Recent Advancements of Polymeric Membranes in Anion Exchange Membrane Water Electrolyzer (AEMWE): A Critical Review
Abstract
:1. Introduction
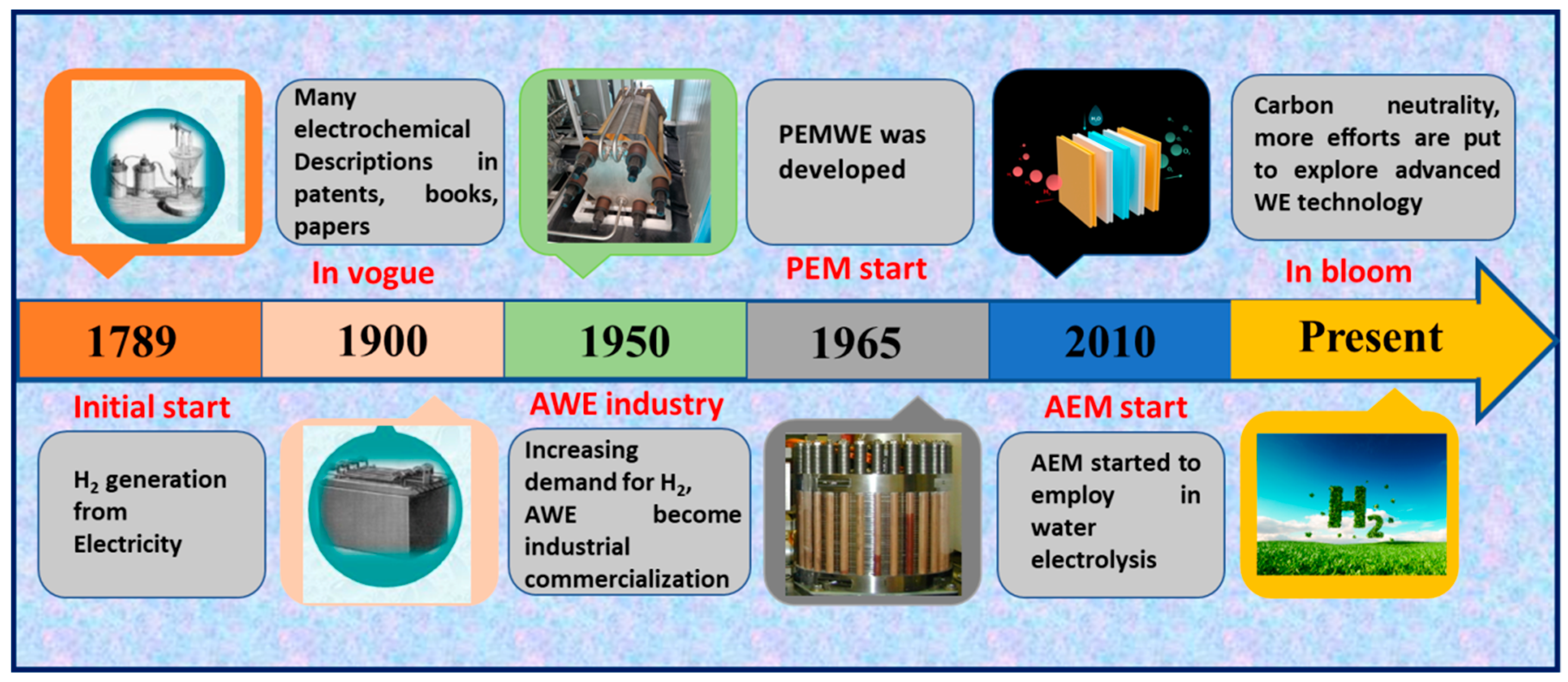
1.1. Water Electrolysis
1.2. Different Types of Water Electrolyzers
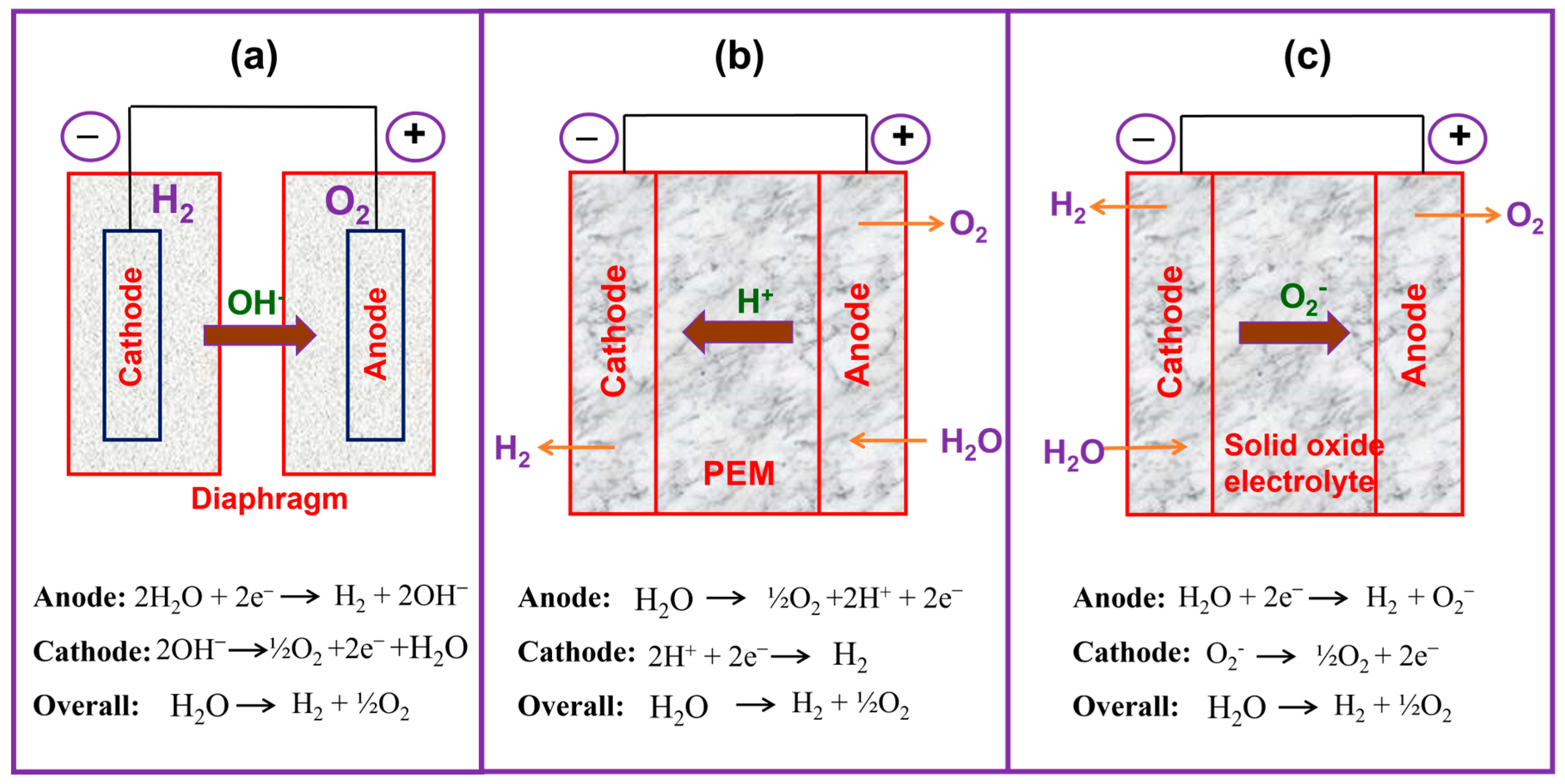
1.2.1. AWE
1.2.2. PEMWE
1.2.3. SOE
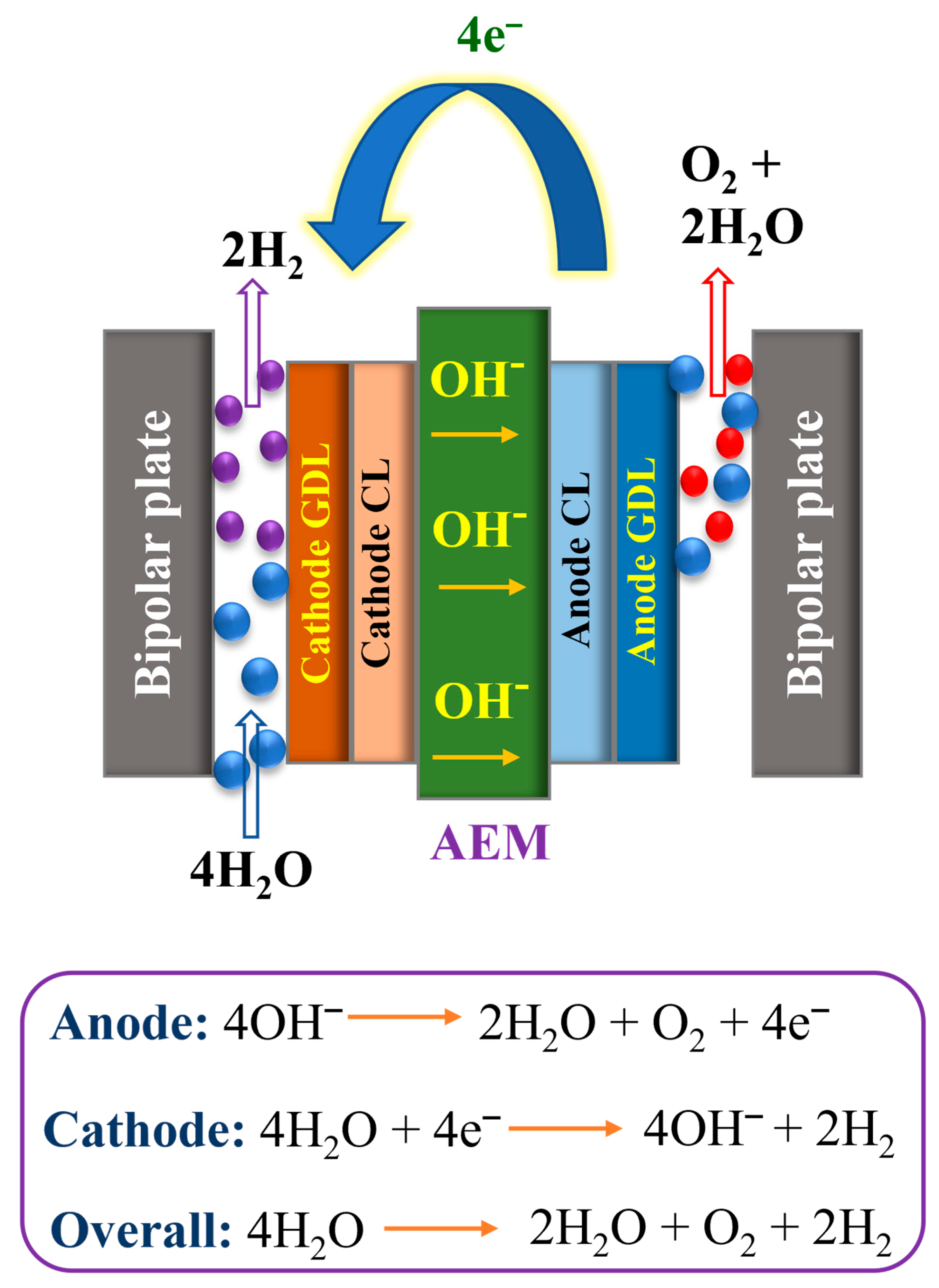
1.3. AEMWE and Its Working Principle
2. Anion Exchange Membrane
2.1. Recent Reports of Anion Exchange Membrane for AEMWEs
2.1.1. Quaternary Ammonium-Based Anion Exchange Membrane
2.1.2. Cross-Linked Anion Exchange Membrane
2.1.3. Piperidinium Functionalized Anion Exchange Membrane
2.1.4. Composite and Blended Anion Exchange Membrane
2.1.5. Morpholine Modified Anion Exchange Membrane
2.1.6. Piperidinium-Based Anion Exchange Membrane
3. Conclusions and Future Perceptions
- (i)
- Membranes: The growth of anion exchange membrane products must preserve several characteristics, namely anion exchange capacity, durability, anionic conductivity, water uptake, and swelling ratio. Basic studies must be carried out to determine in what way the deprivation of the anion exchange membrane occurs in a specific working atmosphere and to engineer supporting constituents. Using in situ examination and calculation tactics can be useful in scheming optimal membranes and understanding the operational mechanisms. Further, the progress of AEMs is important in several dimensions such as molecular design, phase manipulation, electrochemical properties, and application in AEMWEs. Several plausible future trends in AEM design emerge. (A) For cations, densely strung clusters and alkyl substituents are diffused to ensure OH− ion conductivity and alkaline stability, respectively; N-cyclic quaternary ammoniums may direct a new era of enhanced anion exchange membrane behavior. (B) Ether-free polyaromatic supports with reinforcement are now promising for AEM stability necessities; hydrophilic/hydrophobic alterations