Hydrogel/β-FeOOH-Coated Poly(vinylidene fluoride) Membranes with Superhydrophilicity/Underwater Superoleophobicity Facilely Fabricated via an Aqueous Approach for Multifunctional Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Hydrogel-Coated PVDF Membranes
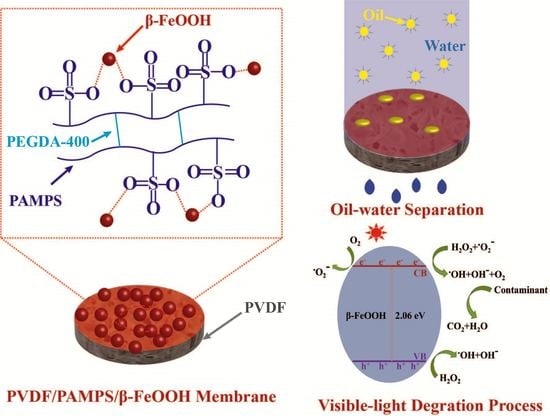
2.3. Fabrication of Hydrogel/β-FeOOH-Coated PVDF Membranes
2.4. Preparation and Separation of Oil-In-Water Emulsion
2.5. Evaluation of the Photo-Fenton Activity
2.6. Characterization of Hydrogel/β-FeOOH Composite Membranes
3. Results
3.1. Preparation and Wetting Behaviors of Membranes
3.2. Characterization of Membranes
3.3. Separation of Oil-In-Water Emulsions
3.4. Photo-Fenton Activity and Water Purification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Baig, N.; Alowaid, A.M.; Abdulazeez, I.; Salhi, B.; Sajid, M.; Kammakakam, I. Designing of nanotextured inorganic-organic hybrid PVDF membrane for efficient separation of the oil-in-water emulsions. Chemosphere 2022, 308, 11. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Patel, R. Review on oil/water separation membrane technology. Membr. J. 2020, 30, 359–372. [Google Scholar]
- Tummons, E.; Han, Q.; Tanudjaja, H.J.; Hejase, C.A.; Chew, J.W.; Tarabara, V.V. Membrane fouling by emulsified oil: A review. Sep. Purif. Technol. 2020, 248, 22. [Google Scholar] [CrossRef]
- Dmitrieva, E.S.; Anokhina, T.S.; Novitsky, E.G.; Volkov, V.V.; Borisov, I.L.; Volkov, A.V. Polymeric Membranes for Oil-Water Separation: A Review. Polymers 2022, 14, 980. [Google Scholar] [CrossRef]
- Xie, H.L.; Shen, L.G.; Xu, Y.C.; Hong, H.C.; Yang, L.N.; Li, R.J.; Lin, H.J. Tannic acid (TA)-based coating modified membrane enhanced by successive inkjet printing of Fe3+ and sodium periodate (SP) for efficient oil-water separation. J. Membr. Sci. 2022, 660, 10. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Chu, L.Y.; Wu, X.M.; Wang, Z.; Jiang, X.B.; Ju, X.J.; Ruan, X.H.; He, G.H. Membrane-based separation technologies: From polymeric materials to novel process: An outlook from China. Rev. Chem. Eng. 2020, 36, 67–105. [Google Scholar] [CrossRef]
- Tanudjaja, H.J.; Hejase, C.A.; Tarabara, V.V.; Fane, A.G.; Chew, J.W. Membrane-based separation for oily wastewater: A practical perspective. Water Res. 2019, 156, 347–365. [Google Scholar] [CrossRef]
- Si, Y.F.; Guo, Z.G. Superwetting Materials of Oil-Water Emulsion Separation. Chem. Lett. 2015, 44, 874–883. [Google Scholar] [CrossRef]
- Jincui, G.; Lei, Z.; Jiawei, Z.; Tao, C. Recent advance of two-dimensional carbon-based films and their polymer functionalized membranes for oil/water separation. Chin. Sci. Bull. 2019, 64, 2316–2331. [Google Scholar]
- Long, M.Y.; Ma, Y.; Yang, C.; Zhang, R.N.; Jiang, Z.Y. Superwetting membranes: From controllable constructions to efficient separations. J. Mater. Chem. A 2021, 9, 1395–1417. [Google Scholar] [CrossRef]
