Photoluminescence Performance and Photocatalytic Activity of Modified Carbon Quantum Dots Derived from Pluronic F127
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CQDs
2.3. Surface Modification of CQDs
2.4. Photocatalytic Degradation Assessment of IC and LF SF
2.5. Characterization
3. Results and Discussion
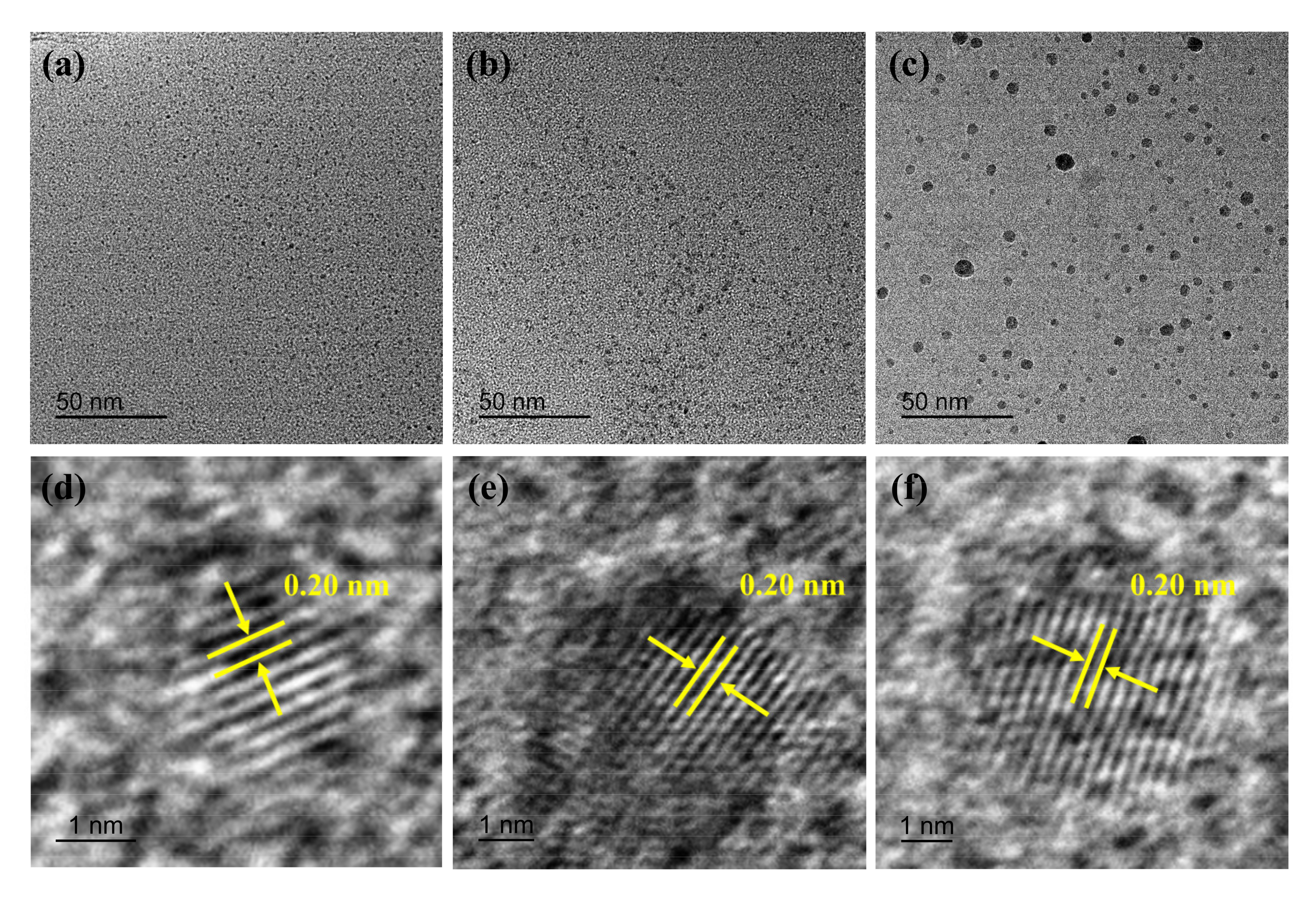
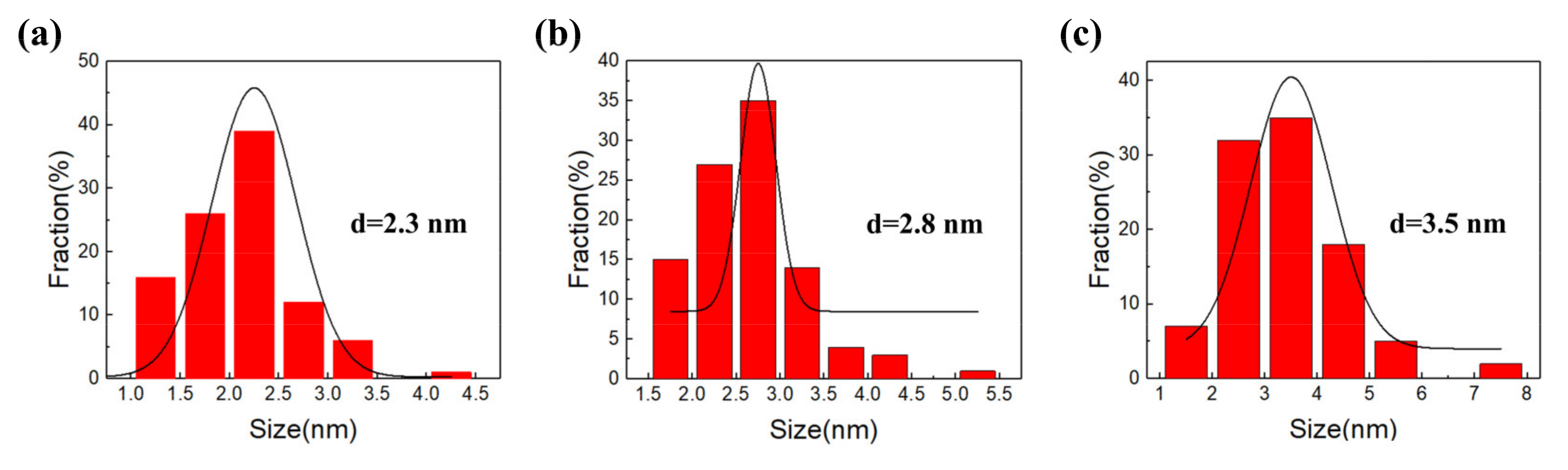
3.1. Morphology of CQDs
3.2. Optical Performance Changes of CQDs with Different Functional Groups
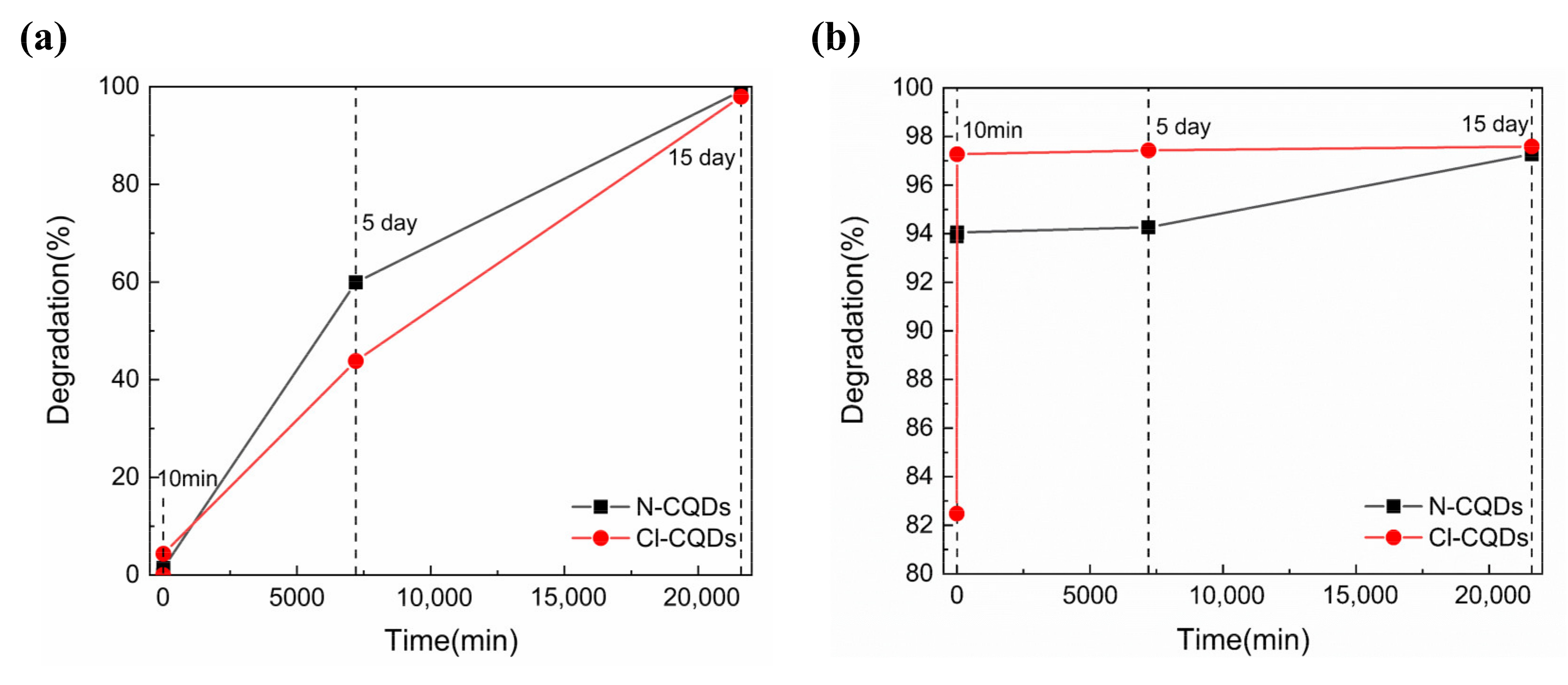
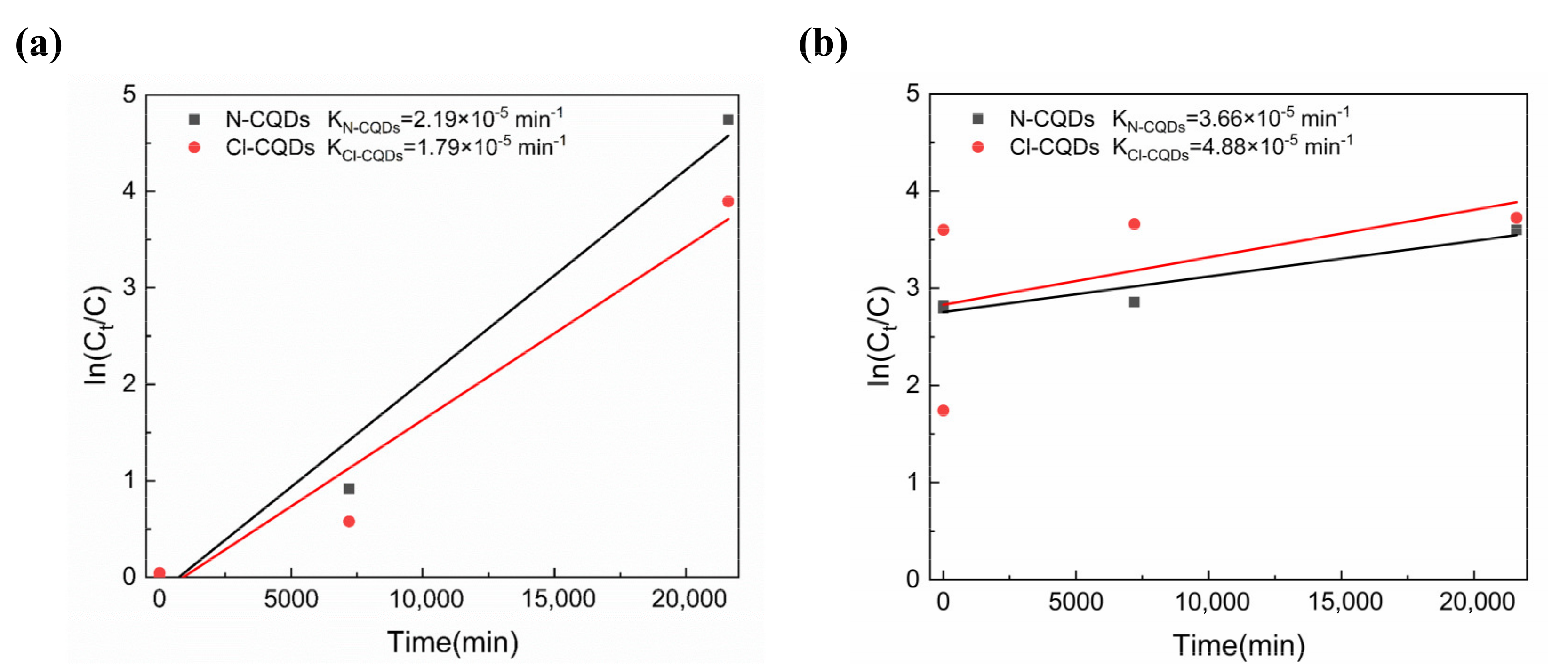
3.3. Photocatalytic Activity of As-Prepared CQDs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, H.B.; Chen, L.; Cao, Z.J.; Hong, F.F. Enhanced decolourization efficiency of textile dye Reactive Blue 19 in a horizontal rotating reactor using strips of BNC-immobilized laccase: Optimization of conditions and comparison of decolourization efficiency. Biochem. Eng. J. 2020, 156, 9. [Google Scholar] [CrossRef]
- Qu, Z.; Wang, J.; Tang, J.; Shu, X.; Liu, X.; Zhang, Z.; Wang, J. Carbon quantum dots/KNbO3 hybrid composites with enhanced visible-light driven photocatalytic activity toward dye waste-water degradation and hydrogen production. Mol. Catal. 2018, 445, 1–11. [Google Scholar] [CrossRef]
- Kiwaan, H.A.; Mohamed, F.S.; El-Ghamaz, N.A.; Beshry, N.M.; El-Bindary, A.A. Experimental and electrical studies of Na-X zeolite for the adsorption of different dyes. J. Mol. Liq. 2021, 332, 13. [Google Scholar] [CrossRef]
- Kiwaan, H.A.; Mohamed, F.S.; El-Bindary, A.A.; El-Ghamaz, N.A.; Abo-Yassin, H.R.; El-Bindary, M.A. Synthesis, identification and application of metal organic framework for removal of industrial cationic dyes. J. Mol. Liq. 2021, 342, 15. [Google Scholar] [CrossRef]
- Hassan, N.; Shahat, A.; El-Didamony, A.; El-Desouky, M.; El-Bindary, A.J. Mesoporous iron oxide nano spheres for capturing organic dyes from water sources. J. Mol. Struct. 2020, 1217, 128361. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Z.; Li, A.; Yang, H. Evaluation of starch-based flocculants for the flocculation of dissolved organic matter from textile dyeing secondary wastewater. Chemosphere 2017, 174, 200–207. [Google Scholar] [CrossRef]
