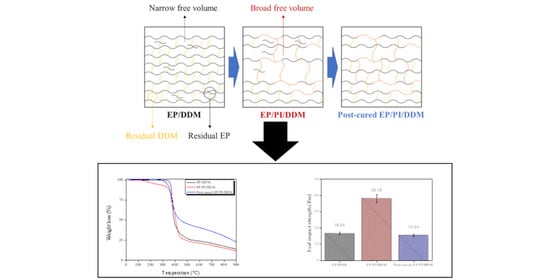
Thermal and Mechanical Characterization of Epoxy/Polyimide Blends via Postcuring Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Thermal Analysis of Epoxy/Polyimide
2.4. Thermomechanical Properties of EPI
2.5. Mechanical Properties of EPI
3. Results and Discussion
3.1. DSC Results of EPI
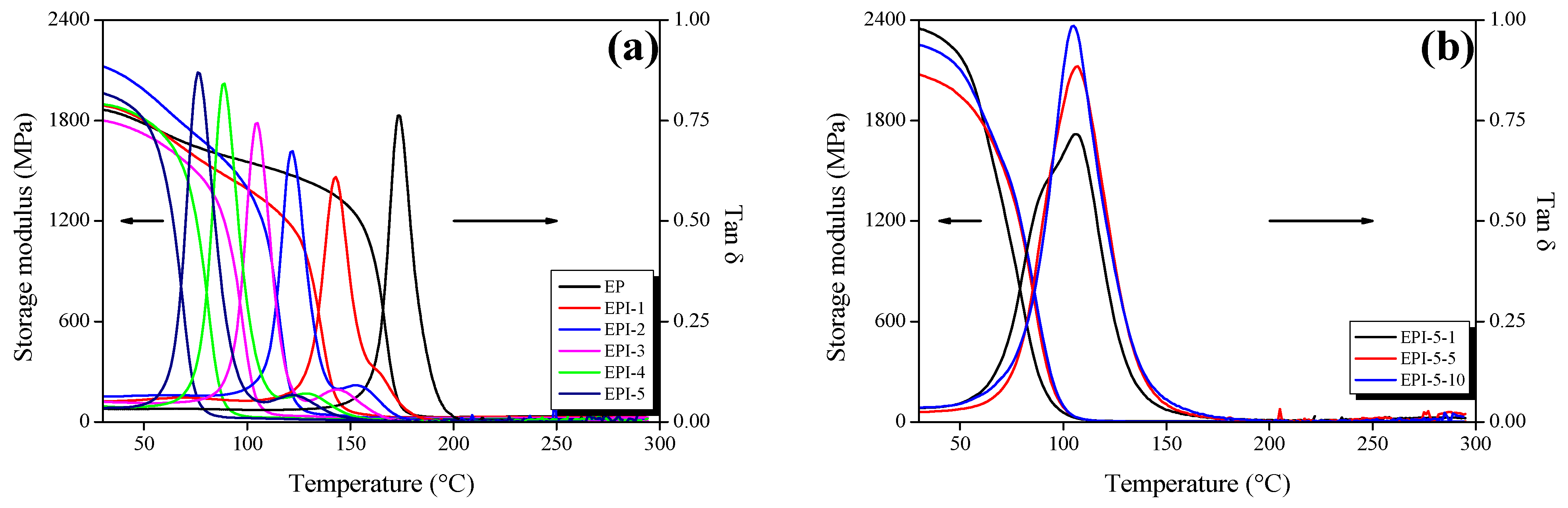
3.2. Crosslinking Density of EPI
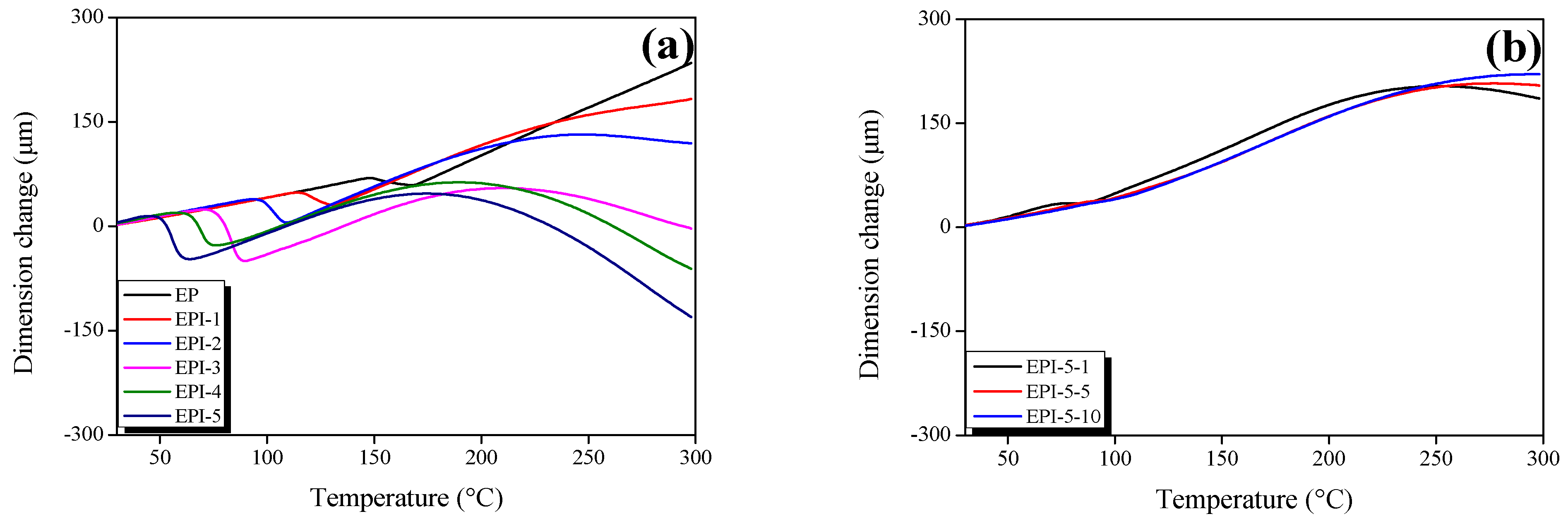
3.3. Thermal Expansion Coefficients of EPI
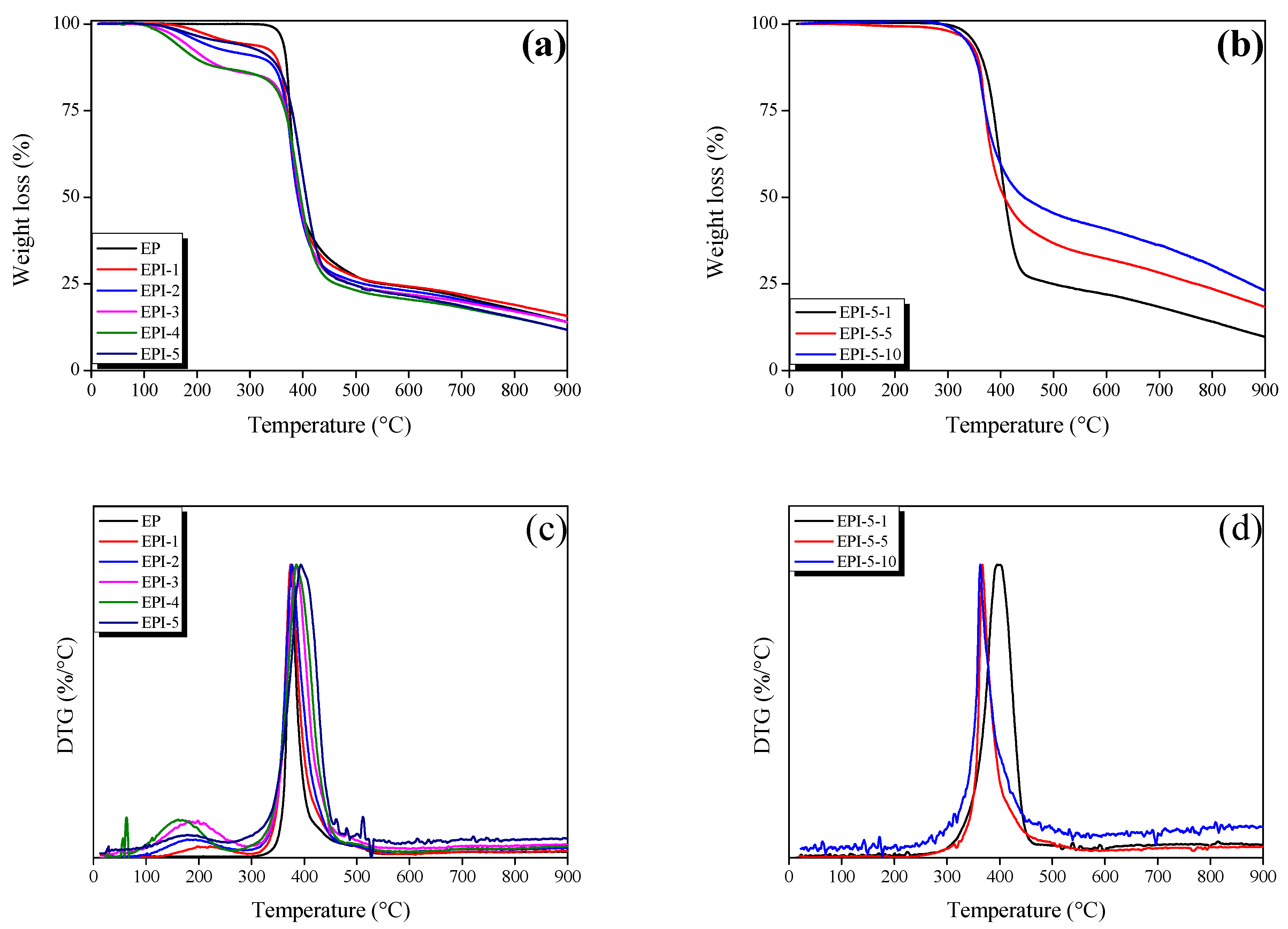
3.4. Thermal Stability of EPI
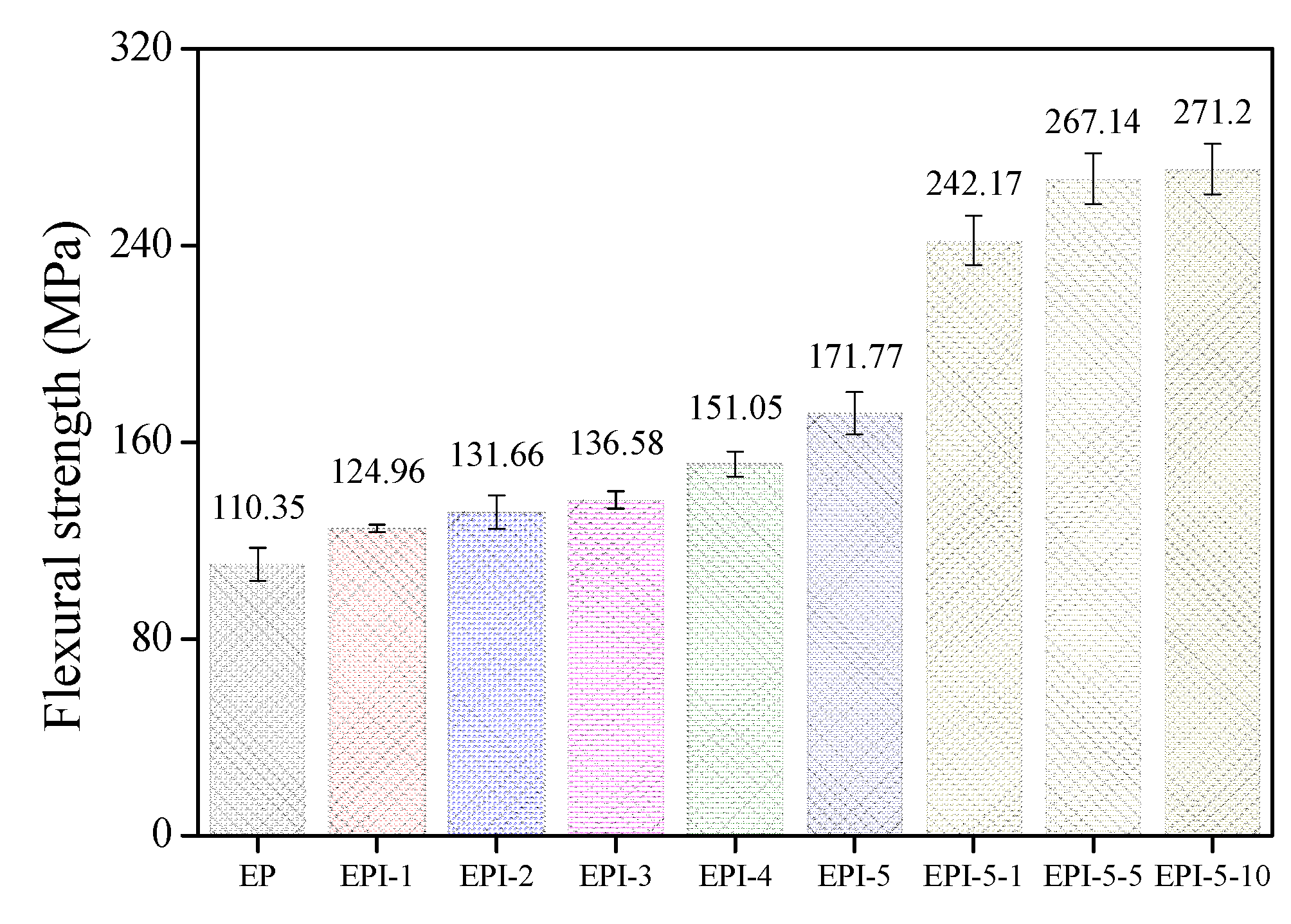
3.5. Mechanical Properties of EPI
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, D.K.; Kim, Y.E.; Kwon, O.H.; Park, W.H.; Cho, D.H. Carbon fiber coating with MWCNT in the presence of polyethyleneimine of different molecular weights and the effect on the interfacial shear strength of thermoplastic and thermosetting carbon fiber composites. Carbon Lett. 2021, 31, 407–417. [Google Scholar] [CrossRef]
- Wan, J.; Bu, Z.Y.; Xu, C.J.; Li, B.G.; Fan, H. Preparation, curing kinetics, and properties of a novel low-volatile starlike aliphatic-polyamine curing agent for epoxy resins. Chem. Eng. J. 2011, 171, 357–367. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, H.Y.; Han, M.J.; Hong, S.K. Thermal and mechanical interfacial properties of the DGEBA/PMR-15 blend system. J. Colloid Interface Sci. 2004, 270, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Kim, D.K.; Kim, B.S.; An, K.H.; Park, S.J.; Rhee, K.Y.; Kim, B.J. Cure behaviors and mechanical properties of carbon fiber-reinforced nylon6/epoxy blended matrix composites. Compos. B Eng. 2017, 112, 15–21. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, S.G. Studies on Surface Free Energy of an Anhydride–Epoxy Cured System: Effect of Side Alkenyl Chain Length of Hardener on Tensile and Impact Properties. J. Colloid Interface Sci. 2000, 228, 90–94. [Google Scholar] [CrossRef]
- Pandit, J.A.; Athawale, A.A. Epoxy-Polyester IPNs modified with aromatic amines. J. Appl. Polym. Sci. 2012, 125, 836–843. [Google Scholar] [CrossRef]
- Zurina, M.; Ismail, H.; Ratnam, C.T. The effect of HVA-2 on properties of irradiated epoxidized natural rubber (ENR-50), ethylene vinyl acetate (EVA), and ENR-50/EVA blend. Polym. Test. 2008, 27, 480–490. [Google Scholar] [CrossRef]
- Polypetchara, N.; Suppakul, P.; Atong, D.; Pechyen, C. Blend of polypropylene/poly(lactic acid) for medical packaging application: Physicochemical, thermal, mechanical, and barrier properties. Energy Procedia 2014, 56, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Kunz, S.C.; Sayre, J.A.; Assink, R.A. Morphology and toughness characterization of epoxy resins modified with amine and carboxyl terminated rubbers. Polymer 1982, 23, 1897–1906. [Google Scholar] [CrossRef]
