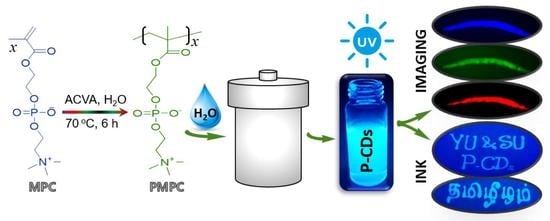
Water Soluble PMPC-Derived Bright Fluorescent Nitrogen/Phosphorous-Doped Carbon Dots for Fluorescent Ink (Anti-Counterfeiting) and Cellular Multicolor Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Poly 2-(methacryloyloxy)ethyl Phosphorylcholine
2.3. Synthesis of Nitrogen/Phosphorous-Doped Carbon Dots
3. Results and Discussion
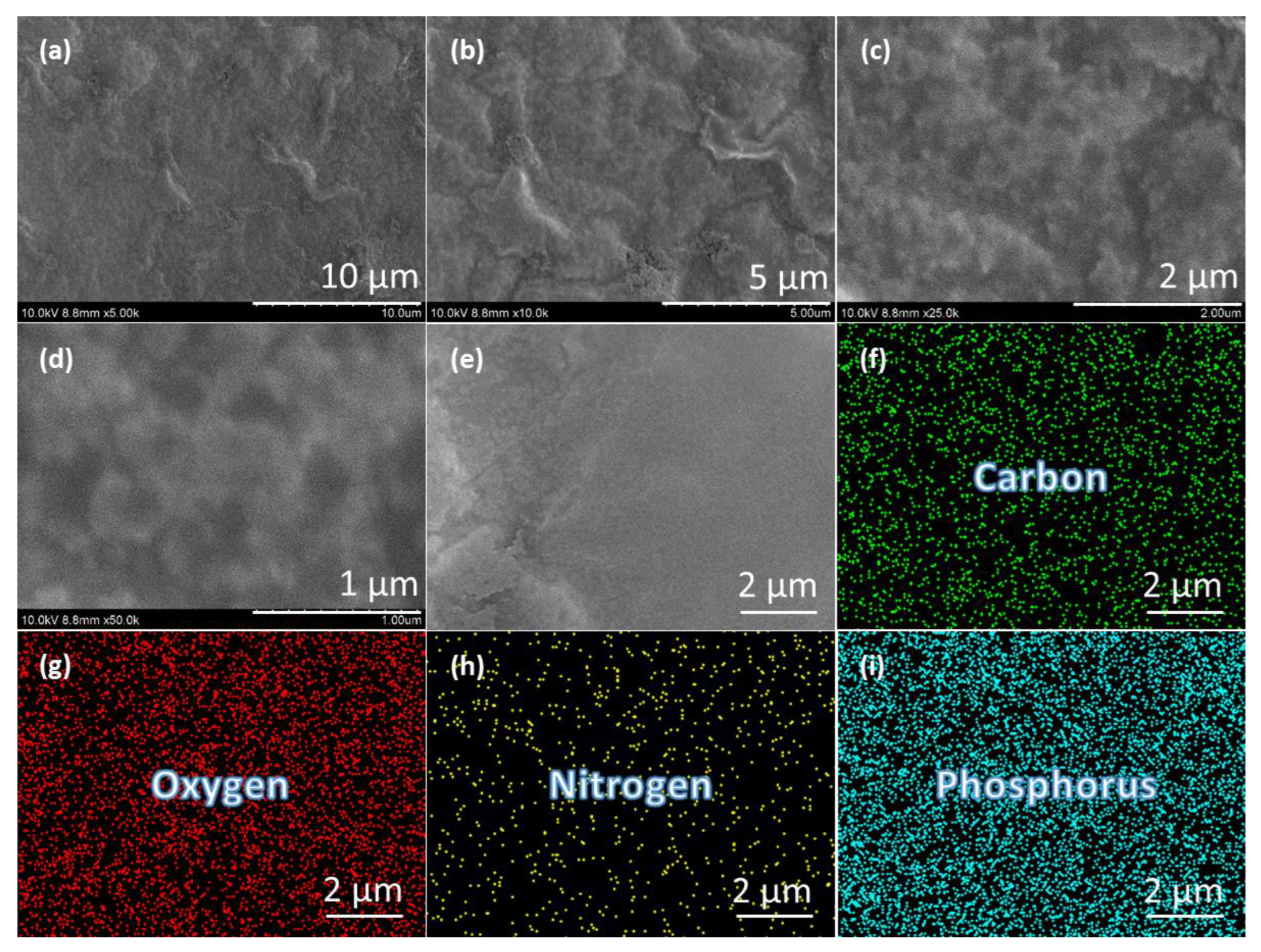
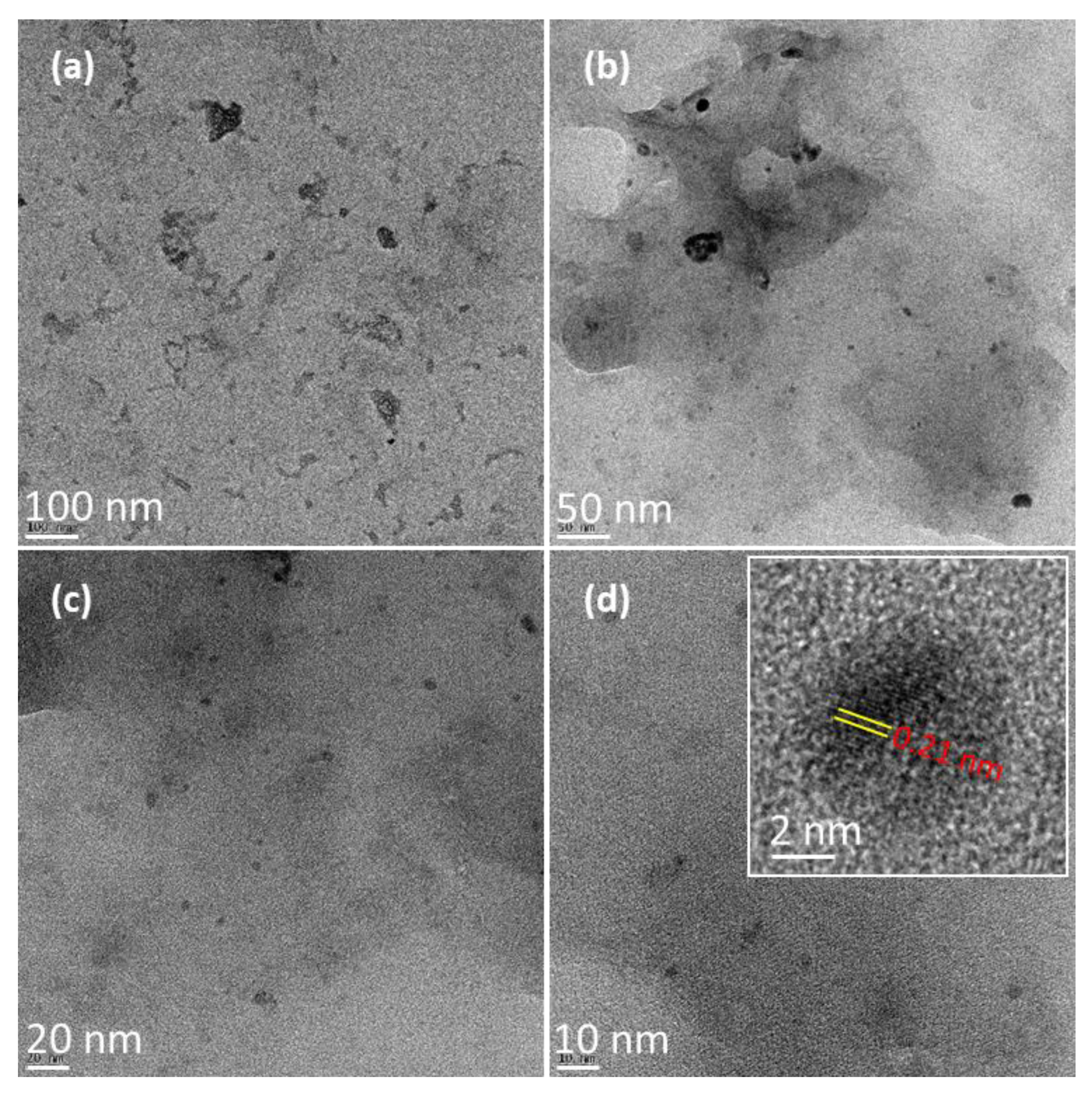
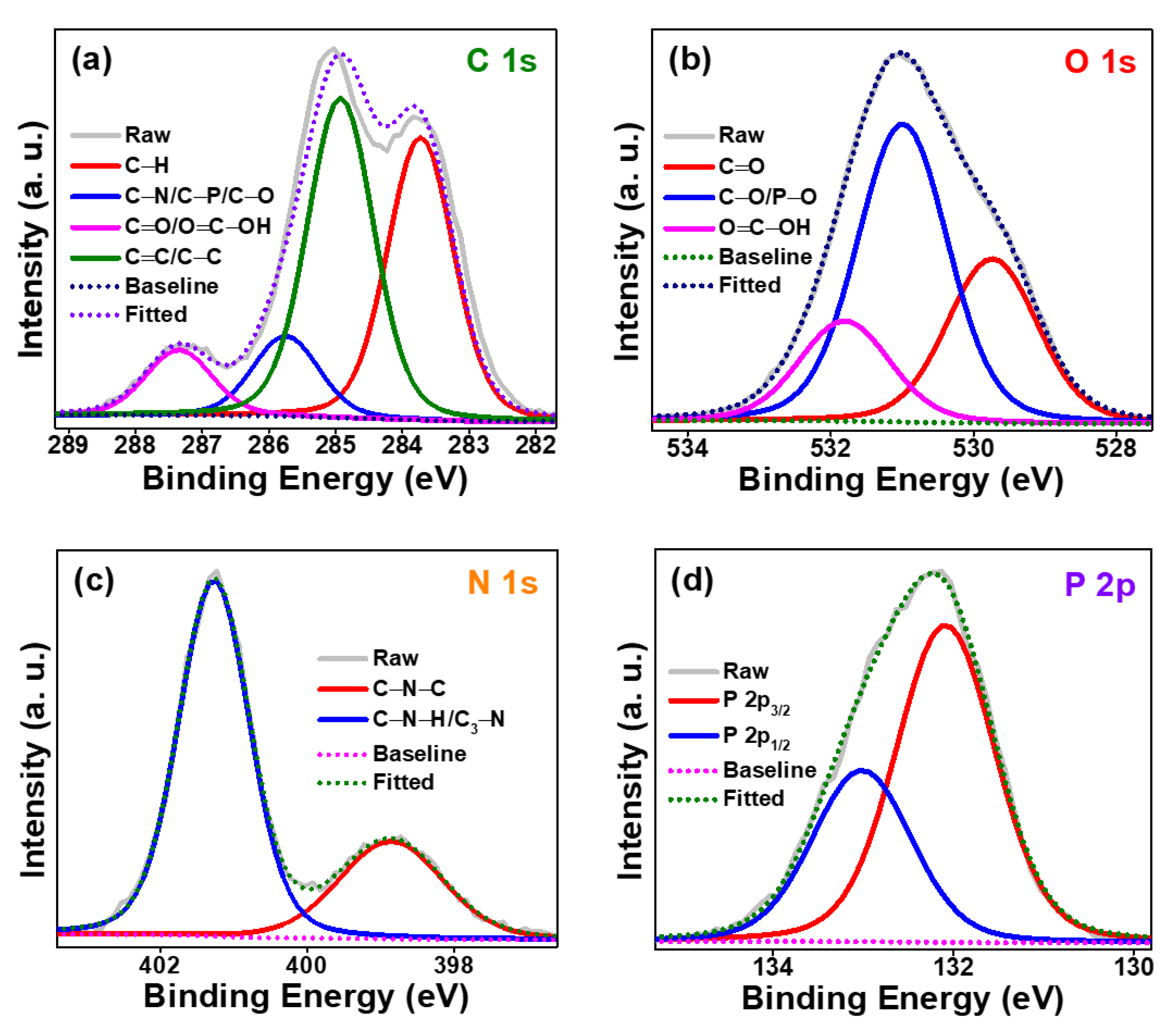
3.1. Structural Studies of Synthesized P-CDs
3.2. Optical Studies of Synthesized P-CDs
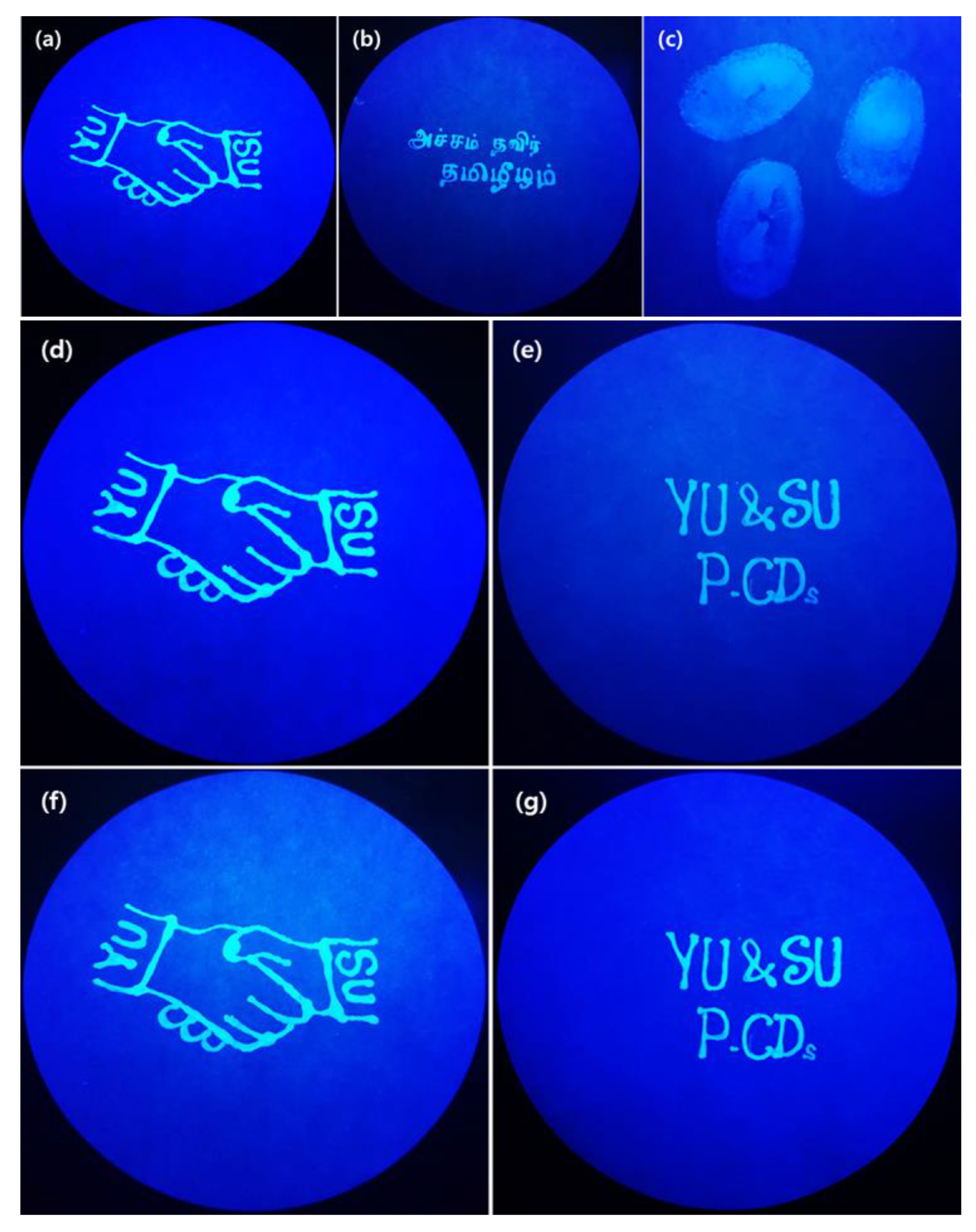
3.3. Fluorescent Ink and Anti-Counterfeiting Studies of Synthesized P-CDs
3.4. Biological Studies of Synthesized P-CDs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Anand, S.R.; Bhati, A.; Saini, D.; Gunture Chauhan, N.; Khare, P.; Sonkar, S.K. Antibacterial Nitrogen-doped Carbon Dots as a Reversible “Fluorescent Nanoswitch” and Fluorescent Ink. ACS Omega 2019, 4, 1581–1591. [Google Scholar] [CrossRef]
- Zhang, X.D.; Li, J.; Niu, J.N.; Bao, X.P.; Zhao, H.D.; Tan, M. Fluorescent carbon dots derived from urine and their application for bio-imaging. Methods 2019, 168, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Rawat, K.S.; Mishra, S.; Baghel, T.; Fatima, S.; John, A.A.; Kalleti, N.; Singh, D.; Nazir, A.; Rath, S.K.; et al. Biocompatible fluorescent carbon quantum dots prepared from beetroot extract for in vivo live imaging in C. elegans and BALB/c mice. J. Mater. Chem. B 2018, 6, 3366–3371. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Cui, L.; Ren, X.; Sun, M.; Liu, H.; Xia, L. Carbon Dots: Synthesis, Properties and Applications. Nanomaterials 2021, 11, 3419. [Google Scholar] [CrossRef]
- Dehvari, K.; Chiu, S.-H.; Lin, J.-S.; Girma, W.M.; Ling, Y.-C.; Chang, J.-Y. Heteroatom doped carbon dots with nanoenzyme like properties as theranostic platforms for free radical scavenging, imaging, and chemotherapy. Acta Biomater. 2020, 114, 343–357. [Google Scholar] [CrossRef]
