Fully Flexible Covalent Organic Frameworks for Fluorescence Sensing 2,4,6-Trinitrophenol and p-Nitrophenol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Fluorescence Spectra
2.3.1. Solid State Fluorescence
2.3.2. Fluorescence Quenching Experiments
2.3.3. Fluorescence Stability Experiments
3. Results and Discussion
3.1. Structural Characterization
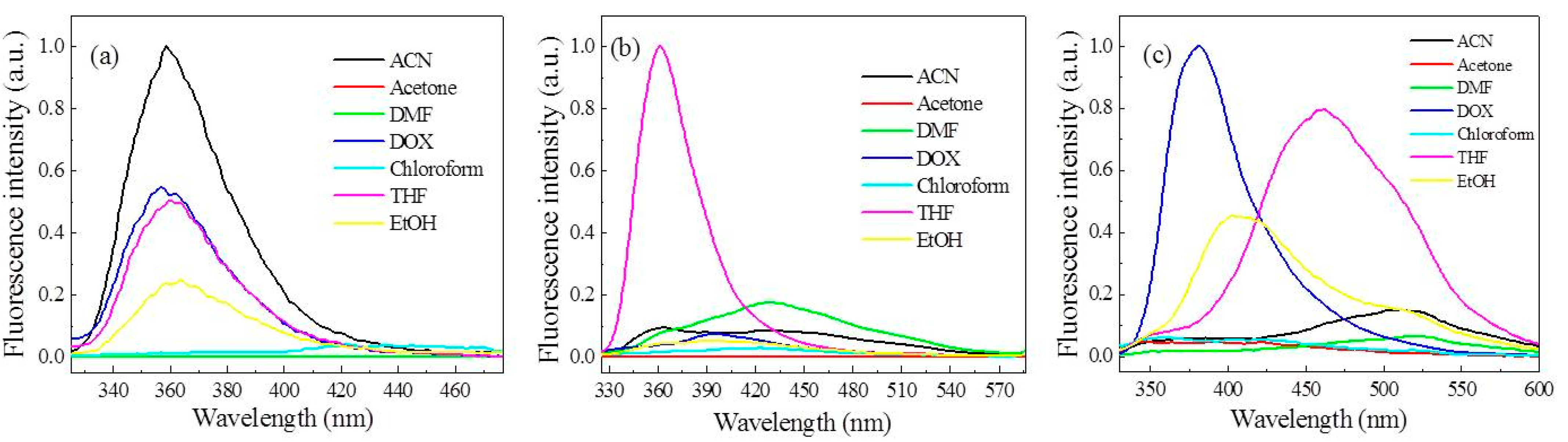
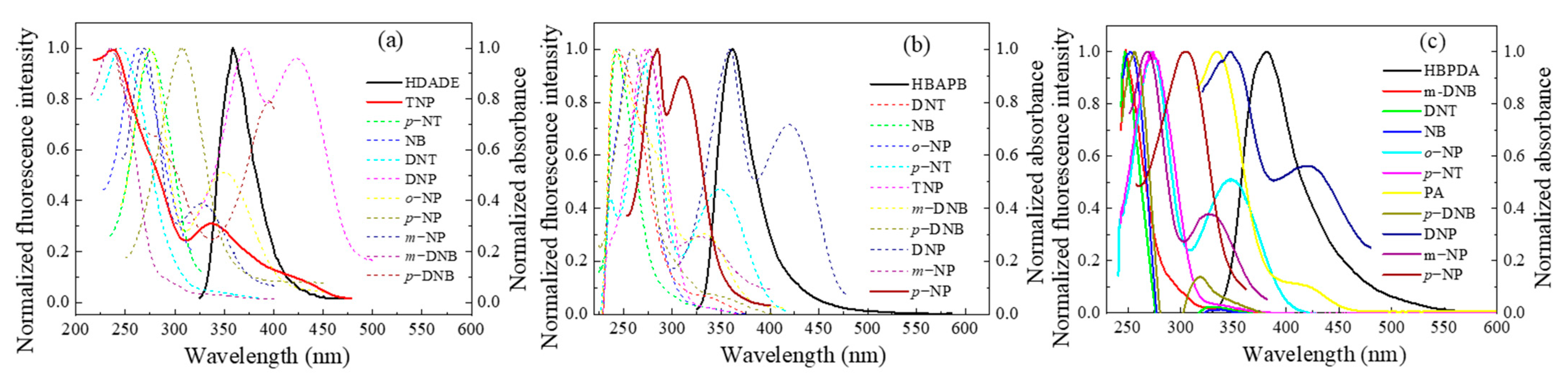
3.2. Effect of Solvent on the Fluorescence Performance of the FFCP COFs
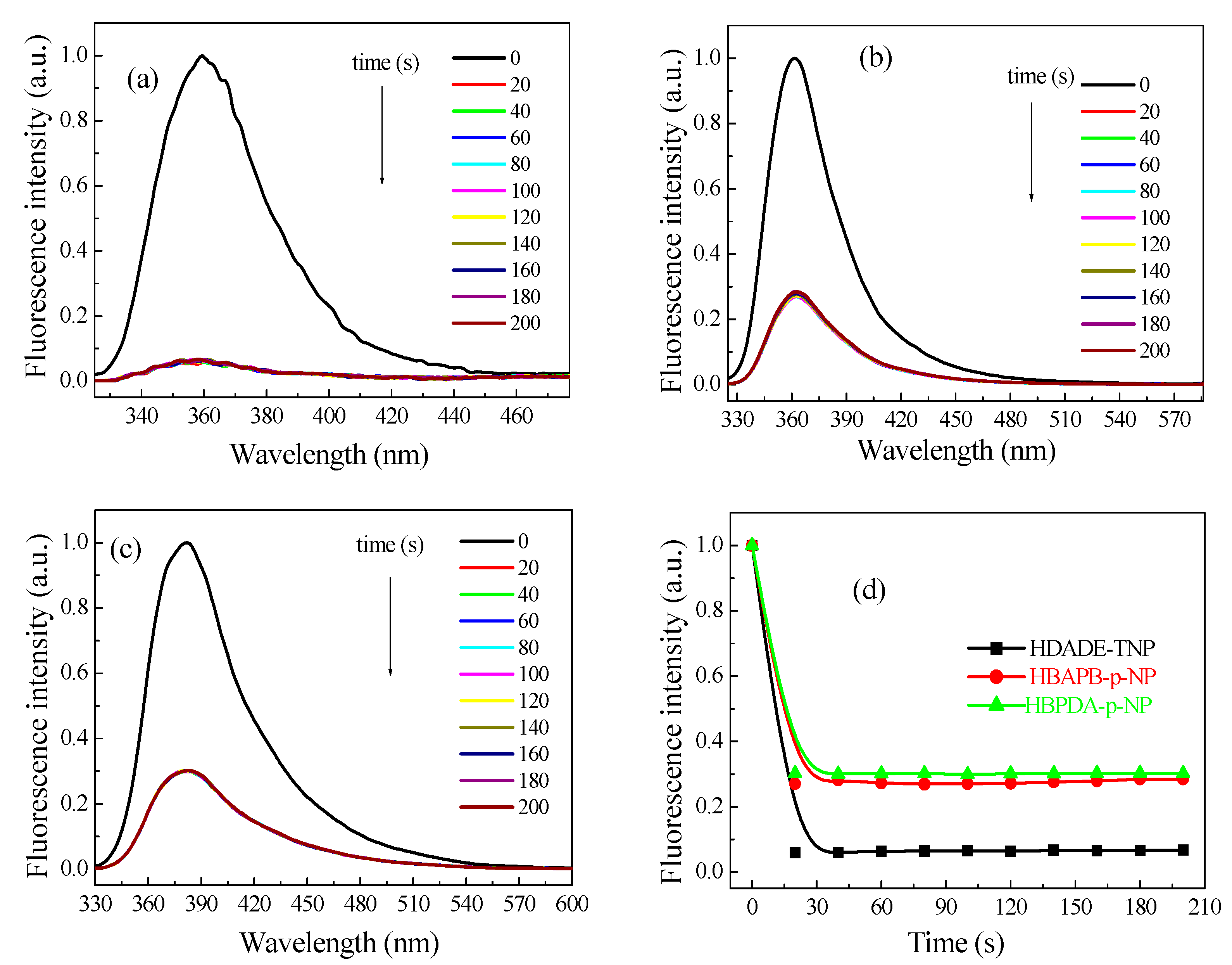
3.3. Response Time
3.4. Sensitivity of the FFCP COFs
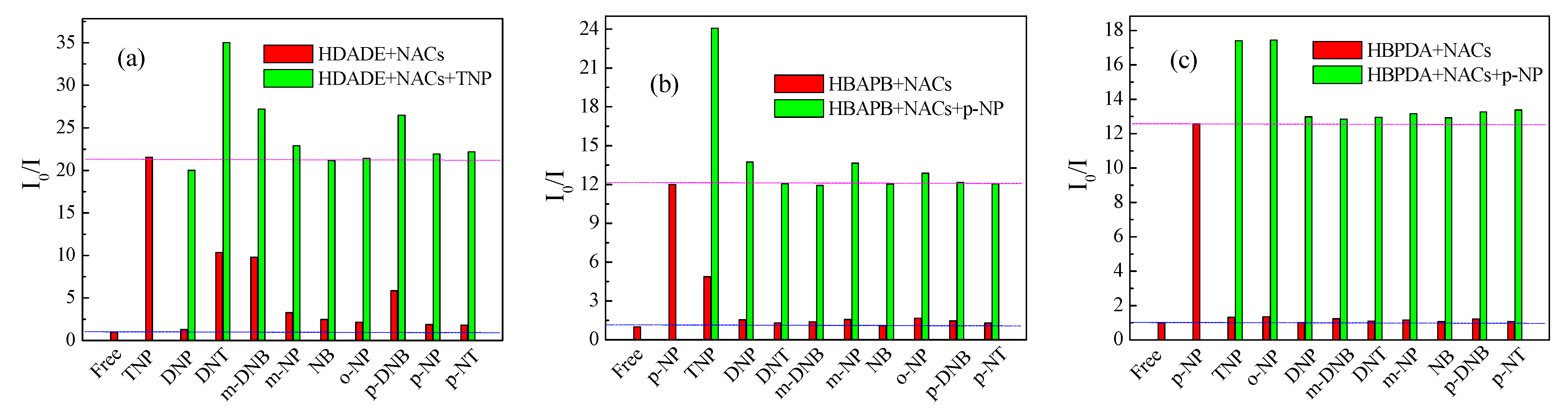
3.5. Selectivity of the FFCP COFs
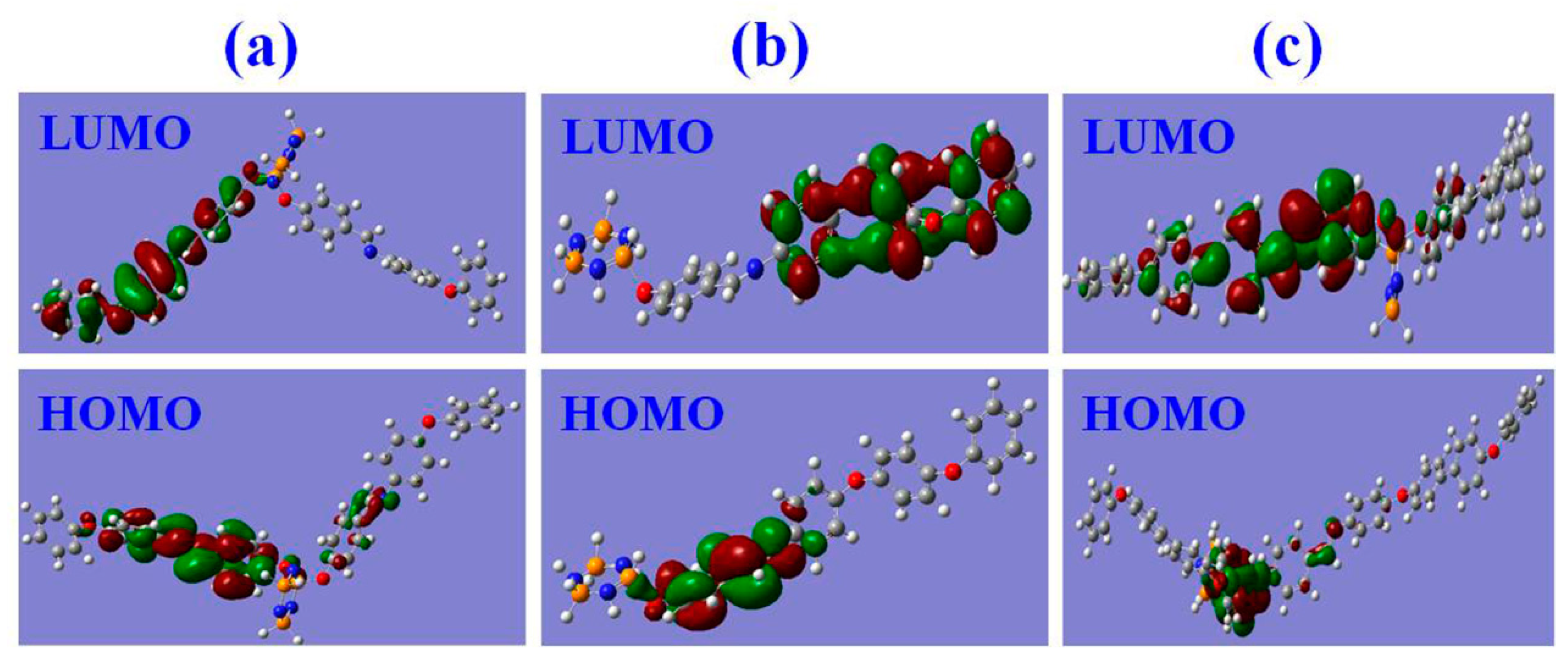
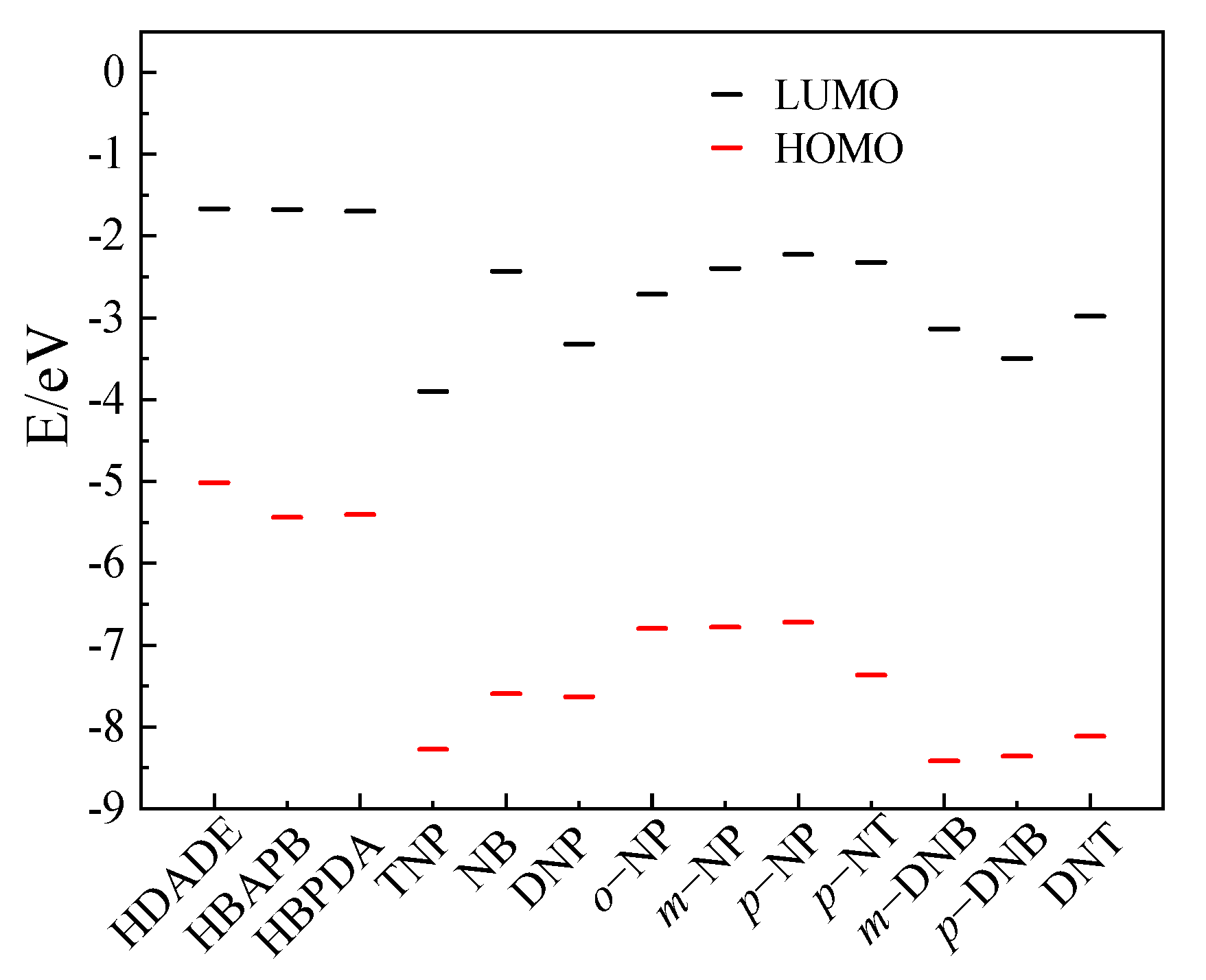
3.6. Fluorescence Sensing Mechanism of the FFCP COFs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yu, J.L.; Wang, X.Y.; Kang, Q.; Li, J.H.; Shen, D.Z.; Chen, L.X. One-pot synthesis of quantum dots based molecular imprinting nanosensor for highly selective and sensitive fluorescent detection of 4-nitrophenol in environmental water. Environ. Sci. Nano 2017, 2, 493–502. [Google Scholar] [CrossRef]
- Wei, X.; Zhou, Z.P.; Hao, T.F.; Li, H.J.; Zhu, Y.Z.; Gao, L.; Yan, Y.S. A novel molecularly imprinted polymer thin film at surface of ZnO nanorods for selective fluorescence detection of para-nitrophenol. RSC Adv. 2015, 5, 44088–44095. [Google Scholar] [CrossRef]
- Tang, J.; Tang, D.P.; Su, B.L.; Huang, J.X.; Qiu, B.; Chen, G.N. Enzyme-free electrochemical immunoassay with catalytic reduction of p-nitrophenol and recycling of p-aminophenol using gold nanoparticles-coated carbon nanotubes as nanocatalysts. Biosens. Bioelectron. 2011, 26, 3219–3226. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, J.H.; Su, D.Y.; Xia, Q.D.; Chai, F.; Wang, C.G.; Qu, F.Y. Fluorescent detection of TNT and 4-nitrophenol by BSA Au nanoclusters. Dalton Trans. 2014, 43, 10057–10063. [Google Scholar] [CrossRef] [PubMed]
- Geng, T.M.; Wang, F.Q.; Fang, X.C.; Xu, H. Dual functional N,O,P containing covalent organic frameworks for adsorping iodine and fluorescence sensing to p-nitrophenol and iodine. Micropor. Mesopor. Mater. 2021, 317, 111001. [Google Scholar] [CrossRef]
