Pyrolytic Modification of Heavy Coal Tar by Multi-Polymer Blending: Preparation of Ordered Carbonaceous Mesophase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Reagent
2.3. Sample Characterization
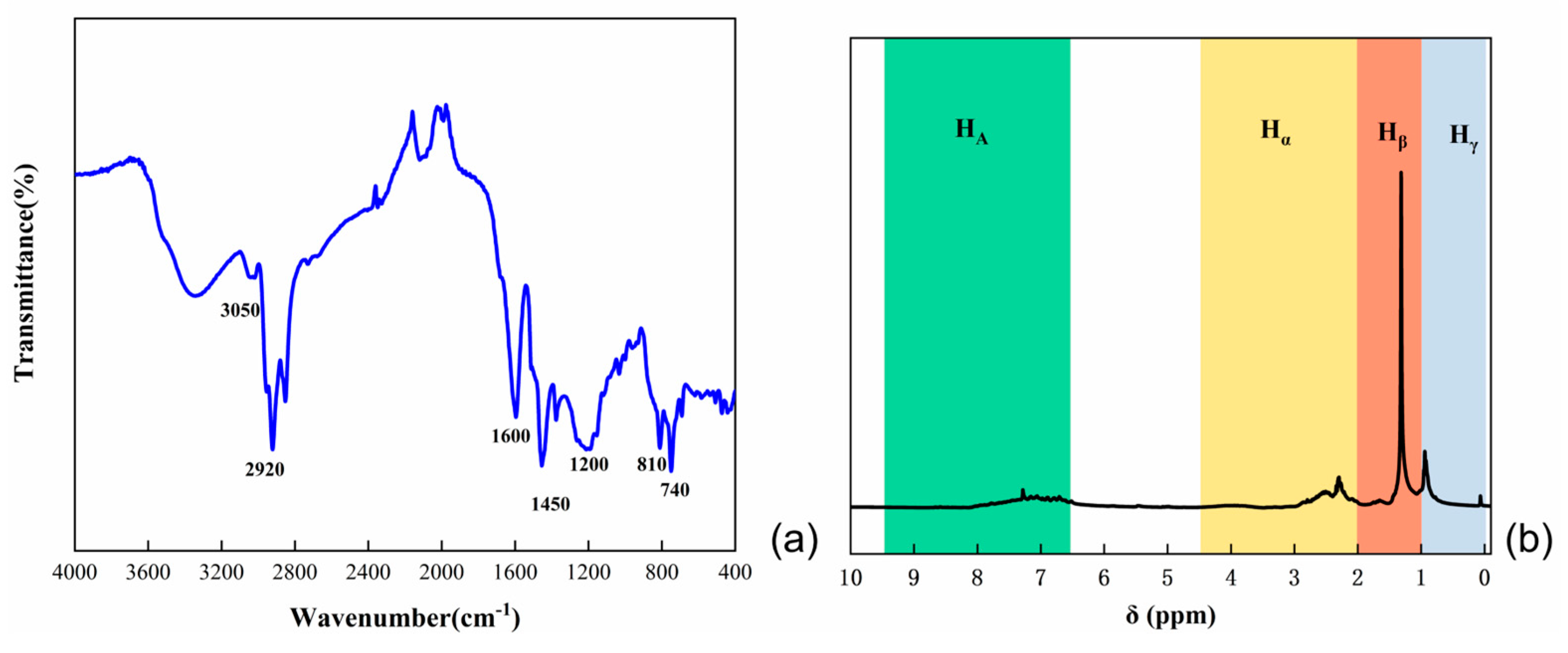
2.3.1. Optical Structure Analysis
2.3.2. FT-IR Analysis
2.3.3. XRD Analysis
2.4. Experimental Protocol
- (1)
- Preparation of Carbonaceous Mesophase through Copolymer-Modified Heavy Coal Tar
- (2)
- Evaluation system of carbonaceous mesophase ordination
3. Results and Discussion
3.1. Analysis of Coal Tar Components
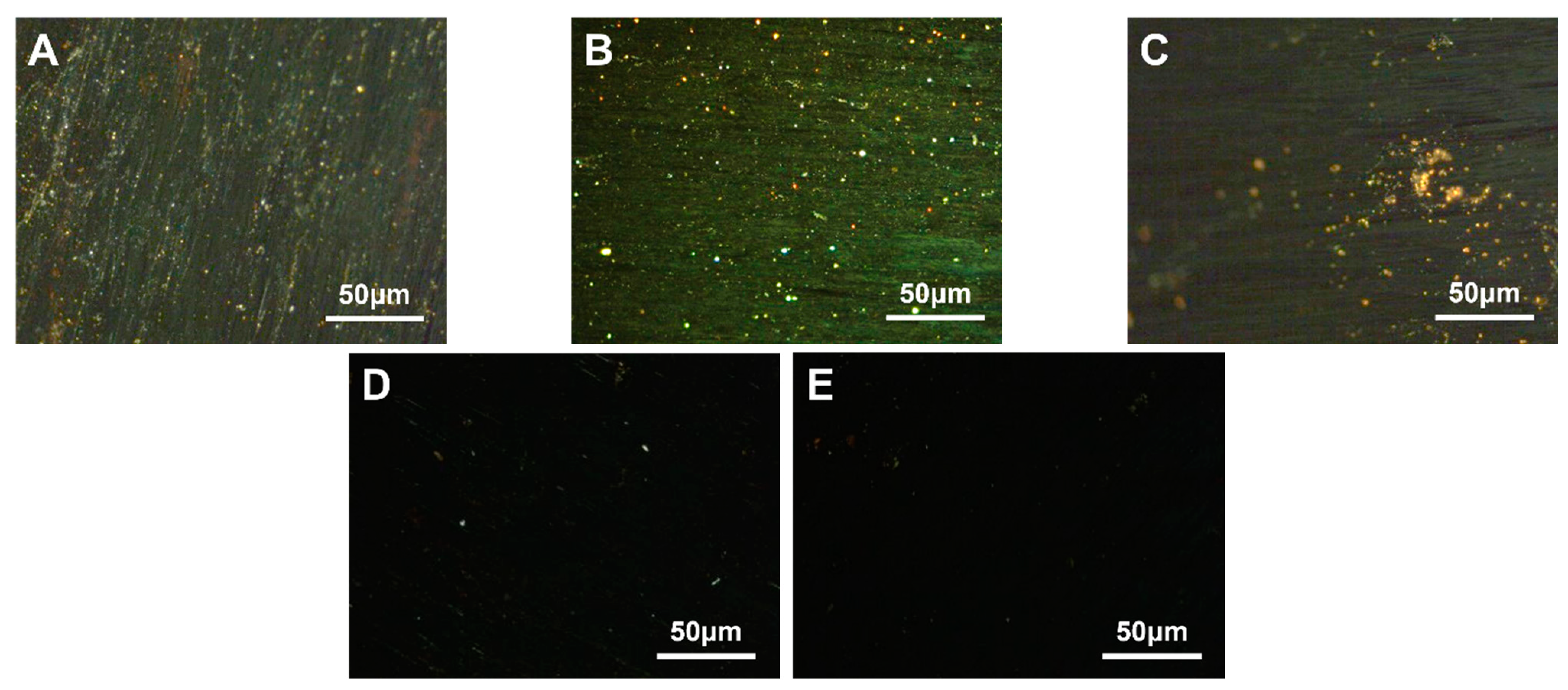
3.2. SBS-Modified Carbonaceous Mesophase
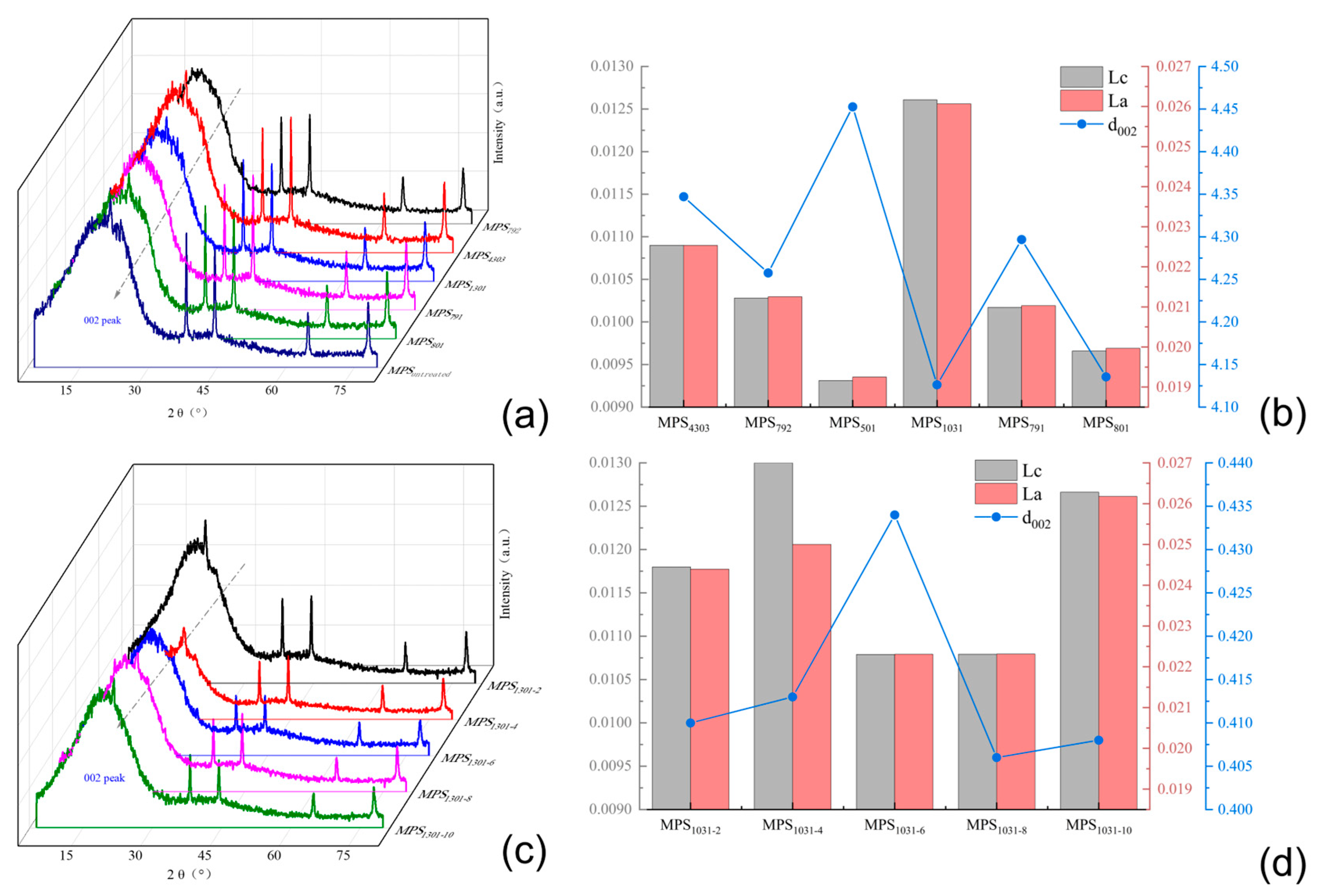
3.2.1. Different SBS Structure Modified Carbonaceous Mesophase
3.2.2. Modified Carbon Mesophase with Different SBS Block Ratios
3.2.3. Modified Carbonaceous Mesophase with Different SBS-1301 Additions
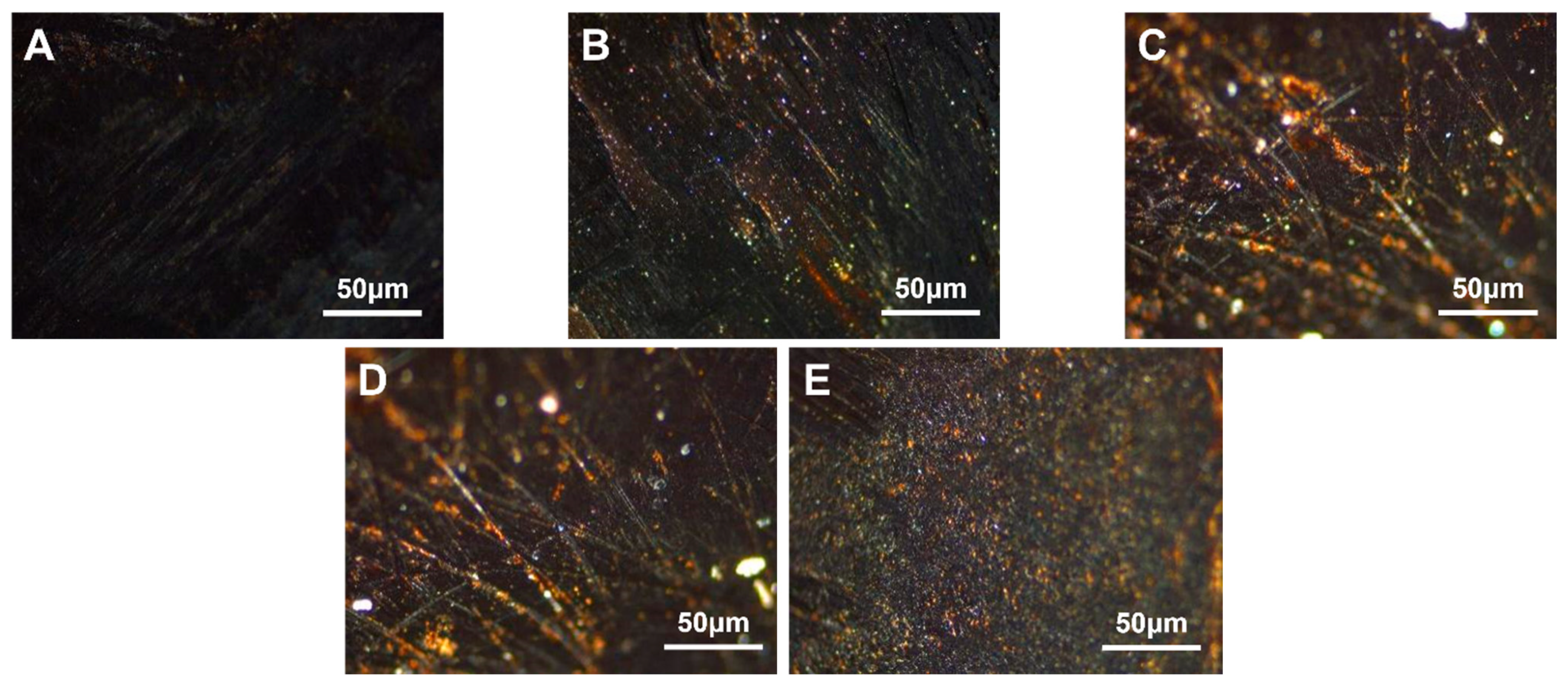
3.3. PE-Modified Carbon Mesophase
3.3.1. PE-Modified Carbon Mesophase with Different Density
3.3.2. Modified Carbonaceous Mesophase with Different HDPE Additions
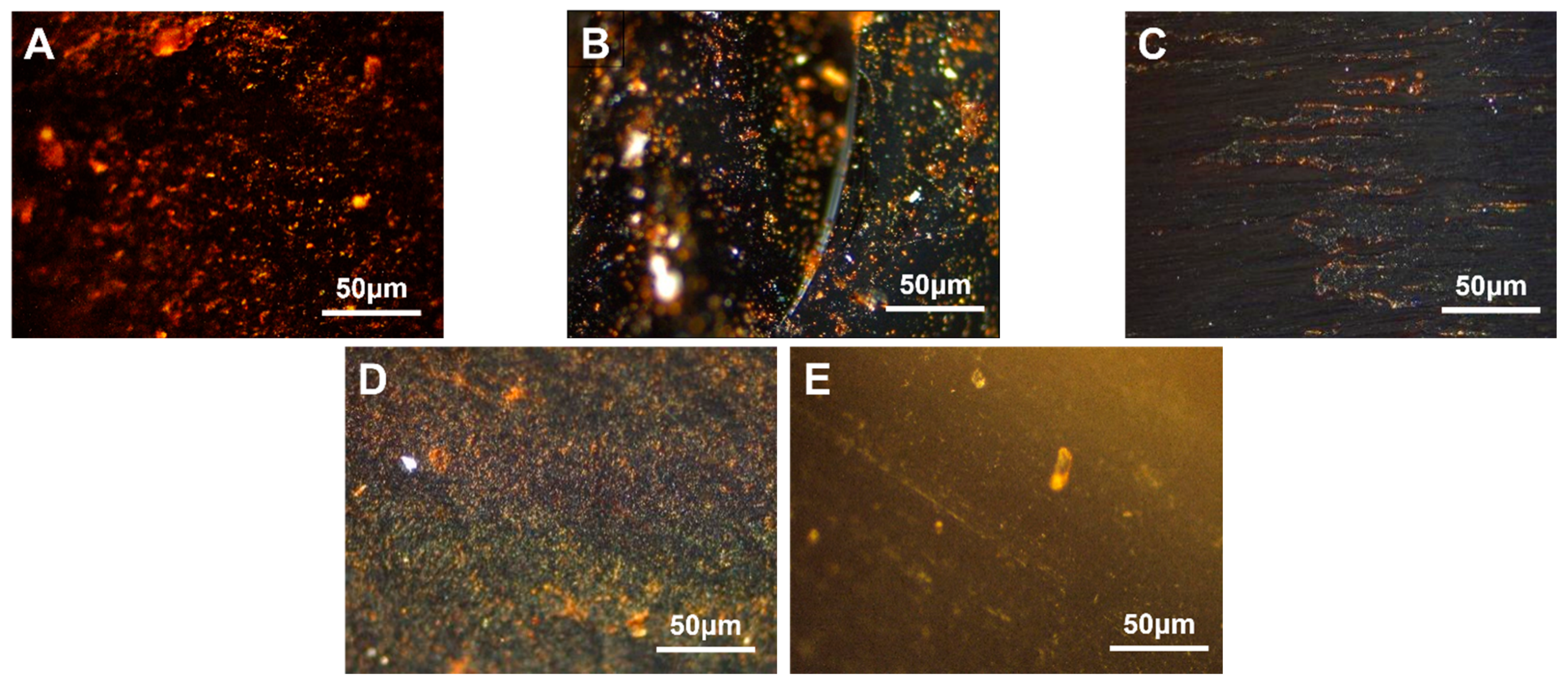
3.4. EVA-Modified Carbon Mesophase
3.4.1. Modified with Different VA Content
3.4.2. Modified with Different EVA Additions
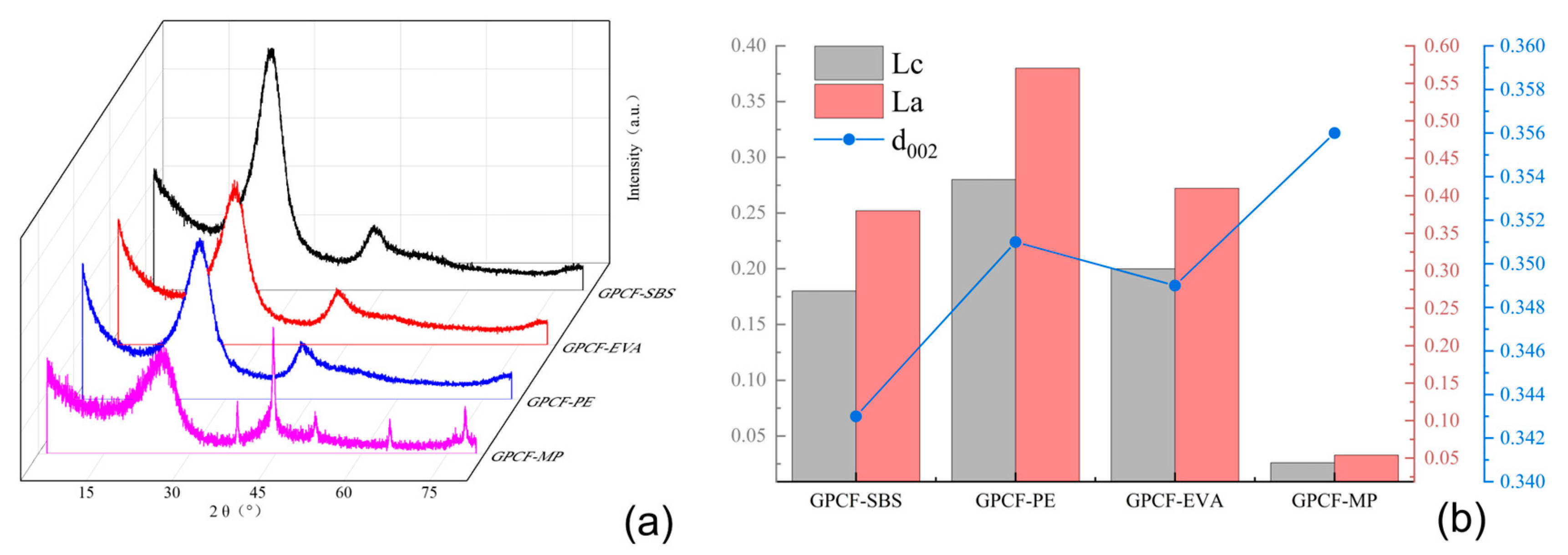
3.5. Comparison of the Effect of Carbonaceous Mesophase Modified by Copolymer
3.6. Analysis of the Modification Mechanism
3.6.1. XRD Analysis
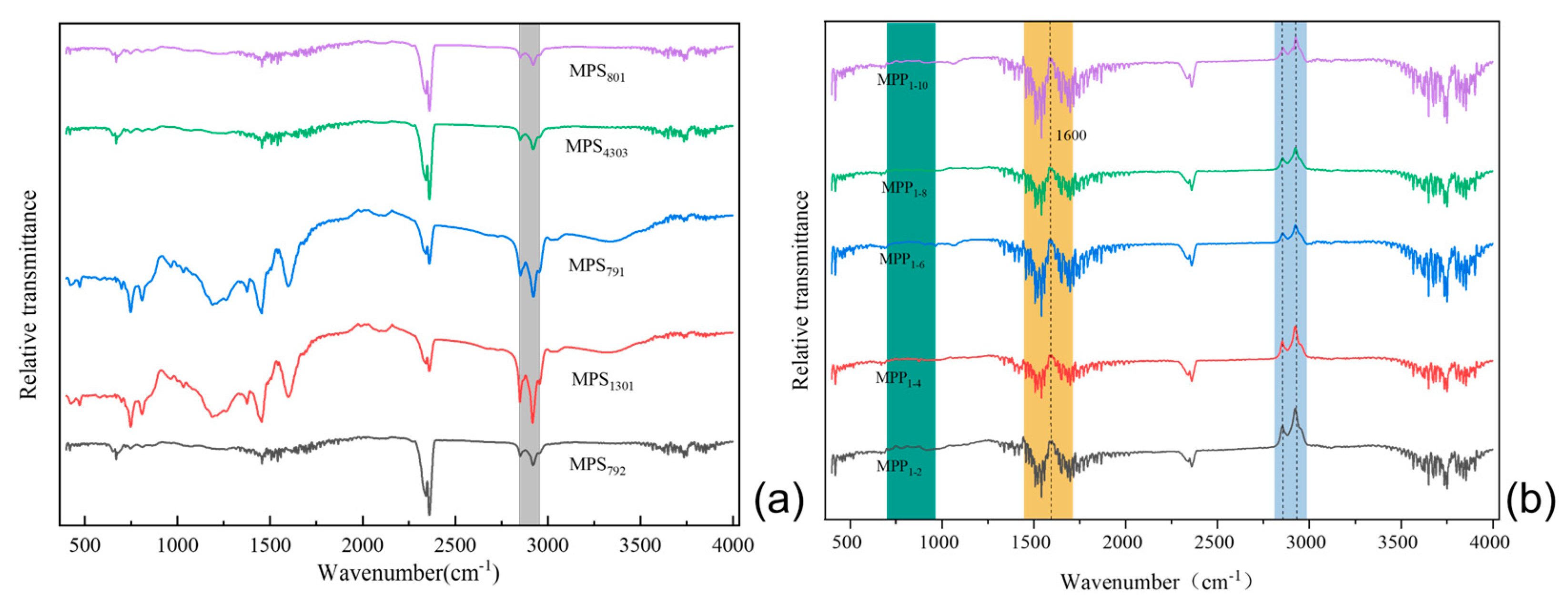
3.6.2. FT-IR Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, M.; Zhu, J.; Wei, B.; Hu, H.; Jin, L. Catalytic upgrading of ex-situ heavy coal tar over modified activated carbon. Fuel 2022, 312, 122912. [Google Scholar] [CrossRef]
- Pasternak, O.; Bannikov, L.; Smirnova, A. Coal Tar Viscosity when Dissolving Coke Oven Gas Deposits. Chem. Chem. Technol. 2017, 11, 125–130. [Google Scholar] [CrossRef]