- Zhou, H.; Guo, Z.G. Superwetting Janus membranes: Focusing on unidirectional transport behaviors and multiple applications. J. Mater. Chem. A 2019, 7, 12921–12950. [Google Scholar] [CrossRef]
- Chen, C.L.; Weng, D.; Mahmood, A.; Chen, S.; Wang, J.D. Separation mechanism and construction of surfaces with special wettability for oil/water separation. ACS Appl. Mater. Interfaces 2019, 11, 11006–11027. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Sun, Y.H.; Guo, Z.G. Designing novel superwetting surfaces for high-efficiency oil-water separation: Design principles, opportunities, trends and challenges. J. Mater. Chem. A 2020, 8, 16831–16853. [Google Scholar] [CrossRef]
- Yao, X.; Liu, J.J.; Yang, C.H.; Yang, X.X.; Wei, J.C.; Xia, Y.; Gong, X.Y.; Suo, Z.G. Hydrogel Paint. Adv. Mater. 2019, 31. [Google Scholar] [CrossRef]
- Su, M.J.; Liu, Y.; Li, S.H.; Fang, Z.P.; He, B.Q.; Zhang, Y.H.; Li, Y.L.; He, P.X. A rubber-like, underwater superoleophobic hydrogel for efficient oil/water separation. Chem. Eng. J. 2019, 361, 364–372. [Google Scholar] [CrossRef]
- Sahoo, B.N.; Gunda, N.S.K.; Nanda, S.; Kozinski, J.A.; Mitra, S.K. Development of dual-phobic surfaces: Superamphiphobicity in air and oleophobicity underwater. ACS Sustain. Chem. Eng. 2017, 5, 6716–6726. [Google Scholar] [CrossRef]
- Dai, L.; Wang, B.B.; An, X.Y.; Zhang, L.Q.; Khan, A.; Ni, Y.H. Oil/water interfaces of guar gum-based biopolymer hydrogels and application to their separation. Carbohydr. Polym. 2017, 169, 9–15. [Google Scholar] [CrossRef]
- Teng, C.; Xie, D.; Wang, J.F.; Zhu, Y.; Jiang, L. A strong, underwater superoleophobic PNIPAMclay nanocomposite hydrogel. J. Mater. Chem. A 2016, 4, 12884–12888. [Google Scholar] [CrossRef]
- Matsubayashi, T.; Tenjimbayashi, M.; Komine, M.; Manabe, K.; Shiratori, S. Bioinspired hydrogel-coated mesh with superhydrophilicity and underwater superoleophobicity for efficient and ultrafast oil/water separation in harsh environments. Ind. Eng. Chem. Res. 2017, 56, 7080–7085. [Google Scholar] [CrossRef]
- Xie, X.L.; Liu, L.J.; Zhang, L.; Lu, A. Strong cellulose hydrogel as underwater superoleophobic coating for efficient oil/water separation. Carbohydr. Polym. 2020, 229, 115467. [Google Scholar] [CrossRef]
- Thi, L.N.; Phan, T.T.T.; Ngoc, T.N.; Viswanath, N.S.M.; Le, H.T.T.; Thi, L.T.; Tien-Trung, N.; Nguyen, L.; Nhiem, D.N.; Huu, H.; et al. Prussian Blue decorated g-C3N4-From novel synthesis to insight study on charge transfer strategy for improving visible-light driven photo-Fenton catalytic activity. J. Alloy. Compd. 2022, 916, 15. [Google Scholar] [CrossRef]
- Wang, B.; Song, Z.J.; Sun, L.S. A review: Comparison of multi-air-pollutant removal by advanced oxidation processes—Industrial implementation for catalytic oxidation processes. Chem. Eng. J. 2021, 409, 128136. [Google Scholar] [CrossRef]