- Hao, T.F.; Wei, X.; Nie, Y.J.; Xu, Y.Q.; Yan, Y.S.; Zhou, Z.P. An eco-friendly molecularly imprinted fluorescence composite material based on carbon dots for fluorescent detection of 4-nitrophenol. Microchim. Acta 2016, 183, 2197–2203. [Google Scholar] [CrossRef]
- Salinas, Y.; Martinez-Manez, R.; Marcos, M.D.; Sancenon, F.; Costero, A.M.; Parra, M.; Gil, S. Optical chemosensors and reagents to detect explosives. Chem. Soc. Rev. 2012, 41, 1261–1296. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gao, M.J.; Deng, L.L.; Kang, X.; Zhang, K.L.; Fu, Q.F.; Xia, Z.N.; Gao, D. A sensitive and selective fluorescent sensor for 2,4,6-trinitrophenol detection based on the composite material of magnetic covalent organic frameworks, molecularly imprinted polymers and carbon dots. Microchem. J. 2020, 154, 104590. [Google Scholar] [CrossRef]
- Letzel, S.; Goen, T.; Bader, M.; Angerer, J.; Kraus, T. Exposure to nitroaromatic explosives and health effects during disposal of military waste. Occup. Environ. Med. 2003, 60, 483–488. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Wang, W.J.; Pan, S.S.; Fu, Y.M.; Dong, B.; Wang, H. Fluorescent self-propelled covalent organic framework as a microsensor for nitro explosive detection. Appl. Mater. Today 2020, 19, 100550. [Google Scholar] [CrossRef]
- Ma, J.; Bian, L.; Zhao, L.; Feng, X.T.; Zhao, L.Z.; Wang, Z.Y.; Pu, Q.S. Dialysed caramel as an effective fluorophore for the simultaneous detection of three nitrophenol. Talanta 2019, 197, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Li, G.F.; Che, W.L.; Zhu, D.X.; Su, Z.M.; Bryce, M.R. Polyurethane derivatives for highly sensitive and selective fluorescent detection of 2,4,6-Trinitrophenol (TNP). J. Mater. Chem. C 2018, 41, 11162–11169. [Google Scholar]
- Jurcic, M.; Peveler, W.J.; Savory, C.N.; Scanlon, D.O.; Kenyone, A.J.; Parkin, I.P. The vapour phase detection of explosive markers and derivatives using two fluorescent metal–organic frameworks. J. Mater. Chem. A 2015, 3, 6351–6359. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Liu, Y.C.; Yu, Y.; Du, J.F.; Cui, Y.Z.; Song, X.W.; Liang, Z.Q. Conjugated microporous polymers based on biphenylene for CO2 adsorption and luminescent detection of nitroaromatic compounds. New J. Chem. 2018, 42, 9482–9487. [Google Scholar] [CrossRef]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; Keeffe, M.O.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.; Dalgarno, S.J.; Xu, Z.; Vilela, F. Conjugated porous polymers: Incredibly versatile materials with far-reaching applications. Chem. Soc. Rev. 2020, 12, 3981–4042. [Google Scholar] [CrossRef]
- Ding, S.Y.; Dong, M.; Wang, Y.W.; Chen, Y.T.; Wang, H.Z.; Su, C.Y.; Wang, W. Thioether-based fluorescent covalent organic framework for selective detection and facile removal of mercury (II). J. Am. Chem. Soc. 2016, 138, 3031–3037. [Google Scholar] [CrossRef]
- Li, Z.P.; Zhang, Y.W.; Xia, H.; Mu, Y.; Liu, X.M. A robust and luminescent covalent organic framework as a highly sensitive and selective sensor for the detection of Cu2+ ions. Chem. Commun. 2016, 52, 6613–6616. [Google Scholar] [CrossRef]
- Ascherl, L.; Evans, E.W.; Gorman, J.; Orsborne, S.; Bessinger, D.; Bein, T.; Friend, R.H.; Auras, F. Perylene-based covalent organic frameworks for acid vapor sensing. J. Am. Chem. Soc. 2019, 141, 15693–15699. [Google Scholar] [CrossRef]
- Jiang, S.; Meng, L.C.; Ma, W.Y.; Pan, G.C.; Zhang, W.; Zou, Y.C.; Liu, L.J.; Xu, B.; Tian, W.J. Dual-functional two-dimensional covalent organic frameworks for water sensing and harvesting. Mater. Chem. Front. 2021, 11, 4193–4201. [Google Scholar] [CrossRef]
- Skorjanc, T.; Shetty, D.; Valant, M. Covalent organic polymers and frameworks for fluorescence-based sensors. ACS Sens. 2021, 6, 1461–1481. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.L.; Yang, L.; Li, M.Y.; Kuang, L.J.; Song, Y.H.; Wang, L. Covalent organic frameworks for fluorescent sensing: Recent developments and future challenges. Coordin. Chem. Rev. 2021, 440, 213957. [Google Scholar] [CrossRef]
- Hu, J.T.; Zhang, J.J.; Lin, Z.X.; Xie, L.L.; Liao, S.H.; Chen, X. Construction of a hollow spherical covalent organic framework with olefin and imine dual linkages based on orthogonal reactions. Chem. Mater. 2022, 34, 5249–5257. [Google Scholar] [CrossRef]
