Luminescent Materials for Volumetric Three-Dimensional Displays Based on Photoactivated Phosphorescence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Spectroscopic Characterization
2.3. Synthesis of PtNI
2.4. Synthesis of PdBuPc
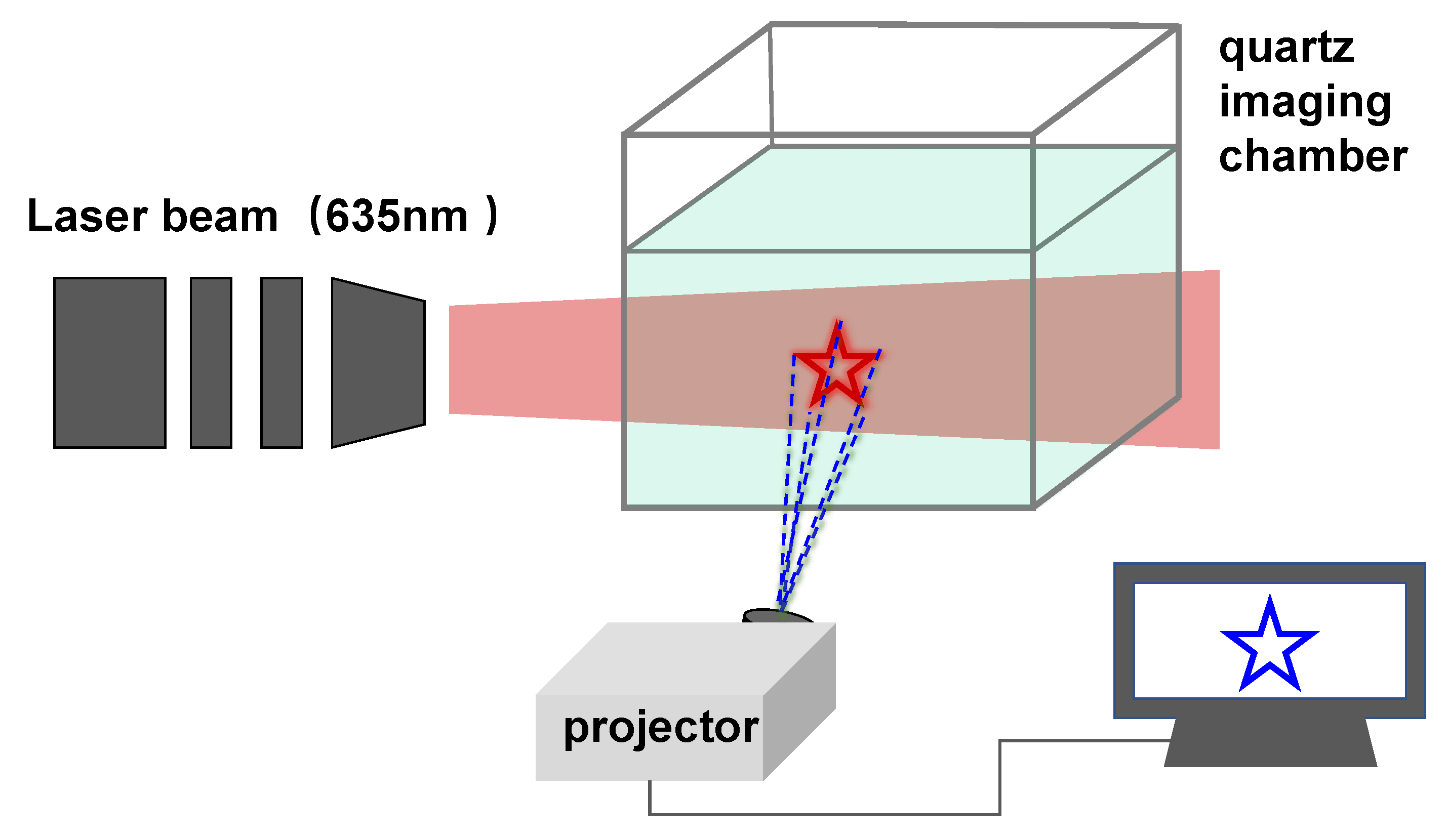
2.5. General Methods for PAP 3D Images