- Prysiazhnyi, Y.; Borbeyiyong, G.I.; Korchak, B.; Pyshyev, S.; Shved, M.; Matlakh, Y. Obtaining and Use of Coumarone-Indene-Carbazole Resin as a Modifier of Road Petroleum Bitumen. 2. Setting the Type and Amount of Catalyst. Chem. Chem. Technol. 2023, 17, 450–459. [Google Scholar] [CrossRef]
- Zhang, J.X. Review of Coal Tar Preparation and Processing Technology. Adv. Mater. Res. 2012, 619, 286–289. [Google Scholar] [CrossRef]
- Liu, D.; Yu, R.; Niu, J.; Lou, B.; Fu, Y.; Guo, S.; Zhao, N.; Yu, E. The effect of accumulation of polycyclic aromatics in FCC decant oil on subsequent pyrolysis behaviors. Fuel 2020, 270, 117529. [Google Scholar] [CrossRef]
- Brooks, J.D.; Taylor, G.H. The formation of graphitizing carbons from the liquid phase. Carbon 1965, 3, 185–193. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Guo, Q.; Yang, J.; Dong, X.; Li, D.; Liu, J.; Shi, J.; Lu, C.; Liu, L. Structure of silicon-modified mesophase pitch-based graphite fibers. Carbon 2015, 94, 335–341. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, J.; Liu, L.; Dong, Y.; Liu, A. Mesophase pitch-based carbon fiber spinning through a filter assembly and the microstructure evolution mechanism. J. Mater. Sci. 2013, 49, 191–198. [Google Scholar] [CrossRef]
- Lim, C.; Ko, Y.; Kwak, C.H.; Kim, S.; Lee, Y.-S. Mesophase pitch production aided by the thermal decomposition of polyvinylidene fluoride. Carbon Lett. 2022, 32, 1329–1335. [Google Scholar] [CrossRef]
- Liu, H.; Li, T.; Shi, Y.; Wang, X.; Lv, J.; Zhang, W. Effect of different secondary quinoline insoluble content on the cellular structure of carbon foam derived from coal tar pitch. J. Anal. Appl. Pyrolysis 2014, 108, 310–315. [Google Scholar] [CrossRef]
- Gul, A.; Yardim, M.F. Preparation and characterization of mesophase pitch based carbon foam produced at low pressure. J. Porous Mater. 2015, 22, 851–857. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Xia, L. Fabrication and characterization of ultrafine graphite/carbon foam composites. J. Porous Mater. 2015, 22, 565–570. [Google Scholar] [CrossRef]