are dependable for microphase separation to make transport highways; a novel network topology understanding develops as a potential method for AEM.Attaining the high performance of AEM material is still in the early stages and will be followed by further development of large-scale processing and low-cost fabrication to satisfy the part of universal energy.
- (ii)
- Catalysts: Effective OER and HER electrocatalysts have been explored in both mathematical calculations and laboratory methodologies. However, based on the working environment of the AEMWE, the essential electrocatalyst resources vary. The AEMWE behaviour is assessed in aqueous potassium hydroxide, carbonate/bicarbonate, and water, and plenty of studies have described potassium hydroxide-fed conditions, where the platinum group of metal-free catalysts shows outstanding electrocatalytic characteristics. Nevertheless, the AEM water electrolyzer must be functioned in a clean water-fed environment eventually. The obstacles stem from the fact that the performance of the most extensively studied nickel catalysts degrades considerably below pH 9. The expansion of durable electrocatalysts in the clean water-fed process is unavoidable.
- (iii)
- Operating conditions: The AEMWE operation is essential. Although the membranes and electrocatalysts have exceptional characteristics, AEMWE performance is lower than the expected outcomes when constructing membrane electrode assemblies or gathering the modules owing to the weight allocation losses, electrochemical resistances, and so on. Further, based on the device’s operating environment, the behavior of an AEM water electrolyzer differs, indicating that the appropriate working environment must be verified to attain higher current densities and long-lasting durability. When executing a stack cell beyond a single unit cell, the performance of electrolytes must be deliberated since the laminar flow or turbulence disturbs the performance of the stack cell.
