barakat, N.A. A novel and chemical stable Co–B nanoflakes-like structure supported over titanium dioxide nanofibers used as catalyst for hydrogen generation from ammonia borane complex. Int. J. Hydrogen Energy 2016, 41, 285–293. [Google Scholar] [CrossRef]
- Chinnappan, A.; Kang, H.-C.; Kim, H. Preparation of PVDF nanofiber composites for hydrogen generation from sodium borohydride. Energy 2011, 36, 755–759. [Google Scholar] [CrossRef]
- Chinnappan, A.; Kim, H. Nanocatalyst: Electrospun nanofibers of PVDF–Dicationic tetrachloronickelate (II) anion and their effect on hydrogen generation from the hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2012, 37, 18851–18859. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Wang, Y.; Kim, H. Preparation of porous PVDF-NiB capsules as catalytic adsorbents for hydrogen generation from sodium borohydride. Fuel Process. Technol. 2011, 92, 1368–1373. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Y.; Lee, D.J.; Li, F.; Kim, H. Preparation of Y-zeolite/CoCl2 doped PVDF composite nanofiber and its application in hydrogen production. Energy 2012, 38, 144–150. [Google Scholar] [CrossRef]
- Kang, H.-C.; Chen, Y.; Arthur, E.E.; Kim, H. Microstructural control of catalyst-loaded PVDF microcapsule membrane for hydrogen generation by NaBH4 hydrolysis. Int. J. Hydrogen Energy 2014, 39, 15656–15664. [Google Scholar] [CrossRef]
- Li, F.; Arthur, E.E.; La, D.; Li, Q.; Kim, H. Immobilization of CoCl2 (cobalt chloride) on PAN (polyacrylonitrile) composite nanofiber mesh filled with carbon nanotubes for hydrogen production from hydrolysis of NaBH4 (sodium borohydride). Energy 2014, 71, 32–39. [Google Scholar] [CrossRef]
- Abbas, M.; Hameed, R.A.; Al-Enizi, A.M.; Thamer, B.M.; Yousef, A.; El-Newehy, M.H. Decorated carbon nanofibers with mixed nickel− manganese carbides for methanol electro-oxidation in alkaline solution. Int. J. Hydrogen Energy 2021, 46, 6494–6512. [Google Scholar] [CrossRef]
- Abutaleb, A.; Zouli, N.; El-Halwany, M.; Ubaidullah, M.; Yousef, A. Graphitic nanofibers supported NiMn bimetallic nanoalloys as catalysts for H2 generation from ammonia borane. Int. J. Hydrogen Energy 2021, 46, 35248–35260. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Brooks, R.M.; El-Halwany, M.; Yousef, A.; Nafady, A.; Hameed, R.A. CoCr7C3-like nanorods embedded on carbon nanofibers as effective electrocatalyst for methanol electro-oxidation. Int. J. Hydrogen Energy 2018, 43, 9943–9953. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; El-Halwany, M.; Al-Abdrabalnabi, M.A.; Bakrey, M.; Ubaidullah, M.; Yousef, A. Novel Low Temperature Route to Produce CdS/ZnO Composite Nanofibers as Effective Photocatalysts. Catalysts 2020, 10, 417. [Google Scholar] [CrossRef] [Green Version]
- Alkaykh, S.; Mbarek, A.; Ali-Shattle, E.E. Photocatalytic degradation of methylene blue dye in aqueous solution by MnTiO3 nanoparticles under sunlight irradiation. Heliyon 2020, 6, e03663. [Google Scholar] [CrossRef]
- Amna, T.; Hassan, M.S.; Yousef, A.; Mishra, A.; Barakat, N.A.; Khil, M.-S.; Kim, H. Inactivation of foodborne pathogens by NiO/TiO2 composite nanofibers: A novel biomaterial system. Food Bioprocess Technol. 2013, 6, 988–996. [Google Scholar] [CrossRef]