- El-Bindary, M.A.; El-Desouky, M.G.; El-Bindary, A.A. Adsorption of industrial dye from aqueous solutions onto thermally treated green adsorbent: A complete batch system evaluation. J. Mol. Liq. 2022, 346, 13. [Google Scholar] [CrossRef]
- Streit, A.F.; Côrtes, L.N.; Druzian, S.P.; Godinho, M.; Collazzo, G.C.; Perondi, D.; Dotto, G.L. Development of high quality activated carbon from biological sludge and its application for dyes removal from aqueous solutions. Sci. Total Environ. 2019, 660, 277–287. [Google Scholar] [CrossRef]
- Altalhi, T.A.; Ibrahim, M.M.; Mersal, G.A.M.; Mahmoud, M.H.H.; Kumeria, T.; El-Desouky, M.G.; El-Bindary, A.A.; El-Bindary, M.A. Adsorption of doxorubicin hydrochloride onto thermally treated green adsorbent: Equilibrium, kinetic and thermodynamic studies. J. Mol. Struct. 2022, 1263, 11. [Google Scholar] [CrossRef]
- Velumani, A.; Sengodan, P.; Arumugam, P.; Rajendran, R.; Santhanam, S.; Palanisamy, M. Carbon quantum dots supported ZnO sphere based photocatalyst for dye degradation application. Curr. Appl. Phys. 2020, 20, 1176–1184. [Google Scholar] [CrossRef]
- Yadav, N.; Gaikwad, R.P.; Mishra, V.; Gawande, M.B. Synthesis and Photocatalytic Applications of Functionalized Carbon Quantum Dots. Bull. Chem. Sci. Jpn. 2022, 95, 1638–1679. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Bai, M.S.; Su, J.; Fang, C.Q.; Li, H.; Chen, J.; Jiao, J. Synthesis of fluorescent carbon quantum dots from aqua mesophase pitch and their photocatalytic degradation activity of organic dyes. J. Mater. Sci. Technol. 2019, 35, 1515–1522. [Google Scholar] [CrossRef]
- Ke, J.; Li, X.Y.; Zhao, Q.D.; Liu, B.J.; Liu, S.M.; Wang, S.B. Upconversion carbon quantum dots as visible light responsive component for efficient enhancement of photocatalytic performance. J. Colloid Interface Sci. 2017, 496, 425–433. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.C.; Ang, W.L.; Sambudi, N.S. Nitrogen and bismuth-doped rice husk-derived carbon quantum dots for dye degradation and heavy metal removal. J. Photochem. Photobiol. A-Chem. 2021, 418, 12. [Google Scholar]