- (iv)
- It is difficult to quantitatively match the behaviour of anion exchange membranes or AEMWEs because the operational conditions or the determining techniques greatly affect the properties. In the event of the prevailing water electrolysis half-cells, the catalysts or the electrode characteristics could be matched with the Tafel slope, overpotential, and so on. It is well established, and it is likely to originate in an analogous working condition. Nevertheless, even with the standardized measurement methods, it is hard to replicate the characteristics of the AEM in real-world working environments. There are discrepancies between the reported characteristics of the marketable MEAs and the results attained from the test center. The electrolysis behaviors of AEMWEs are often disturbed by the working temperature, feed solution, etc. Thus, it is imperative to develop appropriate characterization methods that reflect the actual operating conditions and improve precise standards to assess the efficacy of AEMWEs.
- (v)
- Durability: Regarding stability, accomplishing robust stability of AEMWEs appears to be less challenging than for AMFCs, though there are a few stability-limiting aspects depending on the device’s working conditions. However, it is significant to note that if AEM water electrolysis exchanges with PEM water electrolysis and alkaline water electrolysis methodologies, MEAs of AEMWE must have all the similar behavior and stability necessities. At present, AEMWEs do not have all the necessities, requiring essential development in particular zones before being employed in products. Though it is difficult to tell what working conditions of AEMWEs will be most advantageous for performance, price, stability, and method difficulty, growing research demands aimed at the enhanced behavior and stability of AEMWEs keep this technology encouraging and cautiously feasible. Ultimately, it will reach its own place in the quickly growing H2 economy.
- (vi)
- Feeding solutions: The commonly employed feeding solution is an aqueous hydroxide solution owing to its enhancement of the anionic conductivity of AEMWEs and its consequent performance. Nevertheless, bearing in mind the lack of fresh water, the anticipated choices are seawater or treated wastewater. The above choices represent a challenging situation since AEMWE constituents rapidly worsen with pollutants existing in contaminated waters, and/or the behavior is lower due to modest reactions taking place in the electrodes. Further efforts in making AEMWE systems more robust and less vulnerable to feed-water contaminants are compulsory to enhance their durability and minimize their overall cost.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.A.; Choi, S.; Kim, C.; Yang, J.W.; Kim, S.Y.; Jang, H.W. Si-based water oxidation photoanodes conjugated with earth-abundant transition metal-based catalysts. ACS Mater. Lett. 2020, 2, 107–126. [Google Scholar] [CrossRef]
- Liu, J.; Kang, Z.; Li, D.; Pak, M.; Alia, S.M.; Fujimoto, C.; Bender, G.; Kim, Y.S.; Weber, A.Z. Elucidating the role of hydroxide electrolyte on anion-exchange-membrane water electrolyzer performance. J. Electrochem. Soc. 2021, 168, 54522. [Google Scholar] [CrossRef]
- Vinodh, R.; Deviprasath, C.; Gopi, C.V.V.M.; Kummara, V.G.R.; Atchudan, R.; Ahamad, T.; Kim, H.J.; Yi, M. Novel 13X Zeolite/PANI electrocatalyst for hydrogen and oxygen evolution reaction. Int. J. Hydrogen Energy 2020, 45, 28337–28349. [Google Scholar] [CrossRef]
- Gohil, J.M.; Dutta, K. Structures and properties of polymers in ion exchange membranes for hydrogen generation by water electrolysis. Polym. Adv. Technol. 2021, 32, 4598–4615. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Vincent, I.; Kruger, A.; Bessarabov, D. Development of efficient membrane electrode assembly for low cost hydrogen production by anion exchange membrane electrolysis. Int. J. Hydrogen Energy 2017, 42, 10752–10761. [Google Scholar] [CrossRef]
- Anderson, G.C.; Pivovar, B.S.; Alia, S.M. Establishing Performance Baselines for the Oxygen Evolution Reaction in Alkaline Electrolytes. J. Electrochem. Soc. 2020, 167, 44503. [Google Scholar] [CrossRef]
- Ranjekar, A.M.; Yadav, G.D. Steam Reforming of Methanol for Hydrogen Production: A Critical Analysis of Catalysis, Processes, and Scope. Ind. Eng. Chem. Res. 2021, 60, 89–113. [Google Scholar] [CrossRef]
- El-Shafie, M.; Kambara, S.; Hayakawa, Y. Hydrogen Production Technologies Overview. J. Power Energy Eng. 2019, 7, 107. [Google Scholar] [CrossRef]