- Casco, M.E.; Martínez-Escandell, M.; Kaneko, K.; Silvestre-Albero, J.; Rodríguez-Reinoso, F. Very high methane uptake on activated carbons prepared from mesophase pitch: A compromise between microporosity and bulk density. Carbon 2015, 93, 11–21. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, L.; Liu, Q.; Sun, H.; Wang, Q.; Li, W.; Li, Z.; Wang, B. Ultra-high specific surface area porous carbon derived from chestnut for high-performance supercapacitor. Biomass Bioenergy 2021, 153, 106227. [Google Scholar] [CrossRef]
- Kong, H.J.; Kim, S.; Le, T.H.; Kim, Y.; Park, G.; Park, C.S.; Kwon, O.S.; Yoon, H. Nanostructured mesophase electrode materials: Modulating charge-storage behavior by thermal treatment. Nanoscale 2017, 9, 17450–17458. [Google Scholar] [CrossRef] [PubMed]
- Xu, H. Thermal conversion of an anion-exchange resin: A new catalytic-graphitization route to prepare porous carbons with a high graphitization degree for supercapacitors. RSC Adv. 2016, 6, 112576–112580. [Google Scholar] [CrossRef]
- Huang, Y.S.; Zhang, H.R.; Zhang, X.Q.; Yan, L.W.; Ling, Y.Q.; Zou, H.W.; Chen, Y.; Liang, M. Effect of Mesophase Pitch Incorporation on the Ablation Behavior and Mechanism of Phenolic Composites. Ind. Eng. Chem. Res. 2022, 61, 4612–4624. [Google Scholar] [CrossRef]
- Shimanoe, H.; Ko, S.; Jeon, Y.P.; Nakabayashi, K.; Miyawaki, J.; Yoon, A.S. Shortening Stabilization Time Using Pressurized Air Flow in Manufacturing Mesophase Pitch-Based Carbon Fiber. Polymers 2019, 11, 1911. [Google Scholar] [CrossRef]
- Zhang, K.; Ma, Z.; Song, H.; Zhang, M.; Xu, H.; Zhao, N. Macroporous carbon foam with high conductivity as an efficient anode for microbial fuel cells. Int. J. Hydrogen Energy 2020, 45, 12121–12129. [Google Scholar] [CrossRef]
- Li, H.; Li, T.; Deng, W.; Kong, S. Preparation and Adsorption Properties of Graphene-Modified, Pitch-Based Carbon Foam Composites. Polymers 2022, 14, 4455. [Google Scholar] [CrossRef]