- Dalapati, S.; Jin, S.B.; Gao, J.; Xu, Y.H.; Nagai, A.; Jiang, D.L. An Azine-Linked Covalent Organic Framework. J. Am. Chem. Soc. 2013, 135, 17310–17313. [Google Scholar] [CrossRef]
- Li, Y.; Bi, S.; Liu, F.; Wu, S.Y.; Hu, J.; Wang, L.M.; Liu, H.L.; Hua, Y. Porosity-induced emission: Exploring color-controllable fluorescence of porous organic polymers and their chemical sensing applications. J. Mater. Chem. C 2015, 26, 6876–6881. [Google Scholar] [CrossRef]
- Das, G.; Biswal, B.P.; Kandambeth, S.; Venkatesh, V.; Kaur, G.; Addicoat, M.; Heine, T.; Verm, S.; Banerjee, R. Chemical sensing in two dimensional porous covalent organic nanosheets. Chem. Sci. 2015, 7, 3931–3939. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.Q.; Ding, H.M.; Yuan, D.Q.; Wang, B.S.; Wang, C. A pyrene-based, fluorescent three-dimensional covalent organic framework. J. Am. Chem. Soc. 2016, 10, 3302–3305. [Google Scholar] [CrossRef]
- Zhu, M.W.; Xu, S.Q.; Wang, X.Z.; Chen, Y.Q.; Dai, L.Y.; Zhao, X. The construction of fluorescent heteropore covalent organic frameworks and their applications in spectroscopic and visual detection of trinitrophenol with high selectivity and sensitivity. Chem. Commun. 2018, 18, 2308–2311. [Google Scholar] [CrossRef]
- Jiang, S.; Meng, L.C.; Ma, W.Y.; Qi, Q.K.; Zhang, W.; Xu, B.; Liu, L.J.; Tian, W.J. Morphology controllable conjugated network polymers based on AIE-active building block for TNP detection. Chin. Chem. Lett. 2021, 3, 1037–1040. [Google Scholar] [CrossRef]
- Li, W.T.; Hu, Z.J.; Meng, J.; Zhang, X.; Gao, W.; Chen, M.L.; Wang, J.H. Zn-based metal organic framework-covalent organic framework composites for trace lead extraction and fluorescence detection of TNP. J. Hazard. Mater. 2021, 411, 125021. [Google Scholar] [CrossRef]
- Zhang, M.C.; Li, Y.; Yuan, W.L.; Guo, X.H.; Bai, C.Y.; Zuo, Y.D.; Long, H.H.; Qi, Y.; Li, S.J.; Tao, G.H.; et al. Construction of flexible amine-linked covalent organic frameworks by catalysis and reduction of formic acid via the Eschweiler–Clarke reaction. Angew. Chem. Int. Ed. 2021, 22, 12396–12405. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.H.; Tian, Y.; Zhang, M.C.; Li, Y.; Wen, R.; Li, X.; Li, X.F.; Xue, Y.; Ma, L.J.; Xia, C.Q.; et al. Mechanistic insight into hydrogen-bond-controlled crystallinity and adsorption property of covalent organic frameworks from flexible building blocks. Chem. Mater. 