- Luo, H.W.; Zeng, Y.F.; He, D.Q.; Pan, X.L. Application of iron-based materials in heterogeneous advanced oxidation processes for wastewater treatment: A review. Chem. Eng. J. 2021, 407, 127191. [Google Scholar] [CrossRef]
- Alvarez, M.A.; Ruidiaz-Martinez, M.; Cruz-Quesada, G.; Lopez-Ramon, M.V.; Rivera-Utrilla, J.; Sanchez-Polo, M.; Mota, A.J. Removal of parabens from water by UV-driven advanced oxidation processes. Chem. Eng. J. 2020, 379, 122334. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Ray, A.; Sultana, S.; Paramanik, L.; Parida, K.M. Recent advances in phase, size, and morphology-oriented nanostructured nickel phosphide for overall water splitting. J. Mater. Chem. A 2020, 8, 19196–19245. [Google Scholar] [CrossRef]
- Mills, A.; Davies, R.H.; Worsley, D. Water-purification by semiconductor photocatalysis. Chem. Soc. Rev. 1993, 22, 417–425. [Google Scholar] [CrossRef]
- Liu, J.D.; Zhou, S.Y.; Gu, P.Y.; Zhang, T.Y.; Chen, D.Y.; Li, N.J.; Xu, Q.F.; Lu, J.M. Conjugate Polymer-clothed TiO2@V2O5 nanobelts and their enhanced visible light photocatalytic performance in water remediation. J. Colloid Interface Sci. 2020, 578, 402–411. [Google Scholar] [CrossRef]
- Li, F.R.; Kong, W.T.; Zhao, X.Z.; Pan, Y.L. Multifunctional TiO2-Based Superoleophobic/Superhydrophilic Coating for Oil-Water Separation and Oil Purification. ACS Appl. Mater. Interfaces 2020, 12, 18074–18083. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Chaudhry, S.A. Nanohybrid composite Fe2O3-ZrO2/BC for inhibiting the growth of bacteria and adsorptive removal of arsenic and dyes from water. J. Clean Prod. 2019, 223, 849–868. [Google Scholar] [CrossRef]
- Tahir, M.B.; Sagir, M.; Shahzad, K. Removal of acetylsalicylate and methyl-theobromine from aqueous environment using nano-photocatalyst WO3-TiO2 @g-C3N4 composite. J. Hazard. Mater. 2019, 363, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.; Alexander, B.D.; Dawood, I.; Tillotson, M.; Wells, R.P.K.; Macphee, D.E.; Killham, K. Remediation of a chlorinated aromatic hydrocarbon in water by photoelectrocatalysis. Environ. Pollut. 2009, 157, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Xia, X.Z.; Han, J.C.; Wu, Q.Y.; Yang, H.C. Siphon-driven interfacial photocatalytic reactors enhanced by capillary flow for continuous wastewater treatment. Sep. Purif. Technol. 2022, 300, 8. [Google Scholar] [CrossRef]
- Akharame, M.O.; Oputu, O.U.; Pereao, O.; Olorunfemi, D.I.; Fatoki, O.S.; Opeolu, B.O. Beta-FeOOH/polyamide nanocomposites for the remediation of 4-chlorophenol from contaminated waters. J. Polym. Res. 2022, 29, 16. [Google Scholar] [CrossRef]
- Shi, L.; Shi, Y.; Zhuo, S.F.; Zhang, C.L.; Aldrees, Y.; Aleid, S.; Wang, P. Multi-functional 3D honeycomb ceramic plate for clean water production by heterogeneous photo-Fenton reaction and solar-driven water evaporation. Nano Energy 2019, 60, 222–230. [Google Scholar] [CrossRef]