- Feng, S.; Gao, Z.; Liu, H.; Huang, J.; Li, X.; Yang, Y. Feasibility of detection valence speciation of Cr (III) and Cr (VI) in environmental samples by spectrofluorimetric method with fluorescent carbon quantum dots. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 212, 286–292. [Google Scholar] [CrossRef]
- Ren, L.; Hang, X.; Qin, Z.; Zhang, P.; Wang, W.; Zhang, Y.; Jiang, L. Determination of dopamine by a label-free fluorescent aptasensor based on AuNPs and carbon quantum dots. Optik 2020, 208, 163560. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wang, B.L.; Yu, X.W.; Li, J.Y.; Shang, J.; Yu, J.H. Carbon Dots in Porous Materials: Host-Guest Synergy for Enhanced Performance. Angew. Chem.-Int. Edit. 2020, 59, 19390–19402. [Google Scholar] [CrossRef]
- Lin, H.T.; Huang, J.; Ding, L.Y. Preparation of Carbon Dots with High-Fluorescence Quantum Yield and Their Application in Dopamine Fluorescence Probe and Cellular Imaging. J. Nanomater. 2019, 2019, 9. [Google Scholar] [CrossRef]
- Donate-Buendia, C.; Torres-Mendieta, R.; Pyatenko, A.; Falomir, E.; Fernandez-Alonso, M.; Minguez-Vega, G. Fabrication by Laser Irradiation in a Continuous Flow Jet of Carbon Quantum Dots for Fluorescence Imaging. ACS Omega 2018, 3, 2735–2742. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, W.L.; Liu, X.P.; Zhang, Y.; Bai, Y. Hydrothermal synthesis and photoluminescent mechanistic investigation of highly fluorescent nitrogen doped carbon dots from amino acids. Mater. Res. Bull. 2017, 89, 26–32. [Google Scholar] [CrossRef]
- Luo, X.L.; Han, Y.; Chen, X.M.; Tang, W.Z.; Yue, T.L.; Li, Z.H. Carbon dots derived fluorescent nanosensors as versatile tools for food quality and safety assessment: A review. Trends Food Sci. Technol. 2020, 95, 149–161. [Google Scholar] [CrossRef]