- Babu, K.J.; Senthilkumar, N.; Kim, A.R. Freestanding and binder free PVdF-HFP/Ni-Co nanofiber membrane as a versatile platform for the electrocatalytic oxidation and non-enzymatic detection of urea. Sens. Actuators B Chem. 2017, 241, 541–551. [Google Scholar] [CrossRef]
- Kumar, A.; Logapperumal, S.; Sharma, R.; Das, M.K.; Kar, K.K. Li-ion transport, structural and thermal studies on lithium triflate and barium titanate incorporated poly (vinylidene fluoride-co-hexafluoropropene) based polymer electrolyte. Solid State Ion. 2016, 289, 150–158. [Google Scholar] [CrossRef]
- Elwan, H.A.; Mamlouk, M.; Scott, K. A review of proton exchange membranes based on protic ionic liquid/polymer blends for polymer electrolyte membrane fuel cells. J. Power Sources 2021, 484, 229197. [Google Scholar] [CrossRef]
- Nunes-Pereira, J.; Kundu, M.; Gören, A.; Silva, M.M.; Costa, C.M.; Liu, L.; Lanceros-Méndez, S. Optimization of filler type within poly (vinylidene fluoride-co-trifluoroethylene) composite separator membranes for improved lithium-ion battery performance. Compos. Part B Eng. 2016, 96, 94–102. [Google Scholar] [CrossRef]
- Tian, X.; Jiang, X. Poly (vinylidene fluoride-co-hexafluoropropene)(PVDF-HFP) membranes for ethyl acetate removal from water. J. Hazard. Mater. 2008, 153, 128–135. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Yousef, A.; Shaikh, S.F.; Pandit, B.; El-Halwany, M.M. Electrospun Nickel Nanoparticles@ Poly (vinylidene fluoride-hexafluoropropylene) Nanofibers as Effective and Reusable Catalyst for H2 Generation from Sodium Borohydride. Arab. J. Chem. 2022, 15, 104207. [Google Scholar] [CrossRef]
- Aydın, K.; Kulaklı, B.N.; Coşkuner Filiz, B.; Alligier, D.; Demirci, U.B.; Kantürk Figen, A. Closing the hydrogen cycle with the couple sodium borohydride-methanol, via the formation of sodium tetramethoxyborate and sodium metaborate. Int. J. Energy Res. 2020, 44, 11405–11416. [Google Scholar] [CrossRef]
- Lo, C.-t.F.; Karan, K.; Davis, B.R. Kinetic studies of reaction between sodium borohydride and methanol, water, and their mixtures. Ind. Eng. Chem. Res. 2007, 46, 5478–5484. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, G.; Zhang, L.; Wang, W.; Miao, W.; Chen, K.; Cheng, L.; Li, Y.; Han, S. Ultrafine cobalt nanoparticles supported on carbon nanospheres for hydrolysis of sodium borohydride. J. Renew. Energy 2020, 162, 345–354. [Google Scholar] [CrossRef]
- Gibson, P.; Schreuder-Gibson, H.; Rivin, D. Transport properties of porous membranes based on electrospun nanofibers. Colloids Surf. A Physicochem. Eng. Asp. 2001, 187, 469–481. [Google Scholar] [CrossRef]
- Yousef, A.; Barakat, N.A.; Al-Deyab, S.S.; Nirmala, R.; Pant, B.; Kim, H.Y. Encapsulation of CdO/ZnO NPs in PU electrospun nanofibers as novel strategy for effective immobilization of the photocatalysts. Colloids Surf. A Physicochem. Eng. Asp. 2012, 401, 8–16. [Google Scholar] [CrossRef]
- Shin, J.; Nho, Y.-C.; Hwang, I.S.; Fei, G.; Kim, A.R.; Nahm, K.S. Irradiated PVdF-HFP–tin oxide composite membranes for the applications of direct methanol fuel cells. J. Membr. Sci. 2010, 350, 92–100. [Google Scholar]