- Smolinka, T.; Bergmann, H.; Garche, J.; Kusnezoff, M. The history of water electrolysis from its beginnings to the present. In Electrochemical Power Sources: Fundamentals, Systems, and Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 83–164. [Google Scholar]
- Nicholson, R.S. Theory and application of cyclic voltammetry for measurement of electrode reaction kinetics. Anal. Chem. 1965, 37, 1351–1355. [Google Scholar] [CrossRef]
- Cipriani, G.; Di Dio, V.; Genduso, F.; La Cascia, D.; Liga, R.; Miceli, R.; Galluzzo, G.R. Perspective on hydrogen energy carrier and its automotive applications. Int. J. Hydrogen Energy 2014, 39, 8482–8494. [Google Scholar] [CrossRef]
- Dunn, S. Hydrogen futures: Toward a sustainable energy system. Int. J. Hydrogen Energy 2002, 27, 235–264. [Google Scholar] [CrossRef]
- Barbir, F. PEM electrolysis for production of hydrogen from renewable energy sources. Sol. Energy 2005, 78, 661–669. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Ramakrishna, S.U.B.; Vijaya Krishna, S.; Srilatha, K.; Rama Devi, B.; Himabindu, V. Synthesis of titanium (IV) oxide composite membrane for hydrogen production through alkaline water electrolysis. S. Afr. J. Chem. Eng. 2018, 25, 54–61. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Ramakrishna, S.U.B.; Srinivasulu Reddy, D.; Bhagawan, D.; Himabindu, V. Synthesis of polysulfone and zirconium oxide coated asbestos composite separators for alkaline water electrolysis. Int. J. Chem. Eng. Process Technol. 2017, 3, 1035. [Google Scholar]
- Sapountzi, F.M.; Gracia, J.M.; Weststrate, C.J.; Kee, J.; Fredriksson, H.O.A.; Niemantsverdriet, J.W. Electrocatalysts for the generation of hydrogen, oxygen and synthesis gas. Prog. Energy Combust. Sci. 2017, 58, 1–35. [Google Scholar] [CrossRef]
- Arico, A.S.; Siracusano, S.; Briguglio, N.; Baglio, V.; Di Blasi, A.; Antonucci, V. Polymer electrolyte membrane water electrolysis: Status of technologies and potential applications in combination with renewable power sources. J. Appl. Electrochem. 2013, 43, 107–118. [Google Scholar] [CrossRef]
- Lee, S.A.; Kim, K.; Kwon, K.C.; Park, S.H.; Jang, H.W. Anion exchange membrane water electrolysis for sustainable large-scale hydrogen production. Carbon Neutr. 2022, 1, 26–48. [Google Scholar] [CrossRef]
- Xing, J.; Zeng, Z.; Best, W.; Liu, Z.; Bonville, L.; Maric, R.; Bliznakov, S. Long-term durability test of highly efficient membrane electrode assemblies for anion exchange membrane seawater electrolyzers. J. Power Sources 2023, 558, 232564. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C. Technological development of hydrogen production by solid oxide electrolyzer cell (SOEC). Int. J. Hydrogen Energy 2008, 33, 2337–2354. [Google Scholar] [CrossRef]
- Laguna-Bercero, M.A. Recent advances in high temperature electrolysis using solid oxide fuel cells: A review. J. Power Sources 2012, 203, 4–16. [Google Scholar] [CrossRef]
- Lee, L.; Kim, D. Poly(arylene ether ketone)-based bipolar membranes for acid–alkaline water electrolysis applications. J. Mater. Chem. A 2021, 9, 5485. [Google Scholar] [CrossRef]
- Oener, S.Z.; Foster, M.J.; Boettcher, S.W. Accelerating water dissociation in bipolar membranes and for electrocatalysis. Science 2020, 369, 1099–1103. [Google Scholar] [CrossRef]
- Lei, Q.; Wang, B.; Wang, P.; Liu, S. Hydrogen generation with acid/alkaline amphoteric water electrolysis. J. Energy Chem. 2019, 38, 162–169. [Google Scholar] [CrossRef]
- Xu, J.; Amorim, I.; Li, Y.; Li, J.; Yu, Z.; Zhang, B.; Araujo, A.; Zhang, N.; Liu, L. Stable overall water splitting in an asymmetric acid/alkaline electrolyzer comprising a bipolar membrane sandwiched by bifunctional cobalt-nickel phosphide nanowire electrodes. Carbon Energy 2020, 2, 646–655. [Google Scholar] [CrossRef]
- Sun, K.; Liu, R.; Chen, Y.; Verlage, E.; Lewis, N.S.; Xiang, C. A Stabilized, intrinsically safe, 10% efficient, solar-driven water-splitting cell incorporating earth-abundant electrocatalysts with steady-state pH gradients and product separation enabled by a bipolar membrane. Adv. Energy Mater. 2016, 6, 1600379. [Google Scholar] [CrossRef]
- Carmo, M.; David Fritz, L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Ursua, A.; Gandia, L.M.; Sanchis, P. Hydrogen production from water electrolysis: Current status and future trends. Proc. IEEE 2012, 100, 410–426. [Google Scholar] [CrossRef]
- Seetharaman, S.; Balaji, R.; Ramya, K.; Dhathathreyan, K.S.; Velan, M. Graphene oxide modified non-noble metal electrode for alkaline anion exchange membrane water electrolyzers. Int. J. Hydrogen Energy 2013, 38, 14934–14942. [Google Scholar] [CrossRef]