- Supjaroenpisan, M.; Hanchaina, R.; Kangsamaksin, T.; Paoprasert, P. Effects of Heteroatom Doping of Carbon Dots from Sugar on Optical Properties, Phenolic Content, Antioxidant Activity, Photostability, and Cytotoxicity. ChemistrySelect 2021, 6, 3597–3604. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, H.; Hu, K.; Nie, G.; Yang, Y.; Wang, Y.; Duan, X.; Wang, S. Carbon dots based photocatalysis for environmental applications. J. Environ. Chem. Eng. 2022, 10, 107336. [Google Scholar] [CrossRef]
- Akbar, E.; Moretti, A. Vomiero, Carbon Dots for Photocatalytic Degradation of Aqueous Pollutants: Recent Advancements. Adv. Opt. Mater. 2021, 9, 2100532. [Google Scholar] [CrossRef]
- Saini, D.; Garg, A.K.; Dalal, C.; Anand, S.R.; Sonkar, S.K.; Sonker, A.K.; Westman, G. Visible-Light-Promoted Photocatalytic Applications of Carbon Dots: A Review. ACS Appl. Nano Mater. 2022, 5, 3087–3109. [Google Scholar] [CrossRef]
- Torres, S.; Bogireddy, N.K.; Kaur, I.; Batra, V.; Agarwal, V. Heavy metal ion detection using green precursor derived carbon dots. iScience 2022, 25, 103816. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-J.; Ge, S.; Li, D.-Q.; Xu, Z.-Q.; Wang, E.-J.; Wang, S.-M. Fluorescent carbon dots for sensing metal ions and small molecules. Chin. J. Anal. Chem. 2022, 50, 103–111. [Google Scholar] [CrossRef]
- Batool, M.; Junaid, H.M.; Tabassum, S.; Kanwal, F.; Abid, K.; Fatima, Z.; Shah, A.T. Metal Ion Detection by Carbon Dots—A Review. Crit. Rev. Anal. Chem. 2022, 52, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Zhang, B.; Shi, R.; Zhang, S.; Liu, Y.; Wang, B.; Zhang, K.; Waterhouse, G.I.; Zhang, T.; Lu, S. Carbon Dots as New Building Blocks for Electrochemical Energy Storage and Electrocatalysis. Adv. Energy Mater. 2022, 12, 2103426. [Google Scholar] [CrossRef]
- Siwal, S.S.; Kaur, H.; Saini, A.K.; Thakur, V.K. Recent Progress in Carbon Dots-Based Materials for Electrochemical Energy Storage Toward Environmental Sustainability. Adv. Energy Sustain. Res. 2022, 3, 2200062. [Google Scholar] [CrossRef]
- Wang, B.; Cai, H.; Waterhouse, G.I.N.; Qu, X.; Yang, B.; Lu, S. Carbon Dots in Bioimaging, Biosensing and Therapeutics: A Comprehensive Review. Small Sci. 2022, 2, 2200012. [Google Scholar] [CrossRef]