2018, 7, 2299–2308. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, J.; Zhang, S.; Zhou, X.; He, L.; Sheridan, M.V.; Yuan, M.; Zhang, M.; Chen, L.; Dai, X.; et al. Electron beam irradiation as a general approach for the rapid synthesis of covalent organic frameworks under ambient conditions. J. Am. Chem. Soc. 2020, 20, 9169–9174. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Chakraborty, G.; Mandal, S.K. Comprehensive structural and microscopic characterization of an azine–triazine-functionalized highly crystalline covalent organic framework and its selective detection of dichloran and 4-nitroaniline. ACS Appl. Mater. Inter. 2020, 9, 10224–10232. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Cui, D.; Zhang, S.R.; Zhang, S.R.; Xu, Y.H.; Jiang, D.L. Iodine capture in porous organic polymers and metal–organic frameworks materials. Mater. Horiz. 2019, 8, 1571–1595. [Google Scholar] [CrossRef]
- Guo, X.H.; Li, Y.; Zhang, M.C.; Cao, K.C.; Tian, Y.; Qi, Y.; Li, S.J.; Li, K.; Yu, X.Q.; Ma, L.J. Colyliform crystalline 2D covalent organic frameworks (COFs) with quasi-3D topologies for rapid I2 adsorption. Angew. Chem. 2020, 50, 22886–22894. [Google Scholar] [CrossRef]
- Yin, Z.J.; Xu, S.Q.; Zhan, T.G.; Qi, Q.Y.; Wu, Z.Q.; Zhao, X. Ultrahigh volatile iodine uptake by hollow microspheres formed from a heteropore covalent organic framework. Chem. Commun. 2017, 53, 7266–7269. [Google Scholar] [CrossRef]
- Das, P.; Mandal, S.K. Flexible and semi-flexible amide-hydrazide decorated fluorescent covalent organic frameworks as On-Off pH responsive proton scavengers. ACS Appl. Mater. Inter. 2021, 12, 14160–14168. [Google Scholar] [CrossRef]
- Zhu, H.; Geng, T.M.; Tang, K.B. The cyclotriphosphazene-based covalent organic frameworks constructed entirely by flexible building blocks for adsorping and fluorescence sensing iodine. Micropor. Mesopor. Mat. 2022, 343, 112165. [Google Scholar] [CrossRef]
- Das, G.; Prakasam, T.; Nuryyeva, S.; Han, D.S.; Abdel-Wahab, A.; Olsen, J.-C.; Polychronopoulou, K.; Platas-Iglesias, C.; Ravaux, F.; Jouiad, M.; et al. Multifunctional redox-tuned viologen-based covalent organic polymers. J. Mater. Chem. A 2016, 4, 15361–15369. [Google Scholar] [CrossRef]