- Sugiura, S.; Nishimura, S.; Yasuda, S.; Hosoya, Y.; Katoh, K. Carbon fiber technique for the investigation of single-cell mechanics in intact cardiac myocytes. Nat. Protoc. 2006, 1, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Ogale, A.A. Rheostructural studies on a synthetic mesophase pitch during transient shear flow. Carbon 2006, 44, 2224–2235. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, G.; Hou, X.; Yang, F.; Fan, B.; Zhang, R.; Li, H. Influence of bonding carbon on low carbon Al2O3-C refractory composites. Ceram. Int. 2017, 43, 14599–14607. [Google Scholar] [CrossRef]
- Yang, P.-j.; Li, T.-h.; Li, H.; Dang, A.l.; Yuan, L. Progress in the graphitization and applications of modified resin carbons. New Carbon Mater. 2023, 38, 96–108. [Google Scholar] [CrossRef]
- Ali, N.; Zaman, H.; Zaman, W.; Bilal, M. Rheological properties, structural and thermal elucidation of coal-tar pitches used in the fabrication of multi-directional carbon-carbon composites. Mater. Chem. Phys. 2020, 242, 122564. [Google Scholar] [CrossRef]
- Yuan, G.; Jin, Z.; Zuo, X.; Xue, Z.; Yan, F.; Dong, Z.; Cong, Y.; Li, X. Effect of Carbonaceous Precursors on the Structure of Mesophase Pitches and Their Derived Cokes. Energy Fuels 2018, 32, 8329–8339. [Google Scholar] [CrossRef]
- Forintos, N.; Czigany, T. Multifunctional application of carbon fiber reinforced polymer composites: Electrical properties of the reinforcing carbon fibers—A short review. Compos. Part B Eng. 2019, 162, 331–343. [Google Scholar] [CrossRef]
- Stepanek, P.; Lantto, P. Unexpected NMR shieldings of sp- and sp(2)-hybridized carbon atoms in graphyne systems. Phys. Chem. Chem. Phys. 2022, 24, 25513–25521. [Google Scholar] [CrossRef]