- Shen, C.-L.; Liu, H.-R.; Lou, Q.; Wang, F.; Liu, K.-K.; Dong, L.; Shan, C.-X. Recent progress of carbon dots in targeted bioimaging and cancer therapy. Theranostics 2022, 12, 2860–2893. [Google Scholar] [CrossRef]
- Korah, B.K.; John, N.; John, B.K.; Mathew, S.; Bijimol, D.; Mathew, B. Carbon dots as a fluorescent ink and dual-mode probe for the efficient detection of doxycycline and Hg(II) ions. J. Mater. Res. 2022, 37, 3060–3070. [Google Scholar] [CrossRef]
- Al-Qahtani, S.D.; Hameed, A.; Snari, R.M.; Shah, R.; Alfi, A.A.; El-Metwaly, N.M. Development of fluorescent carbon dots ink from rice straw waste toward security authentication. J. Mol. Liq. 2022, 354, 118927. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Hoffman, J.; Morgiel, J.; Mościcki, T.; Stobiński, L.; Szymański, Z.; Małolepszy, A. Luminescent Carbon Dots Synthesized by the Laser Ablation of Graphite in Polyethylenimine and Ethylenediamine. Materials 2021, 14, 729. [Google Scholar] [CrossRef]
- Ma, Z.; Ming, H.; Huang, H.; Liu, Y.; Kang, Z. One-step ultrasonic synthesis of fluorescent N-doped carbon dots from glucose and their visible-light sensitive photocatalytic ability. New J. Chem. 2012, 36, 861–864. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Liu, Y.; Huang, H.; Lian, S.; Lee, S.-T.; Kang, Z. One-step ultrasonic synthesis of water-soluble carbon nanoparticles with excellent photoluminescent properties. Carbon 2011, 49, 605–609. [Google Scholar] [CrossRef]
- Chao-Mujica, F.J.; Garcia-Hernández, L.; Camacho-López, S.; Camacho-López, M.; Camacho-López, M.A.; Reyes Contreras, D.; Pérez-Rodríguez, A.; Peña-Caravaca, J.P.; Páez-Rodríguez, A.; Darias-Gonzalez, J.G.; et al. Carbon quantum dots by submerged arc discharge in water: Synthesis, characterization, and mechanism of formation. J. Appl. Phys. 2021, 129, 163301. [Google Scholar] [CrossRef]
- Ismail, N.S.; Husain, U.S.; Selvan, S.I.S.; Mordani, N.A.; Juhari, N.; Halim, N.H.A. Effect of heating power towards synthesis of carbon dots through microwave pyrolysis method for optical-based biosensor. AIP Conf. Proc. 2020, 2203, 020057. [Google Scholar]