- Caglayan, M.O.; Mindivan, F.; Sahin, S. Sensor and Bioimaging Studies Based on Carbon Quantum Dots: The Green Chemistry Approach. Crit. Rev. Anal. Chem. 2022, 52, 814–847. [Google Scholar] [CrossRef] [PubMed]
- Muthusankar, G.; Sasikumar, R.; Chen, S.M.; Gopu, G.; Sengottuvelan, N.; Rwei, S.P. Electrochemical synthesis of nitrogen-doped carbon quantum dots decorated copper oxide for the sensitive and selective detection of nonsteroidal anti-inflammatory drug in berries. J. Colloid Interface Sci. 2018, 523, 191–200. [Google Scholar] [CrossRef]
- Sabet, M.; Mahdavi, K. Green synthesis of high photoluminescence nitrogen-doped carbon quantum dots from grass via a simple hydrothermal method for removing organic and inorganic water pollutions. Appl. Surf. Sci. 2019, 463, 283–291. [Google Scholar] [CrossRef]
- Preethi, M.; Murugan, R.; Viswanathan, C.; Ponpandian, N. Potato starch derived N-doped carbon quantum dots as a fluorescent sensing tool for ascorbic acid. J. Photochem. Photobiol. A-Chem. 2022, 431, 8. [Google Scholar] [CrossRef]
- John, V.L.; Nair, Y.; Vinod, T.P. Doping and Surface Modification of Carbon Quantum Dots for Enhanced Functionalities and Related Applications. Part. Part. Syst. Charact. 2021, 38, 28. [Google Scholar] [CrossRef]
- Remli, U.; Aziz, A. Photocatalytic degradation of methyl orange using Carbon Quantum Dots (CQDs) derived from watermelon rinds. IOP Conf. Ser. Mater. Sci. Eng. IOP Publ. 2020, 736, 042038. [Google Scholar] [CrossRef]
- Najjar, M.; Nasseri, M.A.; Allahresani, A.; Darroudi, M. Green and efficient synthesis of carbon quantum dots from Cordia myxa L. and their application in photocatalytic degradation of organic dyes. J. Mol. Struct. 2022, 1266, 10. [Google Scholar] [CrossRef]
- Dejpasand, M.T.; Saievar-Iranizad, E.; Bayat, A.; Montaghemi, A.; Ardekani, S.R. Tuning HOMO and LUMO of three region (UV, Vis and IR) photoluminescent nitrogen doped graphene quantum dots for photodegradation of methylene blue. Mater. Res. Bull. 2020, 128, 8. [Google Scholar] [CrossRef]