- Burnat, D.; Schlupp, M.; Wichser, A.; Lothenbach, B.; Gorbar, M.; Züttel, A.; Vogt, U.F. Composite membranes for alkaline electrolysis based on polysulfone and mineral fillers. J. Power Sources 2015, 291, 163–172. [Google Scholar] [CrossRef]
- Marini, S.; Salvi, P.; Nelli, P.; Pesenti, R.; Villa, M.; Berrettoni, M.; Zangari, G.; Kiros, Y. Advanced alkaline water electrolysis. Electrochim. Acta 2012, 82, 384–391. [Google Scholar] [CrossRef]
- Ayers, K.E.; Anderson, E.B.; Capuano, C.B.; Carter, B.D.; Dalton, L.T.; Hanlon, G.; Manco, J.; Niedzwiecki, M. Research advances towards low cost, high efficiency PEM electrolysis. ECS Trans. 2010, 3, 3–15. [Google Scholar] [CrossRef]
- Saba, S.M.; Muller, M.; Robinius, M.; Stolten, D. The investment costs of electrolysis—A comparison of cost studies from the past 30 years. Int. J. Hydrogen Energy 2018, 43, 1209–1223. [Google Scholar] [CrossRef]
- Dönitz, W.; Erdle, E. High-temperature electrolysis of water vapor-status of development and perspectives for application. Int. J. Hydrogen Energy 1985, 10, 291–295. [Google Scholar] [CrossRef]
- Xu, W.; Scott, K. The effects of ionomer content on PEM water electrolyser membrane electrode assembly performance. Int. J. Hydrogen Energy 2010, 35, 12029–12037. [Google Scholar] [CrossRef]
- Brisse, A.; Schefold, J.; Zahid, M. High temperature water electrolysis in solid oxide cells. Int. J. Hydrogen Energy 2008, 33, 5375–5382. [Google Scholar] [CrossRef]
- Liang, M.; Yu, B.; Wen, M.; Chen, J.; Xu, J.; Zhai, Y. Preparation of LSM-YSZ composite powder for anode of solid oxide electrolysis cell and its activation mechanism. J. Power Sources 2009, 190, 341–345. [Google Scholar] [CrossRef]
- Moçoteguy, P.; Brisse, A. A review and comprehensive analysis of degradation mechanisms of solid oxide electrolysis cells. Int. J. Hydrogen Energy 2013, 38, 15887–15902. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.; Christiansen, L.J. Concepts in Syngas Preparation; Catalytic Science Series; Imperial College Press: London, UK, 2011. [Google Scholar]
- Ju, W.; Heinz, M.V.F.; Pusterla, L.; Hofer, M.; Fumey, B.; Castiglioni, R.; Pagani, M.; Battaglia, C.; Vogt, U.F. Lab-scale alkaline water electrolyzer for bridging material fundamentals with realistic operation. ACS Sustain. Chem. Eng. 2018, 6, 4829–4837. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, C.; Jiang, L.; Lin, H.; An, Y.; Zhou, D.; Leung, M.K.H.; Yang, S. Nanohybridization of MoS2 with Layered Double Hydroxides Efficiently Synergizes the Hydrogen Evolution in Alkaline Media. Joule 2017, 1, 383–393. [Google Scholar] [CrossRef]
- Miller, H.A.; Bouzek, K.; Hnat, J.; Loos, S.; Bernacker, C.I.; Weibgarber, T.; Rontzsch, L.; Maeier-Haack, J. Green hydrogen from anion exchange membrane water electrolysis: A review of recent developments in critical materials and operating conditions. Sustain. Energy Fuels 2020, 4, 2114–2133. [Google Scholar] [CrossRef]
- Liu, J.; Gerhardt, M.R.; Li, D.; Pak, M.; Kang, Z.; Alia, S.M.; Bender, G.; Kim, Y.Z.; Weber, A.Z. Mathematical modeling of hydroxide-exchange-membrane water electrolyzer. ECS Meet. Abstr. 2020, MA2020-02, 2443. [Google Scholar] [CrossRef]
- Kim, Y.S. Polymer electrolytes with high ionic concentration for fuel cells and electrolyzers. ACS Appl. Polym. Mater. 2021, 3, 1250–1270. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K. A review of alkaline solid polymer membrane in the application of AEM electrolyzer: Materials and characterization. Int. J. Energy Res. 2021, 45, 18337–18354. [Google Scholar] [CrossRef]
- Maurya, S.; Shin, S.H.; Kim, M.K.; Yun, S.H.; Moon, S.H. Stability of composite anion exchange membranes with various functional groups and their performance for energy conversion. J. Membr. Sci. 2013, 443, 28–35. [Google Scholar] [CrossRef]
- Zhang, M.; Kim, H.K.; Chalkova, E.; Mark, F.; Lvov, S.N.; Chung, T.C.M. New polyethylene based anion exchange membranes (PE–AEMs) with high ionic conductivity. Macromolecules 2011, 44, 5937–5946. [Google Scholar] [CrossRef]
- Lin, X.; Varcoe, J.R.; Poynton, S.D.; Liang, X.; Ong, A.L.; Ran, J.; Li, Y.; Xu, T. Alkaline polymer electrolytes containing pendant dimethylimidazolium groups for alkaline membrane fuel cells. J. Mater. Chem. A 2013, 1, 7262–7269. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Ponkratov, D.O.; Vygodskii, Y.S. Poly(ionic liquid)s: Synthesis, properties, and application. Polym. Sci. Ser. B 2016, 58, 73–142. [Google Scholar] [CrossRef]