- Otten, M.; Hildebrandt, M.; Kühnemuth, R.; Karg, M. Pyrolysis and Solvothermal Synthesis for Carbon Dots: Role of Purification and Molecular Fluorophores. Langmuir 2022, 38, 6148–6157. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Fu, Y.; Yu, H.; Liu, S.; Yu, S.; Liu, Y.; Wu, Q.; Liu, Y.; Nie, G.; Xu, H.; et al. Efficient ethanol solvothermal synthesis of high-performance nitrogen-doped carbon quantum dots from lignin for metal ion nanosensing and cell imaging. Ind. Crops Prod. 2022, 183, 114957. [Google Scholar] [CrossRef]
- Kurian, M.; Paul, A. Recent trends in the use of green sources for carbon dot synthesis–A short review. Carbon Trends 2021, 3, 100032. [Google Scholar] [CrossRef]
- Ayiloor Rajesh, G.; John, V.L.; Pookunnath Santhosh, A.; Krishnan Nair Ambika, A.; Thavarool Puthiyedath, V. Carbon Dots from Natural Sources for Biomedical Applications. Part. Part. Syst. Charact. 2022, 39, 2200017. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, G.; Zhang, Z.; Lei, J.H.; Liu, T.-M.; Xing, G.; Deng, C.-X.; Tang, Z.; Qu, S. One step synthesis of efficient red emissive carbon dots and their bovine serum albumin composites with enhanced multi-photon fluorescence for in vivo bioimaging. Light Sci. Appl. 2022, 11, 113. [Google Scholar] [CrossRef]
- Rabiee, N.; Iravani, S.; Varma, R.S. Biowaste-Derived Carbon Dots: A Perspective on Biomedical Potentials. Molecules 2022, 27, 6186. [Google Scholar] [CrossRef]
- Kang, C.; Huang, Y.; Yang, H.; Yan, X.F.; Chen, Z.P. A Review of Carbon Dots Produced from Biomass Wastes. Nanomaterials 2020, 10, 2316. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Li, P.; Wen, F.; Xu, Q.; Guo, Q.; Su, W. Green synthesis of carbon quantum dots from plant turmeric holds promise as novel photosensitizer for in vitro photodynamic antimicrobial activity. J. Mater. Res. Technol. 2023, 22, 17–34. [Google Scholar] [CrossRef]
- Chahal, S.; Macairan, J.-R.; Yousefi, N.; Tufenkji, N.; Naccache, R. Green synthesis of carbon dots and their applications. RSC Adv. 2021, 11, 25354–25363. [Google Scholar] [CrossRef]