- Rani, U.A.; Ng, L.Y.; Ng, C.Y.; Mahmoudi, E.; Ng, Y.S.; Mohammad, A.W. Sustainable production of nitrogen-doped carbon quantum dots for photocatalytic degradation of methylene blue and malachite green. J. Water Process Eng. 2021, 40, 14. [Google Scholar] [CrossRef]
- Sukhadeve, G.K.; Janbandhu, S.Y.; Kumar, R.; Lataye, D.H.; Ramteke, D.D.; Gedam, R.S. Visible light assisted photocatalytic degradation of Indigo Carmine dye and NO2 removal by Fe doped TiO2 nanoparticles. Ceram. Int. 2022, 48, 29121–29135. [Google Scholar] [CrossRef]
- Naciri, N.; Farahi, A.; Rafqah, S.; Nasrellah, H.; El Mhammedi, M.A.; Lancar, I.; Bakasse, M. Effective photocatalytic decolorization of indigo carmine dye in Moroccan natural phosphate-TiO2 aqueous suspensions. Opt. Mater. 2016, 52, 38–43. [Google Scholar] [CrossRef]
- Mittal, A.; Mittal, J.; Malviya, A.; Kaur, D.; Gupta, V.K. Decoloration treatment of a hazardous triarylmethane dye, Light Green SF (Yellowish) by waste material adsorbents. J. Colloid Interface Sci. 2010, 342, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Haddad, A.; Merouani, S.; Hannachi, C.; Hamdaoui, O.; Hamrouni, B. Intensification of light green SF yellowish (LGSFY) photodegradion in water by iodate ions: Iodine radicals implication in the degradation process and impacts of water matrix components. Sci. Total Environ. 2019, 652, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Liu, Y.L.; Guo, Z.Y.; Lei, B.F.; Zhuang, J.L.; Zhang, X.J.; Liu, Z.; Hu, C. Hydrophobic carbon dots with blue dispersed emission and red aggregation-induced emission. Nat. Commun. 2019, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.Y.; Jia, P.Y.; Chen, D.S.; Wang, L.N. Hydrothermal synthesis of N-doped carbon quantum dots and their application in ion-detection and cell-imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 248, 11. [Google Scholar] [CrossRef]
- Yin, S.D.; Duvigneau, J.; Vancso, G.J. Fluorescent Polyethylene by In Situ Facile Synthesis of Carbon Quantum Dots Facilitated by Silica Nanoparticle Agglomerates. ACS Appl. Polym. Mater. 2021, 3, 5517–5526. [Google Scholar] [CrossRef]