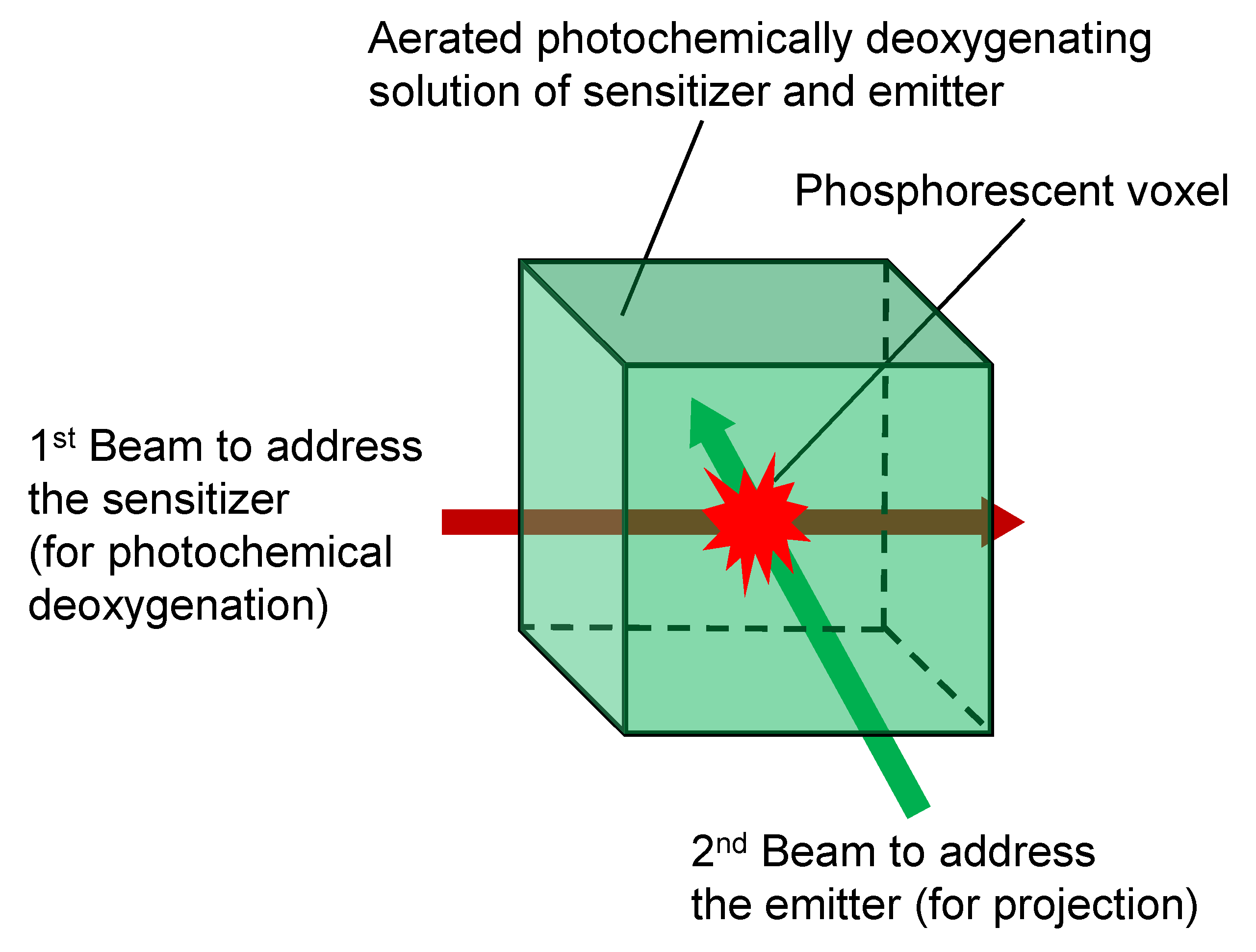
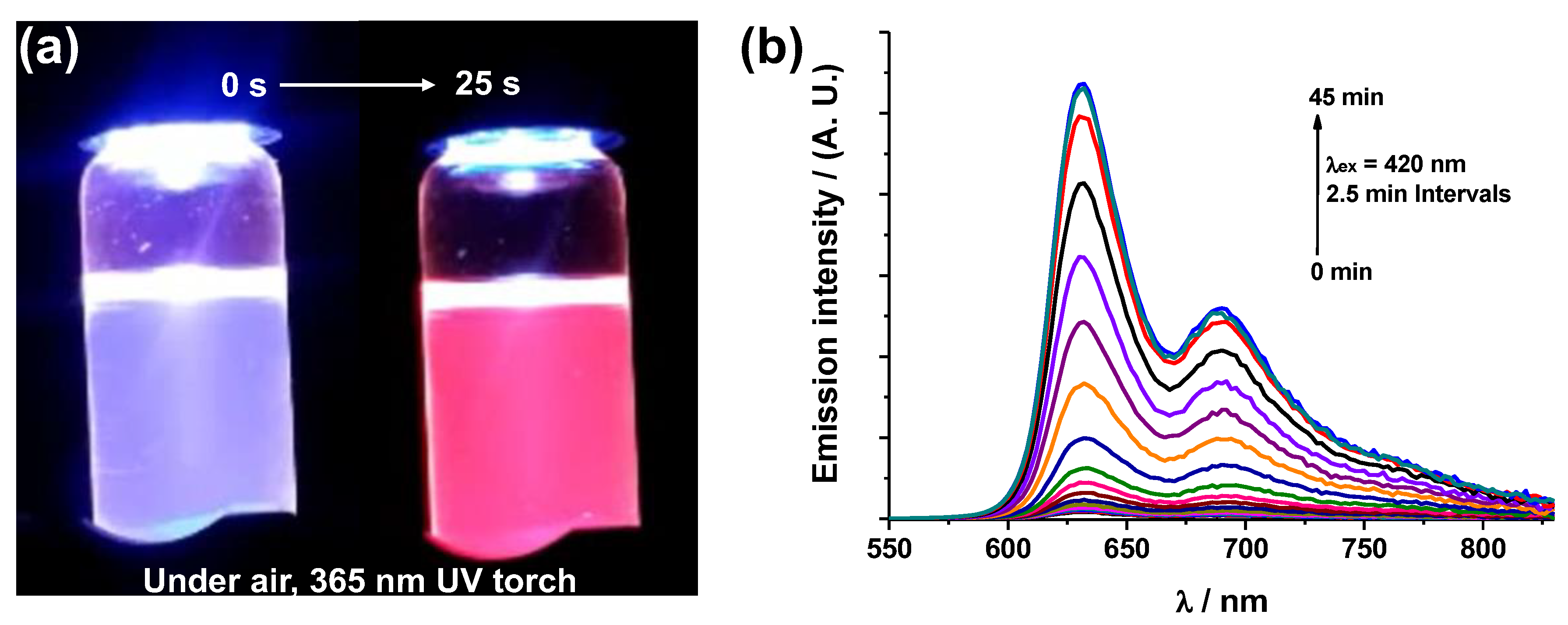
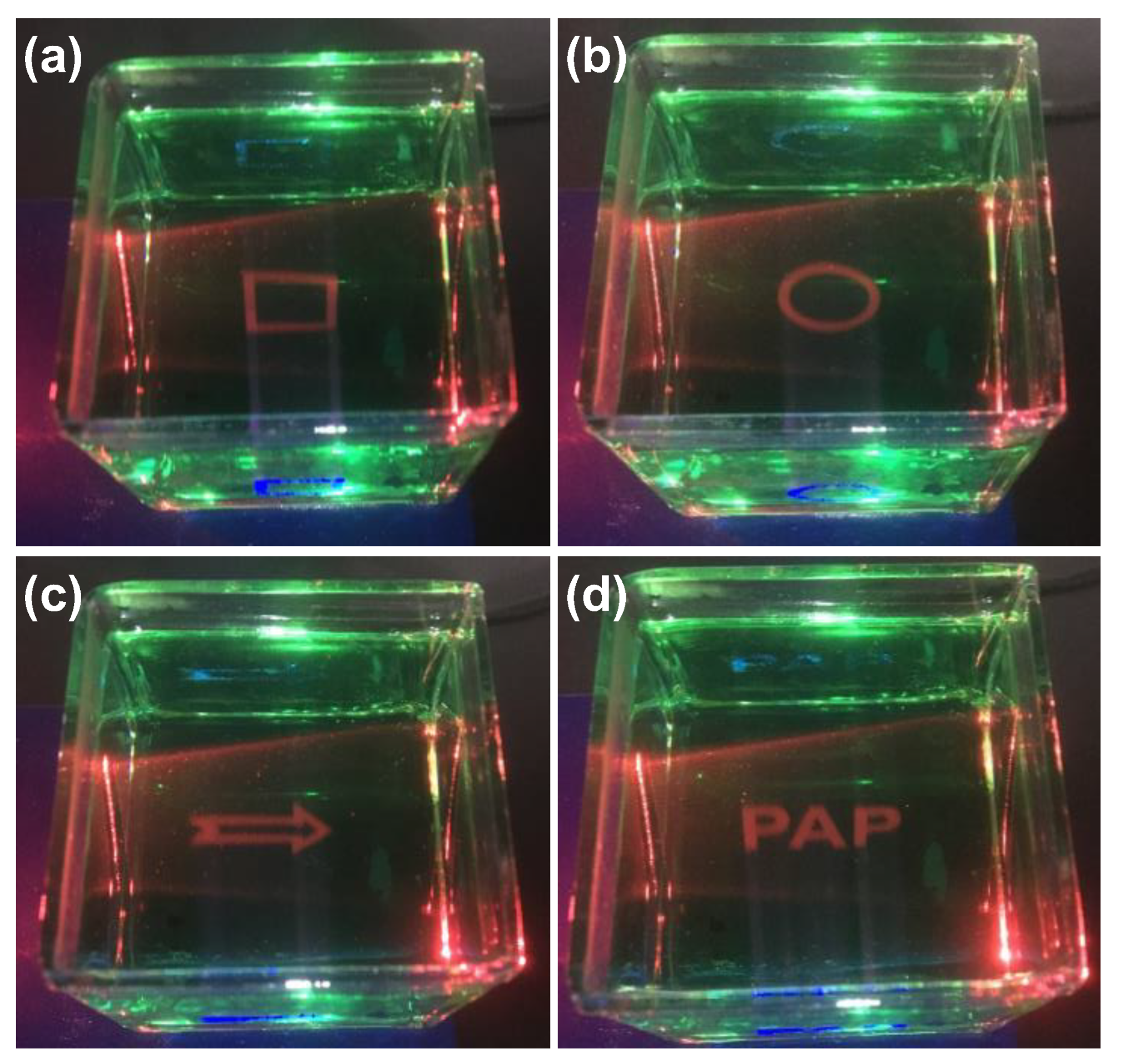
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- MacFarlane, D.L. Volumetric three-dimensional display. Appl. Opt. 1994, 33, 7453–7457. [Google Scholar] [CrossRef] [PubMed]
- Blundell, B.G.; Schwarz, A.J. Volumetric Three-Dimensional Display Systems; John Wiley & Sons, Inc.: Weinheim, Germany, 2000. [Google Scholar]
- Geng, J. Volumetric 3D display for radiation therapy planning. J. Disp. Technol. 2008, 4, 437–450. [Google Scholar] [CrossRef]
- Refai, H.H. Static volumetric three-dimensional display. J. Disp. Technol. 2009, 5, 391–397. [Google Scholar] [CrossRef]
- McDonald, G. Why Don’t We Have Princess Leia Holograms Yet? All Tech Considered. 2017. Available online: https://www.npr.org/sections/alltechconsidered/2017/08/26/546084473/why-don-t-we-have-princess-leia-holograms-yet (accessed on 26 August 2017).