- Döring, A.; Ushakova, E.; Rogach, A.L. Chiral carbon dots: Synthesis, optical properties, and emerging applications. Light Sci. Appl. 2022, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Kuang, Y.; Fan, Z.; Qin, X.; Hu, S.; Liang, Z.; Liu, Q.; Zhang, W.; Wang, B.; Su, Z. Simultaneous Electrochemical Sensing of Indole-3-Acetic Acid and Salicylic Acid on Poly(L-Proline) Nanoparticles-Carbon Dots-Multiwalled Carbon Nanotubes Composite-Modified Electrode. Sensors 2022, 22, 2222. [Google Scholar] [CrossRef]
- Wang, R.; Gu, W.; Liu, Z.; Liu, Y.; Ma, G.; Wei, J. Simple and Green Synthesis of Carbonized Polymer dots from Nylon 66 Waste Fibers and its Potential Application. ACS Omega 2021, 6, 32888–32895. [Google Scholar] [CrossRef]
- Lu, D.; Yin, K.; Hou, S.; Li, P.; Zhu, H.; Hao, J.; Li, H. AlCl3-promoted growth of alkylated carbon dots with an enhanced nonlinear optical response. J. Mater. Chem. C 2022, 10, 5576–5581. [Google Scholar] [CrossRef]
- Yin, K.; Lu, D.; Tian, W.; Zhang, R.; Yu, H.; Gorecka, E.; Pociecha, D.; Godbert, N.; Hao, J.; Li, H. Ordered structures of alkylated carbon dots and their applications in nonlinear optics. J. Mater. Chem. C 2020, 8, 8980–8991. [Google Scholar] [CrossRef]
- Lu, D.; Yin, K.; Zhao, Y.; Gao, Z.; Godbert, N.; Li, H.; Li, H.; Hao, J. Facile synthesis of alkylated carbon dots with blue emission in halogenated benzene solvents. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126129. [Google Scholar] [CrossRef]
- Jorns, M.; Strickland, S.; Mullins, M.; Pappas, D. Improved citric acid-derived carbon dots synthesis through microwave-based heating in a hydrothermal pressure vessel. RSC Adv. 2022, 12, 32401–32414. [Google Scholar]
- Paloncýová, M.; Langer, M.; Otyepka, M. Structural Dynamics of Carbon Dots in Water and N, N-Dimethylformamide Probed by All-Atom Molecular Dynamics Simulations. J. Chem. Theory Comput. 2018, 14, 2076–2083. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Feng, N.; Yin, K.; Li, H.; Hao, J. Supramolecular structures from structurally persistent and surface active carbon dots in water. Nanoscale 2023, 15, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Siahcheshm, P.; Heiden, P. High quantum yield carbon quantum dots as selective fluorescent turn-off probes for dual detection of Fe2+/Fe3+ ions. J. Photochem. Photobiol. A Chem. 2023, 435, 114284. [Google Scholar] [CrossRef]