- Salerno, H.L.S.; Beyer, F.L.; Elabd, Y.A. Anion exchange membranes derived from nafion precursor for the alkaline fuel cell. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 552–562. [Google Scholar] [CrossRef]
- Zhang, B.; Long, H.; Kaspar, R.B.; Wang, J.; Gu, S.; Zhuang, Z.; Pivovar, B.; Yan, Y. Relating alkaline stability to the structure of quaternary phosphonium cations. RSC Adv. 2018, 8, 26640–26645. [Google Scholar] [CrossRef] [PubMed]
- Kwasny, M.T.; Tew, G.N. Expanding metal cation options in polymeric anion exchange membranes. J. Mater. Chem. A 2017, 5, 1400–1405. [Google Scholar] [CrossRef]
- Liu, D.; Lin, L.; Xie, Y.; Pang, J.; Jiang, Z. Anion exchange membrane based on poly(arylene ether ketone) containing long alkyl densely quaternized carbazole derivative pendant. J. Membr. Sci. 2021, 623, 119079. [Google Scholar] [CrossRef]
- Park, E.J.; Maurya, S.; Hibbs, M.R.; Fujimoto, C.H.; Kreuer, K.D.; Kim, Y.S. Alkaline stability of quaternized Diels–Alder polyphenylenes. Macromolecules 2019, 52, 5419–5428. [Google Scholar] [CrossRef]
- Buggy, N.C.; Du, Y.; Kuo, M.C.; Ahrens, K.A.; Wilkinson, J.S.; Seifert, S.; Coughlin, E.B.; Herring, A.M. A polyethylene-based triblock copolymer anion exchange membrane with high conductivity and practical mechanical properties. ACS Appl. Polym. Mater. 2020, 2, 1294–1303. [Google Scholar] [CrossRef]
- Wang, J.; He, R.; Che, Q. Anion exchange membranes based on semi-interpenetrating polymer network of quaternized chitosan and polystyrene. J. Colloid Interface Sci. 2011, 361, 219–225. [Google Scholar] [CrossRef]
- Xu, P.Y.; Guo, T.Y.; Zhao, C.H.; Broadwell, I.; Zhang, Q.G.; Liu, Q.L. Anion exchange membranes based on poly (vinyl alcohol) and quaternized polyethyleneimine for direct methanol fuel cells. J. Appl. Polym. Sci. 2013, 128, 3853–3860. [Google Scholar] [CrossRef]
- Hibbs, M.R.; Hickner, M.A.; Alam, T.M.; McIntyre, S.K.; Fujimoto, C.H.; Cornelius, C.J. Transport properties of hydroxide and proton conducting membranes. Chem. Mater. 2008, 20, 2566–2573. [Google Scholar] [CrossRef]
- Chen, C.; Tse, Y.L.S.; Lindberg, G.E.; Knight, C.; Voth, G.A. Hydroxide solvation and transport in anion exchange membranes. J. Am. Chem. Soc. 2016, 138, 991–1000. [Google Scholar] [CrossRef]
- Cossar, E.; Barnett, A.O.; Seland, F.; Baranova, E.A. The performance of nickel and nickel-iron catalysts evaluated as anodes in anion exchange membrane water electrolysis. Catalysts 2019, 9, 814. [Google Scholar] [CrossRef]
- Kim, B.J.; Cheng, X.; Abbott, D.F.; Fabbri, E.; Bozza, F.; Graule, T.; Castelli, I.E.; Wiles, L.; Danilovic, N.; Ayers, K.E. Highly active nanoperovskite catalysts for oxygen evolution reaction: Insights into activity and stability of Ba0.5Sr0.5Co0.8Fe0.2O2+δ and PrBaCo2O5+δ. Adv. Funct. Mater. 2018, 28, 1804355. [Google Scholar] [CrossRef]
- Henkensmeier, D.; Najibah, M.; Harms, C.; Žitka, J.; Hnát, J.; Bouzek, K. Overview: State-of-the art commercial membranes for anion exchange membrane water electrolysis. J. Electrochem. Energy Convers. Storage 2021, 18, 024001. [Google Scholar] [CrossRef]
- Kang, S.Y.; Park, J.E.; Jang, G.Y.; Kim, O.-H.; Kwon, O.J.; Cho, Y.-H.; Sung, Y.-E. High-performance and durable water electrolysis using a highly conductive and stable anion-exchange membrane. Int. J. Hydrog. Energy 2022, 47, 9115–9126. [Google Scholar] [CrossRef]
- Krivina, R.A.; Ou, Y.; Xu, Q.; Twight, L.P.; Stovall, T.N.; Boettcher, S.W. Oxygen electrocatalysis on mixed-metal oxides/oxyhydroxides: From fundamentals to membrane electrolyzer technology. Acc. Chem. Res. 2021, 2, 548–558. [Google Scholar] [CrossRef]
- López-Fernández, E.; Sacedón, C.G.; Gil-Rostra, J.; Yubero, F.; González-Elipe, A.R.; de Lucas-Consuegra, A. Recent Advances in Alkaline Exchange Membrane Water Electrolysis and Electrode Manufacturing. Molecules 2021, 26, 6326. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Jeong, I.; Choi, J.; Shin, G.; Kim, J.; Kim, T.-H.; Park, T. Anion exchange membranes and ionomer properties of a polyfluorene-based polymer with alkyl spacers for water electrolysis. Appl. Surf. Sci. 2023, 610, 155601. [Google Scholar] [CrossRef]
- Simari, C.; Ur Rehman, M.H.; Caprì, A.; Gatto, I.; Baglio, V.; Nicotera, I. High-performance anion exchange membrane water electrolysis by polysulfone grafted with tetramethyl ammonium functionalities. Mater. Today Sustain. 2023, 21, 100297. [Google Scholar] [CrossRef]
- Cha, M.S.; Park, J.E.; Kim, S.; Han, S.-H.; Shin, S.-H.; Yang, S.H.; Kim, T.-H.; Yu, D.M.; So, S.; Hong, Y.T.; et al. Poly(carbazole)-based anion-conducting materials with high performance and durability for energy conversion devices. Energy Environ. Sci. 2020, 13, 3633. [Google Scholar] [CrossRef]