- Smalley, D.; Poon, T.C.; Gao, H.Y.; Kvavle, J.; Qaderi, K. Volumetric Displays: Turning 3-D Inside-Out. Opt. Photonics News 2018, 29, 26–33. Available online: https://www.osa-opn.org/home/articles/volume_29/june_2018/features/volumetric_displays_turning_3-d_inside-out/ (accessed on 1 June 2018). [CrossRef]
- Lasher, M.; Stolan, P.; Dahlke, W.; Acantilado, N.; McDonald, M. Laser Projected 3-D Volumetric Displays. In Projection Displays II; SPIE: Anderlecht, Belgium, 1996; Volume 2650, pp. 285–295. [Google Scholar]
- Favalora, G.E.; Napoli, J.; Hall, D.M.; Dorval, R.K.; Giovinco, M.; Richmond, M.J.; Chun, W.S. 100 Million-Voxel Volumetric Display. In Cockpit Displays IX: Displays for Defense Applications; SPIE: Anderlecht, Belgium, 2002; Volume 4712, pp. 300–312. [Google Scholar]
- Geng, J. A volumetric 3D display based on a DLP projection engine. Displays 2013, 34, 39–48. [Google Scholar] [CrossRef]
- Gately, M.; Zhai, Y.; Yeary, M.; Petrich, E.; Sawalha, L. A three-dimensional swept volume display based on LED arrays. J. Disp. Technol. 2011, 7, 503–514. [Google Scholar] [CrossRef]
- Penciu, C.; MacFarlane, D.L. Fabrication and characterization of a volumetric three-dimensional display using ion-exchange integrated waveguides. Opt. Eng. 2000, 39, 565–571. [Google Scholar] [CrossRef]
- Downing, E.; Hesselink, L.; Ralston, J.; Macfarlane, R. A three-color, solid-state, three-dimensional display. Science 1996, 273, 1185–1189. [Google Scholar] [CrossRef]
- Langhans, K.; Bahr, D.; Bezecny, D.; Homann, D.; Oltmann, K.; Guill, C.; Rieper, E.; Ardey, G. FELIX 3D Display: An Interactive Tool for Volumetric Imaging. In Stereoscopic Displays and Virtual Reality Systems IX; SPIE: Anderlecht, Belgium, 2002; Volume 4660, p. 176. [Google Scholar]
- Cheng, L.; Wang, C.; Liu, Z. Upconversion nanoparticles and their composite nanostructures for biomedical imaging and cancer therapy. Nanoscale 2013, 5, 23–37. [Google Scholar] [CrossRef]
- Hinklin, T.R.; Rand, S.C.; Laine, R.M. Transparent, polycrystalline upconverting nanoceramics: Towards 3-D displays. Adv. Mater. 2008, 20, 1270–1273. [Google Scholar] [CrossRef]
- Wang, F.; Han, Y.; Lim, C.S.; Lu, Y.; Wang, J.; Xu, J.; Chen, H.; Zhang, C.; Hong, M.; Liu, X. Simultaneous phase and size control of upconversion nanocrystals through lanthanide doping. Nature 2010, 463, 1061–1065. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Z.; Zheng, W.; Zhu, H.; Lu, S.; Ma, E.; Tu, D.; Zhou, S.; Huang, M.; Chen, X.Y. Lanthanide-doped upconversion nanoparticles electrostatically coupled with photosensitizers for near-infrared-triggered photodynamic therapy. Nanoscale 2014, 6, 8274–8282. [Google Scholar] [CrossRef]
- Zito, R. Rate analysis of multiple-step excitation in mercury vapor. J. Appl. Phys. 1963, 34, 1535. [Google Scholar] [CrossRef]
- Barnes, R.H.; Moeller, C.E.; Kircher, J.F.; Verber, C.M. Two-step excitation of fluorescence in iodine monochloride vapor. Appl. Phys. Lett. 1974, 24, 610–612. [Google Scholar] [CrossRef]
- Kim, I.I.; Korevaar, E.J.; Hakakha, H. Three-Dimensional Volumetric Display in Rubidium Vapor. In Projection Displays II; SPIE: Anderlecht, Belgium, 1996; Volume 2650, p. 274. [Google Scholar]