- Kumar, G.G. Irradiated PVdF-HFP-montmorillonite composite membranes for the application of direct ethanol fuel cells. J. Mater. Chem. 2011, 21, 17382–17391. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, Y.; Wang, Y. Preparation of hollow poly (vinylidene fluoride) capsules containing nickel catalyst for hydrogen storage and production. Int. J. Energy Res. 2015, 39, 634–642. [Google Scholar] [CrossRef]
- Babu, K.J.; Yoo, D.J.; Kim, A.R. Binder free and free-standing electrospun membrane architecture for sensitive and selective non-enzymatic glucose sensors. RSC Adv. 2015, 5, 41457–41467. [Google Scholar]
- Kumar, G.G.; Nahm, K.S.; Elizabeth, R.N. Electro chemical properties of porous PVdF-HFP membranes prepared with different nonsolvents. J. Membr. Sci. 2008, 325, 117–124. [Google Scholar] [CrossRef]
- Mandal, B.P.; Vasundhara, K.; Abdelhamid, E.; Lawes, G.; Salunke, H.G.; Tyagi, A.K. Improvement of magnetodielectric coupling by surface functionalization of nickel nanoparticles in Ni and polyvinylidene fluoride nanohybrids. J. Phys. Chem. C 2014, 118, 20819–20825. [Google Scholar] [CrossRef]
- Nath, A.; Kumar, A. Swift heavy ion irradiation induced enhancement in electrochemical properties of ionic liquid based PVdF-HFP-layered silicate nanocomposite electrolyte membranes. J. Membr. Sci. 2014, 453, 192–201. [Google Scholar] [CrossRef]
- Ozay, O.; Aktas, N.; Inger, E.; Sahiner, N. Hydrogel assisted nickel nanoparticle synthesis and their use in hydrogen production from sodium boron hydride. Int. J. Hydrogen Energy 2011, 36, 1998–2006. [Google Scholar] [CrossRef]
- Kaufman, C.M.; Sen, B. Hydrogen generation by hydrolysis of sodium tetrahydroborate: Effects of acids and transition metals and their salts. J. Chem. Soc. Dalton Trans. 1985, 307–313. [Google Scholar] [CrossRef]
- Al-Thabaiti, S.A.; Khan, Z.; Malik, M.A. Bimetallic Ag-Ni nanoparticles as an effective catalyst for hydrogen generation from hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2019, 44, 16452–16466. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, H.; Han, S.-C.; Kim, H.-S.; Song, M.-S.; Lee, J.-Y. Production of hydrogen from sodium borohydride in alkaline solution: Development of catalyst with high performance. Int. J. Hydrogen Energy 2004, 29, 263–267. [Google Scholar] [CrossRef]
- Ingersoll, J.C.; Mani, N.; Thenmozhiyal, J.C.; Muthaiah, A. Catalytic hydrolysis of sodium borohydride by a novel nickel–cobalt–boride catalyst. J. Power Sources 2007, 173, 450–457. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Bai, S.; Qi, K.; Cao, Z.; Zhang, K.; Wu, S.; Wang, D. Catalytic hydrolysis of sodium borohydride via nanostructured cobalt–boron catalysts. Int. J. Hydrogen Energy 2016, 41, 276–284. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Peng, X.; Jing, C.; Hu, W.; Tian, S.; Tian, J. In situ synthesis of cobalt-based tri-metallic nanosheets as highly efficient catalysts for sodium borohydride hydrolysis. Int. J. Hydrogen Energy 2016, 41, 219–226. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, K.; Zhang, D.; Cao, Z.; Zhang, K.; Xie, Y.; Li, G.; Bai, S. Cobalt–copper–boron nanoparticles as catalysts for the efficient hydrolysis of alkaline sodium borohydride solution. Int. J. Hydrogen Energy 2020, 45, 9845–9853. [Google Scholar] [CrossRef]