- Wang, F.Q.; Geng, T.M.; Fang, X.C.; Xu, H. The effects of the linker length on iodine adsorption and fluorescence sensing property of the N,O,P containing covalent organic frameworks. J. Appl. Polym. Sci. 2022, 139, 52889. [Google Scholar] [CrossRef]
- Li, Y.; Chen, W.B.; Hao, W.J.; Li, Y.S.; Chen, L. Covalent organic frameworks constructed from flexible building blocks with high adsorption capacity for pollutants. ACS Appl. Nano Mater. 2018, 9, 4756–4761. [Google Scholar] [CrossRef]
- Xu, L.Q.; Ding, S.Y.; Liu, J.M.; Sun, J.L.; Wang, W.; Zheng, Q.Y. Highly crystalline covalent organic frameworks from flexible building blocks. Chem. Commun. 2016, 52, 4706–4709. [Google Scholar] [CrossRef]
- Ren, S.J.; Dawson, R.; Adams, D.J.; Cooper, A.I. Low band-gap benzothiadiazole conjugated microporous polymers. Polym. Chem. 2013, 22, 5585–5590. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, D.P.; Luo, M.; Feng, L.J.; Zhao, Y.C.; Han, B.H. Nitrogen-containing microporous conjugated polymers via carbazole-based oxidative coupling polymerization: Preparation, porosity, and gas uptake. Small 2014, 2, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, D.P.; Zhu, J.H.; Han, B.H. Mesoporous conjugated polycarbazole with high porosity via structure tuning. Macromolecules 2014, 17, 5926–5931. [Google Scholar] [CrossRef]
- Xiang, Z.H.; Cao, D.P. Synthesis of luminescent covalent–organic polymers for detecting nitroaromatic explosives and small organic molecules. Macromol. Rapid Commun. 2012, 14, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Sigen, A.; Zou, Y.C.; Luo, X.L.; Li, Z.P.; Xia, H.; Liu, X.M.; Mu, Y. Gas uptake, molecular sensing and organocatalytic performances of a multifunctional carbazole-based conjugated microporous polymer. J. Mater. Chem. A 2014, 33, 13422–13430. [Google Scholar] [CrossRef]
- Zhao, W.X.; Zhuang, X.D.; Wu, D.Q.; Zhang, F.; Gehrig, D.; Laquai, F.; Feng, X.L. Boron-π-nitrogen-based conjugated porous polymers with multi-functions. J. Mater. Chem. A 2013, 44, 13878–13884. [Google Scholar] [CrossRef]