- Zhai, X.; Liu, J.; Zhang, Y.; Fan, Q.; Li, Z.; Zhou, Y. Microcrystal structure evolution of mesophase pitch-based carbon fibers with enhanced oxidation resistance and tensile strength induced by boron doping. Ceram. Int. 2019, 45, 11734–11738. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, W.; Cheng, L.; Cao, W.; Sain, M.; Tan, J.; Wang, A.; Jia, H. Carbon Fibers with High Electrical Conductivity: Laser Irradiation of Mesophase Pitch Filaments Obtains High Graphitization Degree. ACS Sustain. Chem. Eng. 2020, 8, 17629–17638. [Google Scholar] [CrossRef]
- Lee, S.; Eom, Y.; Kim, B.-J.; Mochida, I.; Yoon, S.-H.; Kim, B.C. The thermotropic liquid crystalline behavior of mesophase pitches with different chemical structures. Carbon 2015, 81, 694–701. [Google Scholar] [CrossRef]
- Liu, H.; Li, T.; Wang, X.; Zhang, W.; Zhao, T. Preparation and characterization of carbon foams with high mechanical strength using modified coal tar pitches. J. Anal. Appl. Pyrolysis 2014, 110, 442–447. [Google Scholar] [CrossRef]
- Mochida, I.; Amamoto, K.; Maeda, K.; Takeshita, K.; Marsh, H. Co-carbonization of solvent fractions of hydrogenated and alkylated SRC pitches in studies of formation of needle-cokes. Fuel 1979, 58, 482–488. [Google Scholar] [CrossRef]
- Li, J.; Lou, B.; Chai, L.; Fu, Y.; Yu, R.; Gong, X.; Liu, D. Influence of boron trifluoride complex addition on structure and composition of mesophase pitch from FCC decant oil via two-stage thermotreatment. Fuel 2022, 325, 124801. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Zhang, Q.L.; Fang, C.Q.; Ouyang, Y.; Chen, J.; Yu, X.; Liu, D.H. Co-carbonization behaviors of petroleum pitch/waste SBS: Influence on morphology and structure of resultant cokes. J. Anal. Appl. Pyrolysis 2018, 129, 154–161. [Google Scholar] [CrossRef]
- Cheng, Y.; Han, H.; Fang, C.; Li, H.; Huang, Z.; Su, J. Preparation and properties of nano-CaCO3/waste polyethylene/styrene-butadiene-styrene block polymer-modified asphalt. Polym. Compos. 2019, 41, 614–623. [Google Scholar] [CrossRef]
- Li, H.; Sengeh, J.; Agboola, O.D.; Seo, J.; Colby, R.; Chung, T.C.M. Preparation and Characterization of Polyethylene Copolymers with PAH Side Groups as Carbon Fiber Precursors. ACS Appl. Polym. Mater. 2022, 5, 791–802. [Google Scholar] [CrossRef]