- Liu, W.T.; Huang, W.Y. Enhancing the color gamut of white displays using novel deep-blue organic fluorescent dyes to form color-changed thin films with improved efficiency. Opt. Eng. 2012, 51, 104001. [Google Scholar] [CrossRef]
- Lewis, J.D.; Verber, C.M.; McGhee, R.B. A true three-dimensional display. IEEE Trans. Electron Devices 1971, 18, 724. [Google Scholar] [CrossRef]
- Honda, T.; Doumuki, T.; Akella, A.; Galambos, L.; Hesselink, L. One-color one-beam pumping of Er3+-doped ZBLAN glasses for a three-dimensional two-step excitation display. Opt. Lett. 1998, 23, 1108–1110. [Google Scholar] [CrossRef]
- Adamson, A.W. Method and Apparatus for Generating Three-Dimensional Patterns. US3609706, 28 September 1971. [Google Scholar]
- Worthington, J.D.L.; Adelman, A.H. Method and Apparatus for Generating Three-Dimensional Patterns. US3609707, 28 September 1971. [Google Scholar]
- Patel, S.K.; Cao, J.; Lippert, A.R. A volumetric three-dimensional digital light photoactivatable dye display. Nat. Commun. 2017, 8, 15239. [Google Scholar] [CrossRef]
- Li, B.; Haris, U.; Aljowni, M.; Nakatsuka, A.; Patel, S.K.; Lippert, A.R. Tuning the photophysical properties of spirolactam rhodamine photoswitches. Isr. J. Chem. 2020, 61, 244–252. [Google Scholar] [CrossRef]
- Zhong, H.Z.; Wang, Z.W.; Lu, W.G.; Liu, J.; Wang, Y.T. Luminescent materials for 3D display technology. In Chapter 18, Phosphors, up Conversion Nano Particles, Quantum Dots and Their Applications; Springer Science+Business Media: Singapore, 2016. [Google Scholar]
- Cho, J.-H.; Bass, M.; Jenssen, H.P. Volumetric three-dimensional up-conversion display medium. J. SID 2007, 15, 1029–1036. [Google Scholar] [CrossRef]
- Yang, Z.M.; Feng, Z.M.; Jiang, Z.H. Upconversion emission in multi-doped glasses for full colour display. J. Phys. D Appl. Phys. 2005, 38, 1629. [Google Scholar] [CrossRef]
- Deng, R.; Qin, F.; Chen, R.F.; Huang, W.; Hong, M.; Liu, X.G. Temporal full-colour tuning through non-steady-state upconversion. Nat. Nanotechnol. 2015, 10, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Rapaport, A.; Ayrault, K.; Mattew-Daniel, S.E.; Bass, M. Visible light emission from dyes excited by simultaneous absorption of two different frequency beams of light. Appl. Phys. Lett. 1999, 74, 329–331. [Google Scholar] [CrossRef]
- Kim, J.H.; Deng, F.; Castellano, F.N.; Kim, J.H. High efficiency low-power upconverting soft materials. Chem. Mater. 2012, 24, 2250–2252. [Google Scholar] [CrossRef]
- Hayase, G.; Funatomi, T.; Kumagai, K. Ultralow-bulk-density transparent boehmite nanofiber cryogel monoliths and their optical properties for a volumetric three-dimensional display. ACS Appl. Nano. Mater. 2018, 1, 26–30. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Chen, G.; Peng, Q.; Yuan, W.Z.; Xie, Y.J.; Li, S.H.; Zhang, Y.M.; Tang, B.Z. Achieving persistent room temperature phosphorescence and remarkable mechanochromism from pure organic Luminogens. Adv. Mater. 2015, 27, 6195–6201. [Google Scholar] [CrossRef]
- Wang, J.G.; Gu, X.G.; Ma, H.L.; Peng, Q.; Huang, X.B.; Zheng, X.Y.; Sung, H.P.S.; Shan, G.G.; Lam, W.Y.J.; Shuai, Z.G.; et al. A facile strategy for realizing room temperature phosphorescence and single molecule white light emission. Nat. Commun. 2018, 9, 2963. [Google Scholar] [CrossRef]