- Tao, S.; Song, Y.; Zhu, S.; Shao, J.; Yang, B. A new type of polymer carbon dots with high quantum yield: From synthesis to investigation on fluorescence mechanism. Polymer 2017, 116, 472–478. [Google Scholar] [CrossRef] [Green Version]
- Seetasang, S.; Xu, Y. Recent progress and perspectives in applications of 2-methacryloyloxyethyl phosphorylcholine polymers in biodevices at small scales. J. Mater. Chem. B 2022, 10, 2323–2337. [Google Scholar] [CrossRef] [PubMed]
- Perumal, S.; Gangadaran, P.; Bae, Y.W.; Ahn, B.C.; Cheong, I.W. Noncovalent Functionalized Graphene Nanocarriers from Graphite for Treating Thyroid Cancer Cells. ACS Biomater. Sci. Eng. 2021, 7, 2317–2328. [Google Scholar] [CrossRef]
- Hu, Q.; Gong, X.; Liu, L.; Choi, M.M.F. Characterization and Analytical Separation of Fluorescent Carbon Nanodots. J. Nanomater. 2017, 2017, 1804178. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Su, H.; Wang, K.; Wong, W.K.; Zhu, X. Facile synthesis of N-rich carbon quantum dots from porphyrins as efficient probes for bioimaging and biosensing in living cells. Int. J. Nanomed. 2017, 12, 7375–7391. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, A.F.; Tamboli, M.S.; Patil, R.H.; Bhan, A.; Ambekar, J.D.; Kale, B.B. Bioinspired Carbon Quantum Dots: An Antibiofilm Agents. J. Nanosci. Nanotechnol. 2019, 19, 2339–2345. [Google Scholar] [CrossRef]
- Rai, S.; Singh, B.K.; Bhartiya, P.; Singh, A.; Kumar, H.; Dutta, P.K.; Mehrotra, G.K. Lignin derived reduced fluorescence carbon dots with theranostic approaches: Nano-drug-carrier and bioimaging. J. Lumin. 2017, 190, 492–503. [Google Scholar] [CrossRef]
- Wu, W.; Zhan, L.; Ohkubo, K.; Yamada, Y.; Wu, M.; Fukuzumi, S. Photocatalytic H2 evolution from NADH with carbon quantum dots/Pt and 2-phenyl-4-(1-naphthyl)quinolinium ion. J. Photochem. Photobiol. B 2015, 152, 63–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edison, T.N.; Atchudan, R.; Sethuraman, M.G.; Shim, J.J.; Lee, Y.R. Microwave