- Du, J.W.; Zhou, T.T.; Zhang, R.; Hu, S.F. Influence of TPU/EVA Phase Morphology Evolution on Supercritical Carbon Dioxide Extrusion Foaming. Polymers 2023, 15, 3134. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, L.; Luo, T.; Fang, C.; Su, J.; Hui, J. Preparation and Characterization of Mesophase Pitch via Co-Carbonization of Waste Polyethylene/Petroleum Pitch. J. Mater. Sci. Technol. 2015, 31, 857–863. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Guo, B.H.; Xu, J. Polarized Optical Imaging and Its Application in Characterization of Polymer Crystalline Structures. Acta Polym. Sin. 2023, 54, 130–150. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, H.; Ibrahim, U.-K.; Xue, W.; Liu, H.; Guo, A. The quantitative assessment of coke morphology based on the Raman spectroscopic characterization of serial petroleum cokes. Fuel 2019, 246, 60–68. [Google Scholar] [CrossRef]
- Hou, H.; Pu, Z.; Wang, X.; Zhu, R.; Li, X.; Zhong, J. Effect of surface modification of SiO2 particles on the interfacial and mechanical properties of PBS composites. Polym. Compos. 2022, 43, 5087–5094. [Google Scholar] [CrossRef]
- Yu, J.; Ma, X.L.; Song, Q.F.; Zhao, Y.K.; Cao, J.H.; Chen, X. The effect of B4C on the properties of graphite foam prepared by template method. Mater. Res. Innov. 2015, 19, S5-118–S115-122. [Google Scholar] [CrossRef]
- Li, W.-w.; Kang, H.-l.; Xu, J.; Liu, R.-g. Effects of ultra-high temperature treatment on the microstructure of carbon fibers. Chin. J. Polym. Sci. 2017, 35, 764–772. [Google Scholar] [CrossRef]
- Ouzilleau, P.; Gheribi, A.E.; Chartrand, P.; Soucy, G.; Monthioux, M. Why some carbons may or may not graphitize? The point of view of thermodynamics. Carbon 2019, 149, 419–435. [Google Scholar] [CrossRef]
- Zhao, L.; Guanhua, N.; Hui, W.; Qian, S.; Gang, W.; Bingyou, J.; Chao, Z. Molecular structure characterization of lignite treated with ionic liquid via FTIR and XRD spectroscopy. Fuel 2020, 272, 117705. [Google Scholar] [CrossRef]