- Wang, F.F.; Yu, X.L.; Ge, M.F.; Wu, S.J.; Guan, J.; Tang, J.W.; Wu, X.; Ritchie, R.O. Facile self-assembly synthesis of gamma-Fe2O3/graphene oxide for enhanced photo-Fenton reaction. Environ. Pollut. 2019, 248, 229–237. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, H.C.; Wan, L.S.; Liang, H.Q.; Li, H.Y.; Xu, Z.K. Polydopamine-coated porous substrates as a platform for mineralized beta-FeOOH nanorods with photocatalysis under sunlight. ACS Appl. Mater. Interfaces 2015, 7, 11567–11574. [Google Scholar] [CrossRef]
- Wang, J.M.; Li, X.X.; Cheng, Q.Y.; Lv, F.Z.; Chang, C.Y.; Zhang, L.N. Construction of beta-FeOOH@tunicate cellulose nanocomposite hydrogels and their highly efficient photocatalytic properties. Carbohydr. Polym. 2020, 229, 115470. [Google Scholar] [CrossRef]
- Wang, M.L.; Gao, Q.H.; Zhang, M.J.; Zhang, M.X.; Zhang, Y.M.; Hu, J.T.; Wu, G.Z. In-situ formation of durable akaganeite (beta-FeOOH) nanorods on sulfonate-modified poly(ethylene terephthalate) fabric for dual-functional wastewater treatment. J. Hazard. Mater. 2020, 386, 121647. [Google Scholar] [CrossRef]
- Xie, A.T.; Cui, J.Y.; Yang, J.; Chen, Y.Y.; Dai, J.D.; Lang, J.H.; Li, C.X.; Yan, Y.S. Photo-Fenton self-cleaning membranes with robust flux recovery for an efficient oil/water emulsion separation. J. Mater. Chem. A 2019, 7, 8491–8502. [Google Scholar] [CrossRef]
- Zhang, L.Y.; He, Y.; Ma, L.; Chen, J.Y.; Fan, Y.; Zhang, S.H.; Shi, H.; Li, Z.Y.; Luo, P.Y. Hierarchically stabilized PAN/beta-FeOOH nanofibrous membrane for efficient water purification with excellent antifouling performance and robust solvent resistance. ACS Appl. Mater. Interfaces 2019, 11, 34487–34496. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, F.R.; Zhu, W.X.; Su, D.; Sang, Z.Y.; Yan, X.; Li, S.; Liang, J.; Dou, S.X. A multifunctional hierarchical porous SiO2/GO membrane for high efficiency oil/water separation and dye removal. Carbon 2020, 160, 88–97. [Google Scholar] [CrossRef]
- Li, Q.Q.; Deng, W.J.; Li, C.H.; Sun, Q.Y.; Huang, F.Z.; Zhao, Y.; Li, S.K. High-Flux Oil/Water Separation with Interfacial Capillary Effect in Switchable Superwetting Cu(OH)(2)@ZIF-8 Nanowire Membranes. ACS Appl. Mater. Interfaces 2018, 10, 40265–40273. [Google Scholar] [CrossRef]
- Lu, W.L.; Duan, C.; Zhang, Y.L.; Gao, K.; Dai, L.; Shen, M.X.; Wang, W.L.; Wang, J.; Ni, Y.H. Cellulose-based electrospun nanofiber membrane with core-sheath structure and robust photocatalytic activity for simultaneous and efficient oil emulsions separation, dye degradation and Cr(VI) reduction. Carbohydr. Polym. 2021, 258, 11. [Google Scholar] [CrossRef]
- Liu, N.; Qu, R.X.; Chen, Y.N.; Cao, Y.Z.; Zhang, W.F.; Lin, X.; Wei, Y.; Feng, L.; Jiang, L. In situ dual-functional water purification with simultaneous oil removal and visible light catalysis. Nanoscale 2016, 8, 18558–18564. [Google Scholar] [CrossRef]