- Ding, J.; Li, Q.; Su, Y.; Yue, Q.; Gao, B.; Zhou, W. Preparation and catalytic activity of wheat straw cellulose based hydrogel-nanometal composites for hydrogen generation from NaBH4 hydrolysis. Int. J. Hydrogen Energy 2018, 43, 9978–9987. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Q.; Su, Y.; Yue, Q.; Gao, B. A novel Enteromorpha based hydrogel for copper and nickel nanoparticle preparation and their use in hydrogen production as catalysts. Int. J. Hydrogen Energy 2017, 42, 6746–6756. [Google Scholar] [CrossRef]
- Soltani, M.; Zabihi, M. Hydrogen generation by catalytic hydrolysis of sodium borohydride using the nano-bimetallic catalysts supported on the core-shell magnetic nanocomposite of activated carbon. Int. J. Hydrogen Energy 2020, 45, 12331–12346. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Wang, L.; Liu, T.; Zhang, T.; Wang, G.; Xie, G. Hydrogen generation from catalytic hydrolysis of sodium borohydride solution using supported amorphous alloy catalysts (Ni–Co–P/γ-Al2O3). Int. J. Hydrogen Energy 2014, 39, 14935–14941. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, Y.; Liu, X.; Zhang, Y. Preparation of polyvinylidene fluoride–nickel hollow fiber catalytic membranes for hydrogen generation from sodium borohydride. Fuel 2015, 140, 685–692. [Google Scholar] [CrossRef]
- Chinnappan, A.; Kim, H.; Baskar, C.; Hwang, I.T. Hydrogen generation from the hydrolysis of sodium borohydride with new pyridinium dicationic salts containing transition metal complexes. Int. J. Hydrogen Energy 2012, 37, 10240–10248. [Google Scholar] [CrossRef]





| Catalyst | Ea (KJ/mol) | Ref. |
|---|---|---|
| Ni | 42.28 | [70] |
| Ni | 71 | [71] |
| Raney Ni | 63 | [71] |
| Ni-Ag | 16.2 | [72] |
| Ni-Co | 38 | [73] |
| Ni-Co-B | 62 | [74] |
| Co-B/Cu | 43.3 | [75] |
| Co-Cu-Ni | 40.6 | [76] |
| Co-Cu-B/Ni foam | 52 | [77] |
| WSC-Cu | 32.7 | [78] |
| P(EP-g-AA)-Cu | 42.6 | [79] |
| Co-Ni/AC | 68.9 | [19] |
| Co-Ni/MWAC | 40.7 | [80] |
| Sm- Ni-Co-P/g-Al2O3 | 52.1 | [81] |
| Ni–Co–B | 62 | [75] |
| Ni-hollow PVDF capsules | 49.3 | [65] |
| Ni-PVDF hollow fiber | 55.3 | [82] |
| ([C6(mpy)2][NiCl4]2- | 56.4 | [83] |
| PVDF-[C6(mpy)2][NiCl4]2- | 44.6 | [41] |
| NiCu/PVDF-HFP | 27.8 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abutaleb, A. CuNi Alloy NPs Anchored on Electrospun PVDF-HFP NFs Catalyst for H2 Production from Sodium Borohydride. Polymers 2023, 15, 474. https://doi.org/10.3390/polym15030474
Abutaleb A. CuNi Alloy NPs Anchored on Electrospun PVDF-HFP NFs Catalyst for H2 Production from Sodium Borohydride. Polymers. 2023; 15(3):474. https://doi.org/10.3390/polym15030474
Chicago/Turabian StyleAbutaleb, Ahmed. 2023. "CuNi Alloy NPs Anchored on Electrospun PVDF-HFP NFs Catalyst for H2 Production from Sodium Borohydride" Polymers 15, no. 3: 474. https://doi.org/10.3390/polym15030474