- Zhao, W.J.; He, Z.K.; Tang, B.Z. Room-temperature phosphorescence from organic aggregates. Nat. Rev. Mater. 2020, 5, 869–885. [Google Scholar] [CrossRef]
- Chen, B.B.; Wang, Y.; Liu, M.L.; Chang, S.; Lv, J.; Gao, Y.T.; Li, D.W. Bandgap engineering of scandium microspheres for anti-counterfeiting and multicolor imaging. Adv. Opt. Mater. 2023, 2202850. [Google Scholar] [CrossRef]
- Balzani, V.; Ceroni, P.; Juris, A. Photochemistry and Photophysics—Concepts, Research, Applications; Wiley-VCH: Weinheim, Germany, 2014; pp. 82–83. [Google Scholar]
- Wan, S.G.; Lu, W. Reversible photoactivated phosphorescence of gold(I) arylethynyl complexes in aerated DMSO solutions and gels. Angew. Chem. Int. Ed. 2017, 56, 1784–1788. [Google Scholar] [CrossRef]
- Wan, S.G.; Lin, J.X.; Su, H.M.; Dai, J.F.; Lu, W. Photochemically deoxygenating solvents for triplet–triplet annihilation photon upconversion operating in air. Chem. Commun. 2018, 54, 3907–3910. [Google Scholar] [CrossRef]
- Lin, J.X.; Wan, S.G.; Liu, W.F.; Lu, W. Photo-writing self-erasable phosphorescent images using poly(N-vinyl-2-pyrrolidone) as a photochemically deoxygenating matrix. Chem. Commun. 2019, 55, 4299–4302. [Google Scholar] [CrossRef]
- Wan, S.G.; Zhou, H.Q.; Lin, J.X.; Lu, W. A prototype of a volumetric three-dimensional display based on programmable photo-activated phosphorescence. Angew. Chem. Int. Ed. 2020, 59, 8416–8420. [Google Scholar] [CrossRef]
- Li, C.; Hu, Q.K.; Chen, Q.; Yu, W.J.; Xu, J.J.; Xu, Z.X. Tetrapropyl-substituted palladium phthalocyanine used as an efficient hole transport material in perovskite solar cells. Org. Electron. 2021, 88, 106018. [Google Scholar] [CrossRef]
- Guo, H.M.; Muro-Small, M.L.; Ji, S.M.; Zhao, J.Z.; Castellano, F.N. Naphthalimide phosphorescence finally exposed in a platinum(II) diimine complex. Inorg. Chem. 2010, 49, 6802–6804. [Google Scholar] [CrossRef]
- Venediktov, E.A.; Tokareva, O.G. Reactions of methylated quercetin derivatives with singlet molecular oxygen. Kinet. Catal. 2000, 41, 166–169. [Google Scholar] [CrossRef]
- Freyer, W.; Stiel, H.; Hild, M.; Teuchner, K.; Leupold, D. One- and two-photon-induced photochemistry of modified palladium porphyrazines involving molecular oxygen. Photochem. Photobio. 1997, 66, 596–604. [Google Scholar] [CrossRef]
- Lagorio, M.G.; Dicelio, L.E.; San Román, E.A. Quantum yield of singlet molecular oxygen sensitization by copper(II) tetracarboxyphthalocyanine. J. Photochem. Photobio. B Bio. 1989, 3, 615–624. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Y.; Wan, S.; Liu, Q.; Ye, C. Luminescent Materials for Volumetric Three-Dimensional Displays Based on Photoactivated Phosphorescence. Polymers 2023, 15, 2004. https://doi.org/10.3390/polym15092004
Gu Y, Wan S, Liu Q, Ye C. Luminescent Materials for Volumetric Three-Dimensional Displays Based on Photoactivated Phosphorescence. Polymers. 2023; 15(9):2004. https://doi.org/10.3390/polym15092004
Chicago/Turabian StyleGu, Yuhan, Shigang Wan, Qing Liu, and Changqing Ye. 2023. "Luminescent Materials for Volumetric Three-Dimensional Displays Based on Photoactivated Phosphorescence" Polymers 15, no. 9: 2004. https://doi.org/10.3390/polym15092004