- Tripathi, G.; Srivastava, D. Effect of carboxyl-terminated poly(butadiene-co-acrylonitrile) (CTBN) concentration on thermal and mechanical properties of binary blends of diglycidyl ether of bisphenol-A (DGEBA) epoxy resin. Mater. Sci. Eng. C 2007, 443, 262–269. [Google Scholar] [CrossRef]
- Levita, G.; Marchetti, A.; Butta, E. Influence of the temperature of cure on the mechanical properties of ATBN/epoxy blends. Polymer 1985, 26, 1110–1116. [Google Scholar] [CrossRef]
- Huang, P.; Zheng, S.; Huang, J.; Guo, Q. Miscibility and mechanical properties of epoxy resin/polysulfone blends. Polymer 1997, 38, 5565–5571. [Google Scholar] [CrossRef]
- Pandit, J.A.; Sudarshan, K.; Athawale, A.A. Electrically conductive epoxy-polyester-graphite nanocomposites modified with aromatic amines. Polymer 2016, 104, 49–60. [Google Scholar] [CrossRef]
- Kim, D.K.; Kim, K.W.; Han, W.; Kwac, L.K.; Kim, B.J. Studies on Cure Kinetics and Thermal Stability of Epoxy/Nylon 6 Blend. Appl. Chem. Eng. 2015, 26, 538–542. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.C.; Kim, J.K.; Lim, S.H.; Jo, W.H.; Choe, C.R. Effects of mixing temperatures on the morphology and toughness of epoxy/polyamide blends. J. Appl. Polym. Sci. 1999, 72, 1055–1063. [Google Scholar] [CrossRef]