- Xing, C.; Liu, L.; Li, M. Chemical Composition and Aging Characteristics of Linear SBS Modified Asphalt Binders. Energy Fuels 2020, 34, 4194–4200. [Google Scholar] [CrossRef]
- Sun, G.; Li, B.; Sun, D.; Yu, F.; Hu, M. Chemo-rheological and morphology evolution of polymer modified bitumens under thermal oxidative and all-weather aging. Fuel 2021, 285, 118989. [Google Scholar] [CrossRef]
- Stastna, J.; Zanzotto, L.; Vacin, O.J. Viscosity function in polymer-modified asphalts. J. Colloid Interface Sci. 2003, 259, 200–207. [Google Scholar] [CrossRef]
- Cheng, X.; Zha, Q.; Zhong, J.; Yang, X. Needle coke formation derived from co-carbonization of ethylene tar pitch and polystyrene. Fuel 2009, 88, 2188–2192. [Google Scholar] [CrossRef]
- Castro-Díaz, M.; Vega, M.F.; Barriocanal, C.; Snape, C.E. Utilization of Carbonaceous Materials To Restore the Coking Properties of Weathered Coals. Energy Fuels 2015, 29, 5744–5749. [Google Scholar] [CrossRef]
- Saeaung, K.; Phusunti, N.; Phetwarotai, W.; Assabumrungrat, S.; Cheirsilp, B. Catalytic pyrolysis of petroleum-based and biodegradable plastic waste to obtain high-value chemicals. Waste Manag. 2021, 127, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Melendi, S.; Barriocanal, C.; Alvarez, R.; Diez, M.A. Influence of low-density polyethylene addition on coking pressure. Fuel 2014, 119, 274–284. [Google Scholar] [CrossRef]
- Liang, M.; Ren, S.; Fan, W.; Xin, X.; Shi, J.; Luo, H. Rheological property and stability of polymer modified asphalt: Effect of various vinyl-acetate structures in EVA copolymers. Constr. Build. Mater. 2017, 137, 367–380. [Google Scholar] [CrossRef]
- Nciri, N.; Kim, N.; Cho, N. New insights into the effects of styrene-butadiene-styrene polymer modifier on the structure, properties, and performance of asphalt binder: The case of AP-5 asphalt and solvent deasphalting pitch. Mater. Chem. Phys. 2017, 193, 477–495. [Google Scholar] [CrossRef]