- Preethi, M.; Viswanathan, C.; Ponpandian, N. A metal-free, dual catalyst for the removal of Rhodamine B using novel carbon quantum dots from muskmelon peel under sunlight and ultrasonication: A green way to clean the environment. J. Photochem. Photobiol. A-Chem. 2022, 426, 11. [Google Scholar] [CrossRef]
- Baslak, C.; Demirel, S.; Kocyigit, A.; Alatli, H.; Yildirim, M. Supercapacitor behaviors of carbon quantum dots by green synthesis method from tea fermented with kombucha. Mater. Sci. Semicond. Process. 2022, 147, 7. [Google Scholar] [CrossRef]
- Zhang, T.X.; Zhu, J.Y.; Zhai, Y.; Wang, H.; Bai, X.; Dong, B.; Wang, H.; Song, H. A novel mechanism for red emission carbon dots: Hydrogen bond dominated molecular states emission. Nanoscale 2017, 9, 13042–13051. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Fu, Z.; Cui, F. Carbon quantum dots decorated CuS nanocomposite for effective degradation of methylene blue and antibacterial performance. J. Mol. Liq. 2018, 268, 578–586. [Google Scholar] [CrossRef]
- Ziyaadini, M.; Ghashang, M. Sunlight-induced photocatalytic degradation of indigo carmine using Bi5Ti3FeO15 layered structure. Optik 2021, 228, 7. [Google Scholar] [CrossRef]
- Ryali, S.; Sanasi, P.D. Graphene oxide-nano-titania composites for efficient photocatalytic degradation of indigo carmine. J. Chin. Chem. Soc. 2018, 65, 1423–1430. [Google Scholar] [CrossRef]
- Oppong, S.O.B.; Anku, W.W.; Shukla, S.K.; Agorku, E.S.; Govender, P.P. Photocatalytic degradation of indigo carmine using Nd-doped TiO2-decorated graphene oxide nanocomposites. J. Sol.-Gel. Sci. Technol. 2016, 80, 38–49. [Google Scholar] [CrossRef]
- Sehrawat, P.; Rana, S.; Mehta, S.K.; Kansal, S.K. Optimal synthesis of MoS2/Cu2O nanocomposite to enhance photocatalytic performance towards indigo carmine dye degradation. Appl. Surf. Sci. 2022, 604, 17. [Google Scholar] [CrossRef]