- Reydet, E.G.; Vicard, V.; Pascault, J.P.; Sautereau, H. Polyetherimide-Modified Epoxy Networks: Influence of Cure Conditions on Morphology and Mechanical Properties. J. Appl. Polym. Sci. 1997, 65, 2433–2445. [Google Scholar] [CrossRef]
- Bonnet, A.; Lestriez, B.; Pascault, J.P.; Sautereau, H. Intractable High-Tg Thermoplastics Processed with Epoxy Resin: Interfacial Adhesion and Mechanical Properties of the Cured Blends. J. Polym. Sci. B 2001, 39, 363–373. [Google Scholar] [CrossRef]
- Barral, L.; Cano, J.; Lopez, J.; Bueno, I.L.; Nogueira, P.; Ramirez, C.; Torres, A.; Abad, M.J. Thermal properties of amine cured diglycidyl ether of bisphenol A epoxy blended with poly(ether imide). Thermochim. Acta 2000, 344, 137–143. [Google Scholar] [CrossRef]
- Lee, J.H. Polymer Materials Chemistry for IT in the 21st Century; Samkwang: Seoul, Republic of Korea, 2001; pp. 150–152. [Google Scholar]
- Kalita, D.J.; Tarnavchyk, I.; Chisholm, B.J.; Webster, D.C. Novel bio-based epoxy resins from eugenol as an alternative to BPA epoxy and high throughput screening of the cured coatings. Polymer 2021, 233, 124191. [Google Scholar] [CrossRef]
- Gaw, K.; Jikei, M.; Kakimoto, M.A.; Imai, Y. Preparation of polyimide-epoxy composites. React. Funct. Polym. 1996, 30, 85–91. [Google Scholar] [CrossRef]