- Guo, M.; Ban, T.; Wang, Y.; Wang, X.; Zhu, X. “Thiol-ene” crosslinked polybenzimidazoles anion exchange membrane with enhanced performance and durability. J. Colloid Interface Sci. 2023, 638, 349–362. [Google Scholar] [CrossRef]
- Lu, W.; Yang, Z.; Huang, H.; Wei, F.; Li, W.; Yu, Y.; Gao, Y.; Zhou, Y.; Zhang, G. Piperidinium-functionalized poly(vinylbenzyl chloride) cross-linked by polybenzimidazole as an anion exchange membrane with a continuous ionic transport pathway. Ind. Eng. Chem. Res. 2020, 59, 21077–21087. [Google Scholar] [CrossRef]
- Xu, Z.; Wilke, V.; Chmielarz, J.J.; Tobias, M.; Atanasov, V.; Gago, A.S.; Friedrich, K.A. Novel piperidinium-functionalized crosslinked anion exchange membrane with flexible spacers for water electrolysis. J. Membr. Sci. 2023, 670, 121302. [Google Scholar] [CrossRef]
- Rakhshani, S.; Araneo, R.; Pucci, A.; Rinaldi, A.; Giuliani, C.; Pozio, A. Synthesis and characterization of a composite anion exchange membrane for water electrolyzers (AEMWE). Membranes 2023, 13, 109. [Google Scholar] [CrossRef] [PubMed]
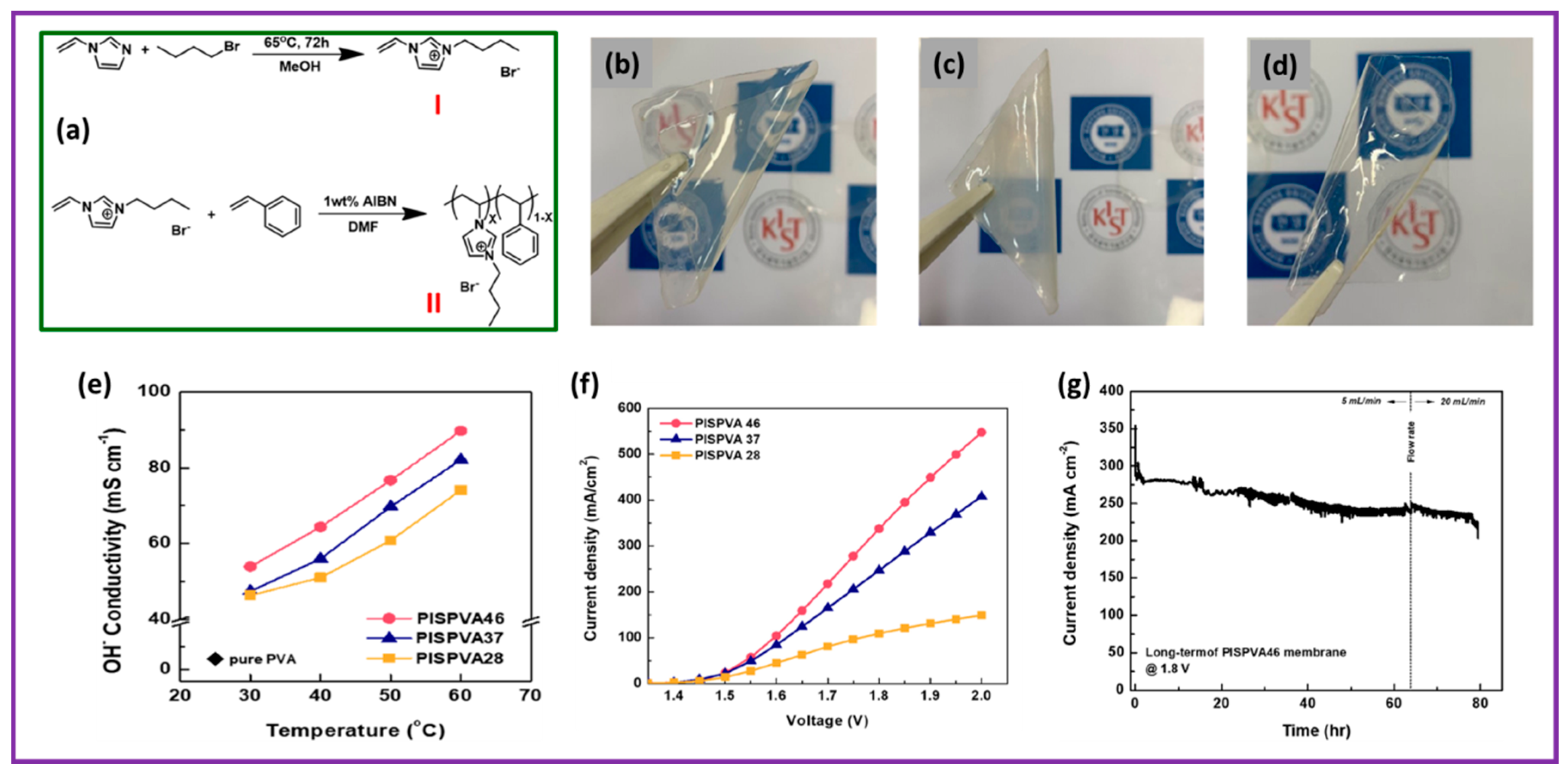
- Park, H.J.; Lee, S.Y.; Lee, T.K.; Kim, H.-J.; Lee, Y.M. N3-butyl imidazolium-based anion exchange membranes blended with Poly (vinyl alcohol) for alkaline water electrolysis. J. Membr. Sci. 2020, 611, 118355. [Google Scholar] [CrossRef]
- Tufa, R.A.; Rugiero, E.; Chanda, D.; Hnat, J.; van Baak, W.; Veerman, J.; Fontananova, E.; Di Profio, G.; Drioli, E.; Bouzek, K.; et al. Salinity gradient power-reverse electrodialysis and alkaline polymer electrolyte water electrolysis for hydrogen production. J. Membr. Sci. 2016, 514, 155–164. [Google Scholar] [CrossRef]
- Tham, D.D.; Kim, D. C2 and N3 substituted imidazolium functionalized poly (arylene ether ketone) anion exchange membrane for water electrolysis with improved chemical stability. J. Membr. Sci. 2019, 581, 139–149. [Google Scholar] [CrossRef]
- You, W.; Padgett, E.; MacMillan, S.N.; Muller, D.A.; Coates, G.W. Highly conductive and chemically stable alkaline anion exchange membranes via ROMP of trans-cyclooctene derivatives. Proc. Natl. Acad. Sci. USA 2019, 116, 9729–9734. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Yang, C.C.; Lin, S.J. Preparation of composite alkaline polymer electrolyte. Mater. Lett. 2002, 57, 873–881. [Google Scholar] [CrossRef]
- Bonizzoni, S.; Stucchi, D.; Caielli, T.; Sediva, E.; Mauri, M.; Mustarelli, P. Morpholinium-modified, polyketone-based anion exchange membranes for water electrolysis. ChemElectroChem. 2023, 10, e202201077. [Google Scholar] [CrossRef]
- Chen, N.; Paek, S.Y.; Lee, J.Y.; Park, J.H.; Lee, S.Y.; Lee, Y.M. High-performance anion exchange membrane water electrolyzers with a current density of 7.68 A cm−2 and a durability of 1000 hours. Energy Environ. Sci. 2021, 14, 6338–6348. [Google Scholar] [CrossRef]
- Liu, L.; Bai, L.; Liu, Z.; Miao, S.; Pan, J.; Shen, L.; Shi, Y.; Li, N. Side-chain structural engineering on poly(terphenyl piperidinium) anion exchange membrane for water electrolysers. J. Membr. Sci. 2023, 665, 121135. [Google Scholar] [CrossRef]
- Caielli, T.; Ferrari, A.R.; Bonizzoni, S.; Sediva, E.; Caprì, A.; Santoro, M.; Gatto, I.; Baglio, V.; Mustarelli, P. Synthesis, characterization and water electrolyzer cell tests of poly (biphenyl piperidinium) anion exchange membranes. J. Power Sources 2023, 557, 232532. [Google Scholar] [CrossRef]
- Yan, X.; Yang, X.; Su, X.; Gao, L.; Zhao, J.; Hu, L.; Di, M.; Li, T.; Ruan, X.; He, G. Twisted ether-free polymer based alkaline membrane for high-performance water electrolysis. J. Power Sources 2020, 480, 228805. [Google Scholar] [CrossRef]
- Hu, X.; Huang, Y.; Liu, L.; Ju, Q.; Zhou, X.; Qiao, X.; Zheng, Z.; Li, N. Piperidinium functionalized aryl ether-free polyaromatics as anion exchange membrane for water electrolysers: Performance and durability. J. Membr. Sci. 2021, 621, 118964. [Google Scholar] [CrossRef]
- Leng, Y.; Chen, G.; Mendoza, A.J.; Tighe, T.B.; Hickner, M.A.; Wang, C.Y. Solid-state water electrolysis with an alkaline membrane. J. Am. Chem. Soc. 2012, 134, 9054–9057. [Google Scholar] [CrossRef]
- Mustain, W.E.; Chatenet, M.; Page, M.; Kim, Y.S. Durability challenges of anion exchange membrane fuel cells. Energy Environ. Sci. 2020, 13, 2805–2838. [Google Scholar] [CrossRef]
- Lagadec, M.F.; Grimaud, A. Water electrolysers with closed and open electrochemical systems. Nat. Mater. 2020, 19, 1140–1150. [Google Scholar] [CrossRef]