- Geng, T.M.; Zhu, Z.M.; Wang, X.; Xia, H.Y.; Wang, Y.; Li, D.K. Poly {tris [4-(2-Thienyl) phenyl] amine} fluorescent conjugated microporous polymer for selectively sensing picric acid. Sens. Actuators B-Chem. 2017, 244, 334–343. [Google Scholar] [CrossRef]
- Xu, Y.H.; Nagai, A.; Jiang, D.H. Core-shell conjugated microporous polymers: A new strategy for exploring color-tunable and-controllable light emissions. Chem. Commun. 2013, 16, 1591–1593. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.H.; Chen, L.; Guo, Z.Q.; Nagai, A.; Jiang, D.H. Light-emitting conjugated polymers with microporous network architecture: Interweaving scaffold promotes electronic conjugation, facilitates exciton migration, and improves luminescence. J. Am. Chem. Soc. 2011, 44, 17622–17625. [Google Scholar] [CrossRef] [PubMed]
- Novotney, J.L.; Dichtel, W.R. Conjugated porous polymers for TNT vapor detection. ACS Macro. Lett. 2013, 5, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.; Chatterjee, N.; Das, S.; Saha, K.D.; Bhaumik, A. Porous polyurea network showing aggregation induced white light emission, applications as biosensor and scaffold for drug delivery. ACS Appl. Mater. Inter. 2014, 24, 22569–22576. [Google Scholar] [CrossRef]
- Geng, T.M.; Li, D.K.; Zhu, Z.M.; Zhang, W.Y.; Ye, S.N.; Zhu, H.; Wang, Z.Q. Fluorescent conjugated microporous polymer based on perylene tetraanhydride bisimide for sensing o-nitrophenol. Anal. Chim. Acta 2018, 1011, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.W.; Wang, Y.P.; Geng, W.T.; Liu, H.L. Rapid detection of tryptamine by optosensor with molecularly imprinted polymers based on carbon dots-embedded covalent-organic frameworks. Sens. Actuators B-Chem. 2019, 285, 546–552. [Google Scholar] [CrossRef]
- Li, J.; Sun, F.; Shi, X.; Ren, H.; Li, M.; Zhu, G. A highly crystalline fluorine-based porous organic framework with high photoluminescence quantum yield. Macromol. Rapid Commun. 2019, 11, 1900060. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.F.; Ding, S.Y.; Liu, J.M.; Wang, W.; Zheng, Q.Y. Triazatruxene based covalent organic framework and its quick-response fluorescence-on nature towards electron rich arenes. J. Mater. Chem. C 2015, 3, 10066–10069. [Google Scholar] [CrossRef]