- Xie, A.T.; Cui, J.Y.; Yang, J.; Chen, Y.Y.; Lang, J.H.; Li, C.X.; Yan, Y.S.; Dai, J.D. Photo-Fenton self-cleaning PVDF/NH2-MIL-88B(Fe) membranes towards highly-efficient oil/water emulsion separation. J. Membr. Sci. 2020, 595, 117499. [Google Scholar] [CrossRef]
- Yu, J.; Pan, Y.P.; Lu, Q.F.; Yang, W.; Gao, J.Z.; Li, Y. Synthesis and swelling behaviors of P(AMPS-co-AAc) superabsorbent hydrogel produced by glow-discharge electrolysis plasma. Plasma Chem. Plasma Process. 2013, 33, 219–235. [Google Scholar] [CrossRef]
- Wang, J.; Yu, X.H.; Wang, C.; Xiang, K.C.; Deng, M.D.; Yin, H.B. PAMPS/MMT composite hydrogel electrolyte for solid-state supercapacitors. J. Alloy. Compd. 2017, 709, 596–601. [Google Scholar] [CrossRef]
- Wei, C.Z.; Nan, Z.D. Effects of experimental conditions on one-dimensional single-crystal nanostructure of beta-FeOOH. Mater. Chem. Phys. 2011, 127, 220–226. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Liu, X.M.; Zhao, Y.P.; Dionysiou, D.D. Aligned alpha-FeOOH nanorods anchored on a graphene oxide-carbon nanotubes aerogel can serve as an effective Fenton-like oxidation catalyst. Appl. Catal. B-Environ. 2017, 213, 74–86. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, C.; He, A.; Yang, S.J.; Wu, G.P.; Darling, S.B.; Xu, Z.K. Photocatalytic nanofiltration membranes with self-cleaning property for wastewater treatment. Adv. Funct. Mater. 2017, 27, 1700251. [Google Scholar] [CrossRef]
- Su, S.S.; Liu, Y.Y.; Liu, X.M.; Jin, W.; Zhao, Y.P. Transformation pathway and degradation mechanism of methylene blue through beta-FeOOH@GO catalyzed photo-Fenton-like system. Chemosphere 2019, 218, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Naushad, M.; Sharma, G.; Alothman, Z.A. Photodegradation of toxic dye using Gum Arabic-crosslinked-poly(acrylamide)/Ni(OH)(2)/FeOOH nanocomposites hydrogel. J. Clean Prod. 2019, 241, 118263. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Zhu, T.; Liu, H.; Tang, Z.; Kuang, X.; Qiao, Y.; Zhang, H.; Zhu, C. Hydrogel/β-FeOOH-Coated Poly(vinylidene fluoride) Membranes with Superhydrophilicity/Underwater Superoleophobicity Facilely Fabricated via an Aqueous Approach for Multifunctional Applications. Polymers 2023, 15, 839. https://doi.org/10.3390/polym15040839
Tang Y, Zhu T, Liu H, Tang Z, Kuang X, Qiao Y, Zhang H, Zhu C. Hydrogel/β-FeOOH-Coated Poly(vinylidene fluoride) Membranes with Superhydrophilicity/Underwater Superoleophobicity Facilely Fabricated via an Aqueous Approach for Multifunctional Applications. Polymers. 2023; 15(4):839. https://doi.org/10.3390/polym15040839
Chicago/Turabian StyleTang, Yin, Tang Zhu, Huichao Liu, Zheng Tang, Xingwen Kuang, Yongna Qiao, Hao Zhang, and Caizhen Zhu. 2023. "Hydrogel/β-FeOOH-Coated Poly(vinylidene fluoride) Membranes with Superhydrophilicity/Underwater Superoleophobicity Facilely Fabricated via an Aqueous Approach for Multifunctional Applications" Polymers 15, no. 4: 839. https://doi.org/10.3390/polym15040839