- Gaw, K.; Jikei, M.; Kakimoto, M.A.; Imai, Y. Adhesion behavior of polyamic acid cured epoxy. Polymer 1997, 38, 4413–4415. [Google Scholar] [CrossRef]
- Agag, T.; Takeichi, T. Synthesis and characterization of epoxy film cured with reactive polyimide. Polymer 1999, 40, 6557–6563. [Google Scholar] [CrossRef]
- Doyle, C.D. Estimating Thermal Stability of Experimental Polymers by Empirical Thermogravimetric Analysis. Anal. Chem. 1961, 33, 77–79. [Google Scholar] [CrossRef]
- Menczel, J.D.; Prime, R.B. Thermal Analysis of Polymers; Fundamentals and Applications; Wiley: New York, NY, USA, 2009; pp. 387–491. [Google Scholar]
- Flory, P.J. Molecular theory of rubber elasticity. Polymer 1979, 20, 1317–1320. [Google Scholar] [CrossRef]
- ASTM Standard D790, 2017; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM International: West Conshohocken, PA, USA, 2017. [CrossRef]
- ASTM Standard D256, 2010; Standard Test Methods for Determining the Izod Pendulum Impact Resistance of Plastics. ASTM International: West Conshohocken, PA, USA, 2018, 2018. [CrossRef]
- Brostow, W.; Lobland, E.H. Sliding wear, viscoelasticity, and brittleness of polymers. J. Mater. Res. 2006, 21, 2422–2428. [Google Scholar] [CrossRef] [Green Version]