assisted green synthesis of fluorescent N-doped carbon dots: Cytotoxicity and bio-imaging applications. J. Photochem. Photobiol. B Biol. 2016, 161, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Manimekalai, K.; Jayaprakash, P. Influence of potassium chloride doping on the properties of potassium dihydrogen phosphate crystal for nonlinear optical applications. J. Mater. Sci. Mater. Electron. 2021, 32, 8033–8042. [Google Scholar] [CrossRef]
- Hu, Y.; Geng, X.; Zhang, L.; Huang, Z.; Ge, J.; Li, Z. Nitrogen-doped Carbon Dots Mediated Fluorescent on-off Assay for Rapid and Highly Sensitive Pyrophosphate and Alkaline Phosphatase Detection. Sci. Rep. 2017, 7, 5849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Dong, T. Photoluminescence tuning in carbon dots: Surface passivation or/and functionalization, heteroatom doping. J. Mater. Chem. C 2018, 6, 7944–7970. [Google Scholar] [CrossRef]
- Sengottuvelu, D.; Shaik, A.K.; Mishra, S.; Ahmad, H.; Abbaszadeh, M.; Hammer, N.I.; Kundu, S. Multicolor Nitrogen-Doped Carbon Quantum Dots for Environment-Dependent Emission Tuning. ACS Omega 2022, 7, 27742–27754. [Google Scholar] [CrossRef]
- Ghosh, S.; Ghosh, A.; Ghosh, G.; Marjit, K.; Patra, A. Deciphering the Relaxation Mechanism of Red-Emitting Carbon Dots Using Ultrafast Spectroscopy and Global Target Analysis. J. Phys. Chem. Lett. 2021, 12, 8080–8087. [Google Scholar] [CrossRef]
- Zuo, P.; Chen, Z.; Yu, F.; Zhang, J.; Zuo, W.; Gao, Y.; Liu, Q. An easy synthesis of nitrogen and phosphorus co-doped carbon dots as a probe for chloramphenicol. RSC Adv. 2020, 10, 32919–32926. [Google Scholar] [CrossRef]
- Gong, X.; Lu, W.; Paau, M.C.; Hu, Q.; Wu, X.; Shuang, S.; Dong, C.; Choi, M.M. Facile synthesis of nitrogen-doped carbon dots for Fe3+ sensing and cellular imaging. Anal. Chim. Acta 2015, 861, 74–84. [Google Scholar] [CrossRef]
- Shen, R.; Song, K.; Liu, H.; Li, Y.; Liu, H. Dramatic fluorescence enhancement of bare carbon dots through facile reduction chemistry. Chemphyschem 2012, 13, 3549–3555. [Google Scholar] [CrossRef]