- Janbandhu, S.Y.; Suhaila, C.T.; Munishwar, S.R.; Jayaramaiah, J.R.; Gedam, R.S. Borosilicate glasses containing CdS/ZnS QDs: A heterostructured composite with enhanced degradation of IC dye under visible-light. Chemosphere 2022, 286, 11. [Google Scholar] [CrossRef]




| Photocatalyst | Organic Dyes | Light Source | Concentration of Dyes | Degradation Efficiency | Reaction Time | Reference |
|---|---|---|---|---|---|---|
| graphene oxide (10%)–nano-titania | IC | Visible light | 10 mg/L | 98% | 60 min | [44] |
| Fe (2 mol%) doped TiO2 | IC | 100 W fluorescent bulb | 10 mg/L | 94.76% | 60 min | [32] |
| Nd (2%)-TiO2-GO (graphene oxide) | IC | Simulated solar light | 20 mg/L | 75% | 30 min | [45] |
| Bi5Ti3FeO15 | IC | Sunlight | 30 mg/L | 13% (pH = 8) | 240 min | [43] |
| N-CQDs | IC | Visible light | 30 mg/L | 97% | 240 min | [12] |
| N-CQDs | IC | Natural light | 50 mg/L | 99.13% | 15 day | This work |
| Cl-CQDs | IC | Natural light | 50 mg/L | 97.97% | 15 day | This work |
| UV/IO3− | LG SF | Low-pressure mercury lamp (15 mW cm−2) | 10 mg/L | 79% (0.5 mM IO3−) | 10 min | [35] |
| 93% (1 mM IO3−) | ||||||
| 98% (10 mM IO3−) | ||||||
| 20 mg/L | 43% (0.5 mM IO3−) | |||||
| 60% (1 mM IO3−) | ||||||
| 85% (10 mM IO3−) | ||||||
| N-CQDs | LG SF | Natural light | 50 mg/L | 94.05% | 10 min | This work |
| Cl-CQDs | LG SF | Natural light | 50 mg/L | 97.27% | 10 min | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Zhang, Y.; Cheng, Y.; Chen, J.; Li, F. Photoluminescence Performance and Photocatalytic Activity of Modified Carbon Quantum Dots Derived from Pluronic F127. Polymers 2023, 15, 850. https://doi.org/10.3390/polym15040850
Liu L, Zhang Y, Cheng Y, Chen J, Li F. Photoluminescence Performance and Photocatalytic Activity of Modified Carbon Quantum Dots Derived from Pluronic F127. Polymers. 2023; 15(4):850. https://doi.org/10.3390/polym15040850
Chicago/Turabian StyleLiu, Linlin, Yue Zhang, Youliang Cheng, Jing Chen, and Fengjuan Li. 2023. "Photoluminescence Performance and Photocatalytic Activity of Modified Carbon Quantum Dots Derived from Pluronic F127" Polymers 15, no. 4: 850. https://doi.org/10.3390/polym15040850