- Brostow, W.; Lobland, H.E.H. Brittleness of materials: Implications for composites and a relation to impact strength. J. Mater. Sci. 2010, 45, 242–250. [Google Scholar] [CrossRef]
- Feng, Q.; Yang, J.; Liu, Y.; Xiao, H.; Fu, S. Simultaneously Enhanced Cryogenic Tensile Strength, Ductility and Impact Resistance of Epoxy Resins by Polyethylene Glycol. J. Mater. Sci. Technol. 2014, 30, 90–96. [Google Scholar] [CrossRef]
- Hagen, R.; Salmen, L.; Stenberg, B. Effects of the Type of Crosslink on Viscoelastic Properties of Natural Rubber. J. Polym. Sci. B Polym. Phys. 1996, 34, 1997–2006. [Google Scholar] [CrossRef]

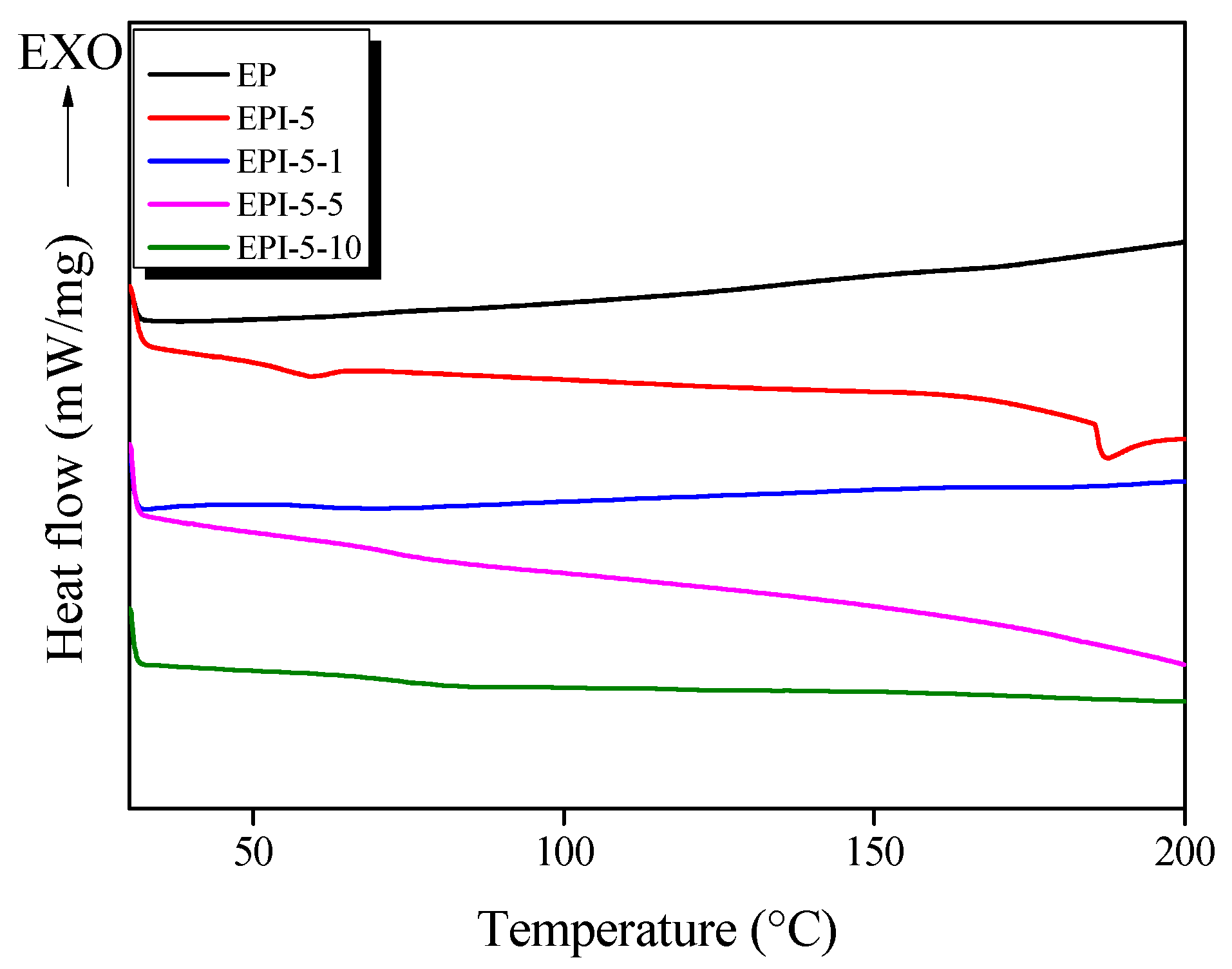

| Nomenclature | Tg (°C) | * Ts (°C) |
|---|---|---|
| EP | 160.71 | - |
| EPI-5 | 55.67 | 187.47 |
| EPI-5-1 | 59.27 | - |
| EPI-5-5 | 72.66 | - |
| EPI-5-10 | 74.52 | - |
| Sample | Crosslinking Density (103 mol/m3) | Tg (°C) |
|---|---|---|
| EP | 2.46 | 173.47 |
| EPI-1 | 2.40 | 142.77 |
| EPI-2 | 2.05 | 121.47 |
| EPI-3 | 1.71 | 104.52 |
| EPI-4 | 1.33 | 88.56 |
| EPI-5 | 1.18 | 76.32 |
| EPI-5-1 | 2.45 | 105.85 |
| EPI-5-5 | 2.67 | 106.30 |
| EPI-5-10 | 3.76 | 104.49 |
| Sample | Before Tg CTE (10−6 μm/μm·°C) | After Tg CTE (10−6 μm/μm·°C) | * Ts (°C) |
|---|---|---|---|
| EP | 68.27 | 169.22 | - |
| EPI-1 | 67.71 | 113.23 | 224.52 |
| EPI-2 | 67.09 | 75.43 | 215.60 |
| EPI-3 | 65.20 | 27.96 | 206.30 |