- Lin, P.; Huang, W.; Li, Y.; Tang, N.; Xiao, F. Investigation of influence factors on low temperature properties of SBS modified asphalt. Constr. Build. Mater. 2017, 154, 609–622. [Google Scholar] [CrossRef]





| HS (wt.%) | HI-TS (wt.%) | TI-QS (wt.%) | QI (wt.%) |
|---|---|---|---|
| 92.28 | 6.88 | 0.84 | - |
| Asphalt | Anthracene Oil | Wash Oil | Naphthalene Oil | Phenol Oil | Light Oil | Other |
|---|---|---|---|---|---|---|
| 90.5% | 3.4% | 2.3% | 1.8% | 1.8% | 0.1% | 0.1% |
| Brand | 1301 | 4303 | 792 | YH-801 | 791-H |
|---|---|---|---|---|---|
| S/B | 30/70 | 30/70 | 40/60 | 30/70 | 30/70 |
| Structure | Linear | Radial | Linear | Radial | Linear |
| Category | High-Density Polyethylene | Linear Low-Density Polyethylene | Low-Density Polyethylene |
|---|---|---|---|
| Specification | HDPE | LLDPE | LDPE |
| Crystallinity (%) | 85~97 | 50~55 | 55~65 |
| Density (g/cm3) | 0.941~0.960 | 0.918~0.935 | 0.91~0.93 |
| VA contents | 12 wt.% | 18 wt.% | 25 wt.% | 32 wt.% | 40 wt.% |
|---|---|---|---|---|---|
| Melt index (g/10 min) (190 °C/2.16 kg) | 8 | 8 | 19 | 43 | 52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Liu, C.; Jia, Y.; Mu, Y.; Yan, Y.; Huang, P. Pyrolytic Modification of Heavy Coal Tar by Multi-Polymer Blending: Preparation of Ordered Carbonaceous Mesophase. Polymers 2024, 16, 161. https://doi.org/10.3390/polym16010161
Zhang L, Liu C, Jia Y, Mu Y, Yan Y, Huang P. Pyrolytic Modification of Heavy Coal Tar by Multi-Polymer Blending: Preparation of Ordered Carbonaceous Mesophase. Polymers. 2024; 16(1):161. https://doi.org/10.3390/polym16010161
Chicago/Turabian StyleZhang, Lei, Chunjiang Liu, Yang Jia, Yidan Mu, Yao Yan, and Pengcheng Huang. 2024. "Pyrolytic Modification of Heavy Coal Tar by Multi-Polymer Blending: Preparation of Ordered Carbonaceous Mesophase" Polymers 16, no. 1: 161. https://doi.org/10.3390/polym16010161