- Gomes, R.; Bhaumik, A. A new triazine functionalized luminescent covalent organic framework for nitroaromatic sensing and CO2 storage. RSC Adv. 2016, 6, 28047–28054. [Google Scholar] [CrossRef]


| COFs | The Equations | Regression Coefficient (R) | Concentration Range of TNP or p-NP (mol L−1) | Limit of Detections (mol L−1) |
|---|---|---|---|---|
| HDADE | I0/I = 1.0016 + 6.29 × 104 [TNP] | 0.966 | 0 to 1.0 × 10−5 | 1.19 × 10−11 |
| HBAPB | I0/I = 0.9883 + 2.17 × 105 [p-NP] | 0.9984 | 0 to 5.0 × 10−5 | 6.91 × 10−12 |
| HBPDA | I0/I = 0.6245 + 2.48 × 105 [p-NP] | 0.9960 | 0 to 7.5 × 10−5 | 6.05 × 10−12 |
| Sample | NACs | Ksv (L mol−1) | LODs (mol L−1) | Refs |
|---|---|---|---|---|
| Py-Azine COF | TNP | 7.6 × 104 | - | [24] |
| o-NP | 5.9 × 102 | - | ||
| FL-SNW-DPP-0.11 | TNP | 5.3 × 104 | - | [25] |
| TfpBDH-CONs | TNP | 2.6 × 104 | - | [26] |
| p-NP | − | |||
| TAT-COF-2 | NB | − | 10 × 10–6 | [58] |
| TRIPTA | PA | 2.7 × 106 | - | [59] |
| p-NP | − | - | ||
| 3D-Py-COF | TNP | 3.1 × 104 | - | [27] |
| COF-BABD-DB | TNP | 5.7 × 105 | - | [28] |
| p-NP | 1.55 × 104 | - | ||
| COF-BABD-BZ | TNP | 4.5 × 105 | - | |
| p-NP | 3.2 × 104 | - | ||
| PAF-130 | TNP | 4.69 × 104 | - | [57] |
| o-NP | − | - | ||
| HBD | p-NP | 7.91 × 104 | 4.6 × 10−11 | [5] |
| HDASD | p-NP | 1.16 × 105 | 2.59 × 10−10 | |
| HDOBD | p-NP | 3.76 × 104 | 7.98 × 10−11 | |
| HDADE | TNP | 6.29 × 104 | 1.19 × 10−11 | This work |
| HBAPB | p-NP | 2.17 × 105 | 6.91 × 10−12 | |
| HBPDA | p-NP | 2.48 × 105 | 6.05 × 10−12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Geng, T.-M.; Tang, K.-B. Fully Flexible Covalent Organic Frameworks for Fluorescence Sensing 2,4,6-Trinitrophenol and p-Nitrophenol. Polymers 2023, 15, 653. https://doi.org/10.3390/polym15030653
Zhu H, Geng T-M, Tang K-B. Fully Flexible Covalent Organic Frameworks for Fluorescence Sensing 2,4,6-Trinitrophenol and p-Nitrophenol. Polymers. 2023; 15(3):653. https://doi.org/10.3390/polym15030653
Chicago/Turabian StyleZhu, Hai, Tong-Mou Geng, and Kai-Bin Tang. 2023. "Fully Flexible Covalent Organic Frameworks for Fluorescence Sensing 2,4,6-Trinitrophenol and p-Nitrophenol" Polymers 15, no. 3: 653. https://doi.org/10.3390/polym15030653