- Lee, S.A.; Yang, J.W.; Choi, S.; Jang, H.W. Nanoscale electrodeposition: Dimension control and 3D conformality. Exploration 2021, 1, 20210012. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Han, G.H.; Hong, S.; Park, J.; Bang, J.; Kim, S.Y.; Ahn, S.H. Electrodeposition: An efficient method to fabricate self-supported electrodes for electrochemical energy conversion systems. Exploration 2022, 2, 20210077. [Google Scholar] [CrossRef]
- Tong, W.; Forster, M.; Dionigi, F.; Dresp, S.; Sadeghi Erami, R.; Strasser, P. Electrolysis of low-grade and saline surface water. Nat. Energy 2020, 5, 367–377. [Google Scholar] [CrossRef]
- Dionigi, F.; Reier, T.; Pawolek, Z.; Gliech, M.; Strasser, P. Design criteria, operating conditions, and nickel-iron hydroxide catalyst materials for selective seawater electrolysis. ChemSusChem 2016, 9, 962–972. [Google Scholar] [CrossRef] [PubMed]

| Parameters | AWE | PEMWE | AEMWE |
|---|---|---|---|
| Separation | Diaphragm | PEM | AEM |
| Cathode | Nickel molybdenum alloys | Pt group metals | Transition metals |
| Anode | Nickel cobalt alloys | RuOx, IrOx | Transition metals |
| Current collector plate | Nickel | Copper | Copper |
| Bipolar plate | --- | Graphite and Ti | Ni or SS |
| Electrolyte | KOH | Pure water | Alkali solution, pure water |
| Current density | <0.5 A cm−2 | 1~2 A cm−2 | 1~2 A cm−2 |
| Operating temperature | 60–90 °C | 50–90 °C | 40–80 °C |
| Gas purity | >99.5% | >99.99% | >99.99% |
| Lifetime | ~100 kh | <10 kh | <2 kh |
| Estimated cost | Low | High | --- |
| Technology status | Mature | Commercial for small scale | Research and Development |
| Electrolysis Methods | Merits | Demerits |
|---|---|---|
| AWE |
|
|
| PEMWE |
|
|
| AEMWE |
|
|
| SOE |
|
|
| Membrane | Anode | Cathode | Electrolyte | Temperature (°C) | Current Density | Durability (h) | Ref. |
|---|---|---|---|---|---|---|---|
| Quaternized poly [9,9-bis(6-bromohexyl) fluorene]-co [4,4-bis((4-phenyl) propyl) biphenyl)] | IrO2 | Pt/C | 1 M KOH | 70 | 1531 mA cm−2 | 1000 h, 80 °C | [68] |
| Polysulfone-g-tetramethyl ammonium | Nickel foam | Pt/C | 1 M KOH | 90 | 1.3 A cm−2 | --- | [69] |
| Quaternized poly-carbazole | IrO2 | Pt/C | 1 M KOH | 50 | 3.5 A cm−2 | --- | [70] |
| Cross-linked polybenzimidazole with norborene | IrO2 | Pt/C | 1 M KOH | 60 | 150 mA cm−2 | 8.3 h | [71] |
| Piperidinium-f-poly(vinyl benzyl chloride) cross-linked by polybenzimidazole | Ir black | Pt/C | 1 M KOH | 60 | 2 V @ 700 mA cm−2 | 200 h 1.9 V (500 mA cm−2) | [72] |
| Piperidinium-f-styrene-ethylene-butylene-styrene copolymer | Ir-Black | Pt/C | Ultra-pure water 0.1 M KOH | 60 | 2 V @ 275 mA cm−2 2V @ 680 mA cm−2 | 300 h | [73] |
| Composite AEM (Celgard/Fumion) | Stainless steel | Stainless steel | 0.5 M KOH | 25 | 560 mA cm−2 | 163 h | [74] |
| N3-butyl Imidazolium blended with poly(vinyl alcohol) | IrO2 | Pt/C | 0.5 M KOH | 60 | 2 V @ 547.7 mA cm−2 | 80 h (1.8 V) | [75] |
| Morpholinium-modified, polyketone-based AEM | Nickel foam | Nickel foam | Pure water | 25 | 2.5 V @ 60 mA cm−2 | --- | [81] |
| Poly(fluorenyl-co-aryl piperidinium) | IrO2 | Pt/C | 1 M KOH | 80 | 7.68 A cm−2 @ 2 V | 1100 h, 0.5 A cm−2, 60 °C, 200 µV h−1 | [82] |
| Poly(terphenyl piperidinium) based AEM | IrO2 | Pt-Ru/C | 0.1 M KOH | 60 | 1.77 V @ 2.5 A cm−2 | 300 h, 1 A cm−2 | [83] |
| Poly(biphenyl piperidinium) | Nickel foam | Pt/C | 1 M KOH | 60 | 2.2 V @ 2.8 A cm−2 | --- | [84] |
| Twisted ether-free poly arylene piperidinium | IrO2 | Pt/C | 5.6 wt.% KOH | 50 | 200 mA cm−2 | 500 h (2.1 V) | [85] |
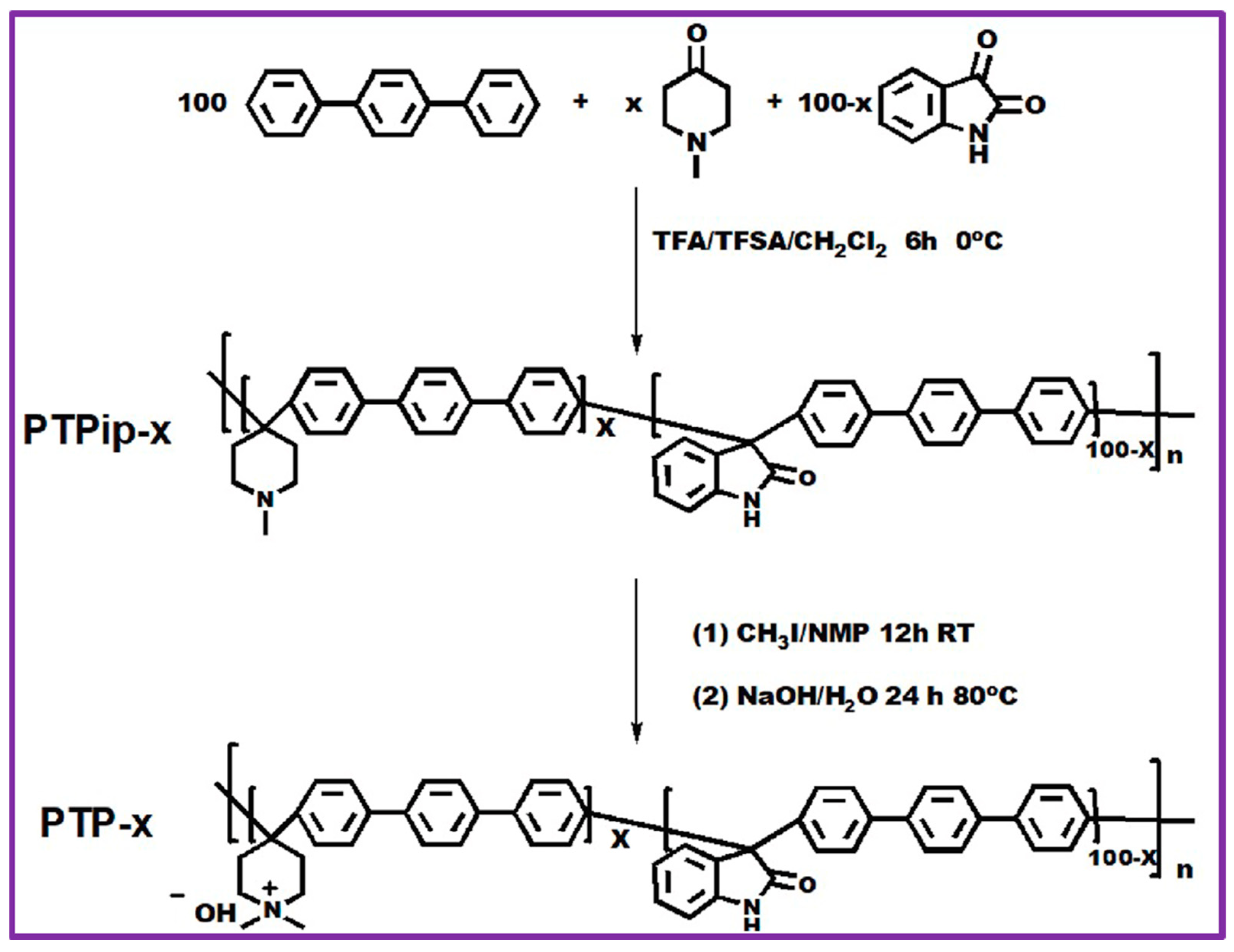
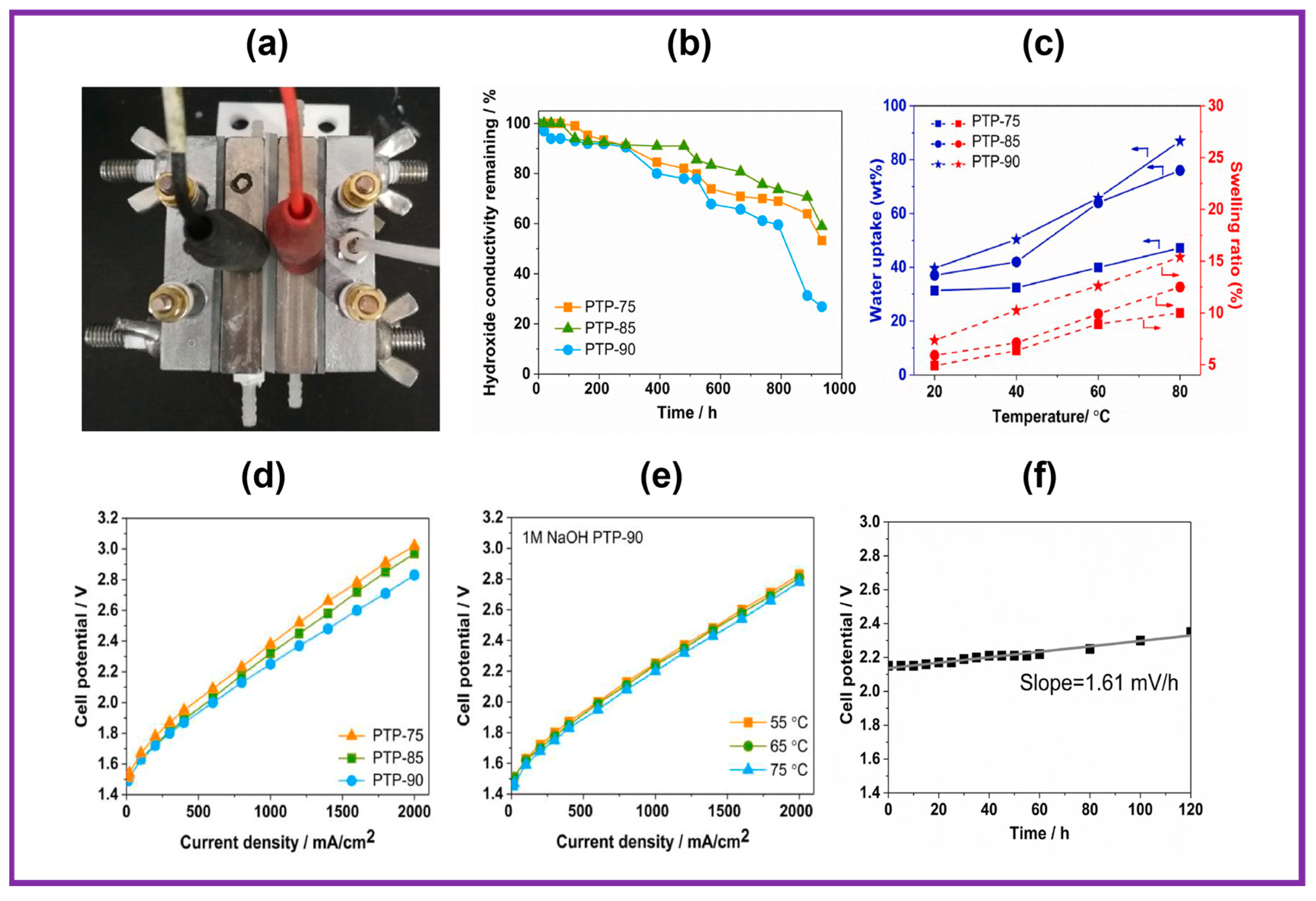
| Quaternized poly (terphenyl piperidinium) co (oxindole terphenylylene) | IrO2 | Pt black | 1 M NaOH | 55 | 2.2 V @ 910 mA cm−2 | 400 mA cm−2, 120 h | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinodh, R.; Kalanur, S.S.; Natarajan, S.K.; Pollet, B.G. Recent Advancements of Polymeric Membranes in Anion Exchange Membrane Water Electrolyzer (AEMWE): A Critical Review. Polymers 2023, 15, 2144. https://doi.org/10.3390/polym15092144
Vinodh R, Kalanur SS, Natarajan SK, Pollet BG. Recent Advancements of Polymeric Membranes in Anion Exchange Membrane Water Electrolyzer (AEMWE): A Critical Review. Polymers. 2023; 15(9):2144. https://doi.org/10.3390/polym15092144
Chicago/Turabian StyleVinodh, Rajangam, Shankara Sharanappa Kalanur, Sadesh Kumar Natarajan, and Bruno G. Pollet. 2023. "Recent Advancements of Polymeric Membranes in Anion Exchange Membrane Water Electrolyzer (AEMWE): A Critical Review" Polymers 15, no. 9: 2144. https://doi.org/10.3390/polym15092144