| EPI-4 | 65.26 | −18.90 | 202.23 |
| EPI-5 | 72.29 | −44.57 | 186.30 |
| EPI-5-1 | 98.44 | 188.30 | 235.13 |
| EPI-5-5 | 81.16 | 181.40 | 245.15 |
| EPI-5-10 | 74.44 | 174.40 | 248.84 |
| Sample | 1 IDT (°C) | 2 Tmax (°C) | 3 A*·K* | 4 IPDT (°C) |
|---|---|---|---|---|
| EP | 361.89 | 374.54 | 0.6313 | 639.07 |
| EPI-1 | 263.42 | 374.14 | 0.6627 | 667.39 |
| EPI-2 | 203.03 | 376.78 | 0.6147 | 619.51 |
| EPI-3 | 172.84 | 385.21 | 0.6090 | 614.46 |
| EPI-4 | 155.23 | 385.33 | 0.5552 | 560.94 |
| EPI-5 | 245.81 | 393.78 | 0.5785 | 584.13 |
| EPI-5-1 | 348.46 | 398.63 | 0.6602 | 598.94 |
| EPI-5-5 | 339.41 | 367.33 | 0.8517 | 769.53 |
| EPI-5-10 | 336.12 | 363.32 | 0.9948 | 895.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-M.; Kim, K.-W.; Kim, B.-J. Thermal and Mechanical Characterization of Epoxy/Polyimide Blends via Postcuring Process. Polymers 2023, 15, 1072. https://doi.org/10.3390/polym15051072
Lee Y-M, Kim K-W, Kim B-J. Thermal and Mechanical Characterization of Epoxy/Polyimide Blends via Postcuring Process. Polymers. 2023; 15(5):1072. https://doi.org/10.3390/polym15051072
Chicago/Turabian StyleLee, Yong-Min, Kwan-Woo Kim, and Byung-Joo Kim. 2023. "Thermal and Mechanical Characterization of Epoxy/Polyimide Blends via Postcuring Process" Polymers 15, no. 5: 1072. https://doi.org/10.3390/polym15051072