- Chen, Y.; Xiong, G.; Zhu, L.; Huang, J.; Chen, X.; Chen, Y.; Cao, M. Enhanced Fluorescence and Environmental Stability of Red-Emissive Carbon Dots via Chemical Bonding with Cellulose Films. ACS Omega 2022, 7, 6834–6842. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gadly, T.; Gupta, A.; Ballal, A.; Ghosh, S.K.; Kumbhakar, M. Origin of Excitation Dependent Fluorescence in Carbon Nanodots. J. Phys. Chem. Lett. 2016, 7, 3695–3702. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wu, F.; Yu, J.; Deng, Q.; Zhang, F.; Wang, G. Titanium carbide (Ti3C2Tx) MXene: A novel precursor to amphiphilic carbide-derived graphene quantum dots for fluorescent ink, light-emitting composite and bioimaging. Carbon 2017, 118, 50–57. [Google Scholar] [CrossRef]
- Holden, M.A.; Cremer, P.S. Light Activated Patterning of Dye-Labeled Molecules on Surfaces. J. Am. Chem. Soc. 2003, 125, 8074–8075. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wei, P.; de Leon, A.; Frey, T.; Pentzer, E. Polymer composites with photo-responsive phthalocyanine for patterning in color and fluorescence. Eur. Polym. J. 2017, 89, 399–405. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perumal, S.; Atchudan, R.; Jebakumar Immanuel Edison, T.N.; Sangaraju, S.; Sathyaraj, W.V.; Lee, Y.R. Water Soluble PMPC-Derived Bright Fluorescent Nitrogen/Phosphorous-Doped Carbon Dots for Fluorescent Ink (Anti-Counterfeiting) and Cellular Multicolor Imaging. Polymers 2023, 15, 1352. https://doi.org/10.3390/polym15061352
Perumal S, Atchudan R, Jebakumar Immanuel Edison TN, Sangaraju S, Sathyaraj WV, Lee YR. Water Soluble PMPC-Derived Bright Fluorescent Nitrogen/Phosphorous-Doped Carbon Dots for Fluorescent Ink (Anti-Counterfeiting) and Cellular Multicolor Imaging. Polymers. 2023; 15(6):1352. https://doi.org/10.3390/polym15061352
Chicago/Turabian StylePerumal, Suguna, Raji Atchudan, Thomas Nesakumar Jebakumar Immanuel Edison, Sambasivam Sangaraju, Weslen Vedakumari Sathyaraj, and Yong Rok Lee. 2023. "Water Soluble PMPC-Derived Bright Fluorescent Nitrogen/Phosphorous-Doped Carbon Dots for Fluorescent Ink (Anti-Counterfeiting) and Cellular Multicolor Imaging" Polymers 15, no. 6: 1352. https://doi.org/10.3390/polym15061352