Biomass- and Carbon Dioxide-Derived Polyurethane Networks for Thermal Interface Material Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CO2-Captured FCT
2.2.1. General Procedure for 2,5-bis((oxiran-2-ylmethoxy)methyl)furan (BOMF) Synthesis
2.2.2. General Procedure for 4,4’-(((furan-2,5-diylbis(methylene))bis(oxy)) bis(methylene))bis(1,3-dioxolan-2-one) Synthesis
2.2.3. General Procedure for FCT Synthesis
2.3. Synthesis of Ag-Nanoparticle-Coated Multiwalled Carbon Nanotubes (nAgMWNTs)
2.4. Network Polyurethane (CPU) Using CO2
2.5. Preparation of CPU–Ag Composites (CPU-TIM) Using Ball Milling Method
2.6. Preparation of CPU and CPU-TIM Film Using Hot Press
2.7. Characterization Methods
3. Results
3.1. Solid-State Synthesis of CO2-Utilizing Network Polyurethanes (CPUs) Using a Biomass-Derived Alcohol and CO2 Fixation Crosslinker
3.2. Thermal and Thermomechanical Characterization of CPU Films
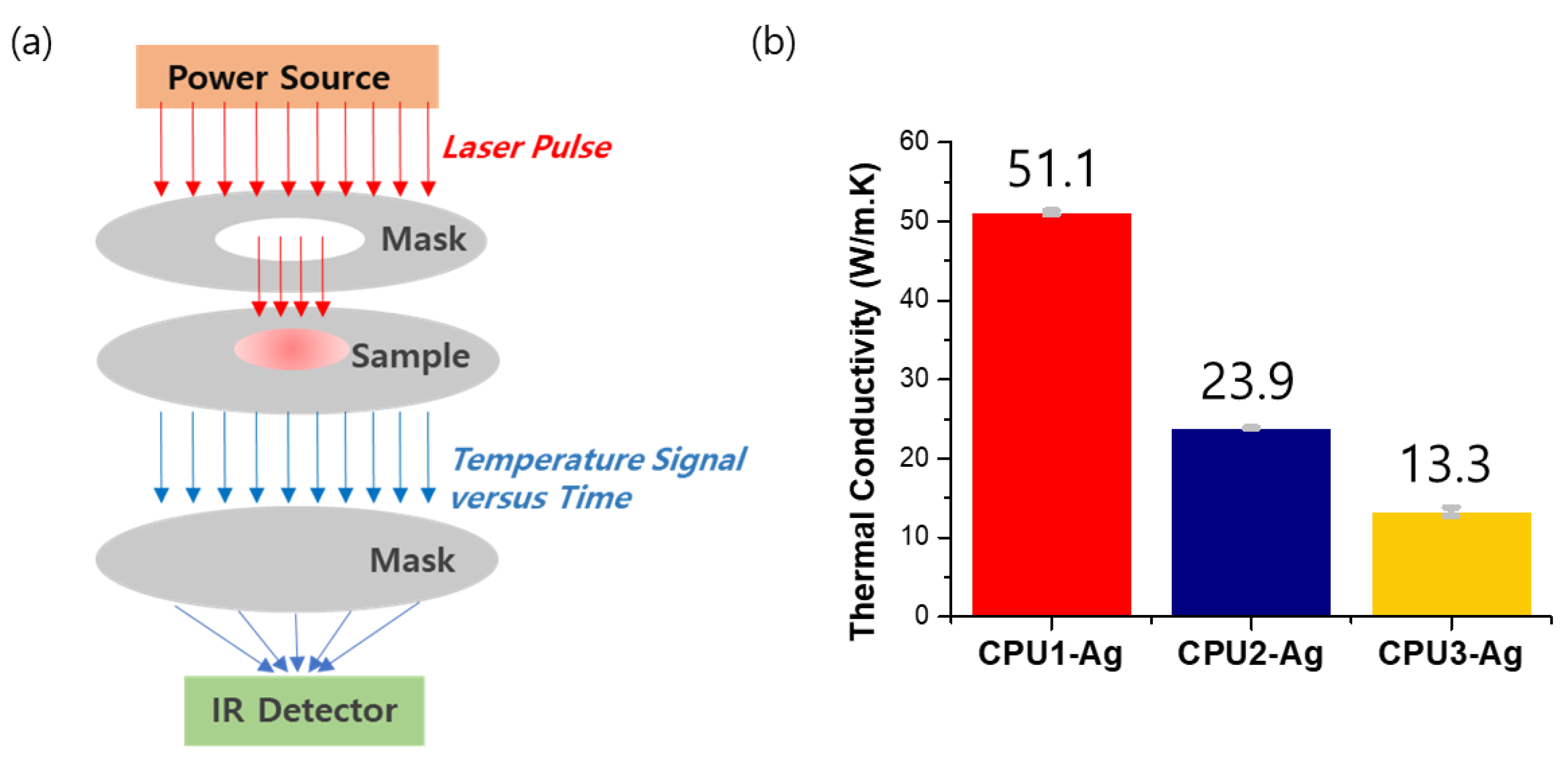
3.3. Thermal Conductivity Analysis of CPU–Ag Composites as Thermal Interfacial Material Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kumari, S.; Rao, A.; Kaur, M.; Dhania, G. Petroleum-Based Plastics versus Bio-Based Plastics: A Review. Nat. Environ. Pollut. Technol. 2023, 22, 1111–1124. [Google Scholar] [CrossRef]
- Maddah, H.A. Polypropylene as a Promising Plastic: A Review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.; Bryant, P.; Kurusingal, V.; Colwell, J.M.; Laycock, B. Degradation and stabilization of polyurethane elastomers. Prog. Polym. Sci. 2019, 90, 211–268. [Google Scholar] [CrossRef]
- Das, A.; Mahanwar, P. A brief discussion on advances in polyurethane applications. Adv. Ind. Eng. Polym. Res. 2020, 3, 93–101. [Google Scholar] [CrossRef]
- da Costa, J.P.; Santos, P.S.M.; Duarte, A.C.; Rocha-Santos, T. (Nano)plastics in the environment—Sources, fates and effects. Sci. Total Environ. 2016, 566–567, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Padervand, M.; Lichtfouse, E.; Robert, D.; Wang, C. Removal of microplastics from the environment. A review. Environ. Chem. Lett. 2020, 18, 807–828. [Google Scholar] [CrossRef]
- Schwarz, A.E.; Ligthart, T.N.; Boukris, E.; van Harmelen, T. Sources, transport, and accumulation of different types of plastic litter in aquatic environments: A review study. Mar. Pollut. Bull. 2019, 143, 92–100. [Google Scholar] [CrossRef]
- Ali, S.S.; Elsamahy, T.; Koutra, E.; Kornaros, M.; El-Sheekh, M.; Abdelkarim, E.A.; Zhu, D.; Sun, J. Degradation of conventional plastic wastes in the environment: A review on current status of knowledge and future perspectives of disposal. Sci. Total Environ. 2021, 771, 144719. [Google Scholar] [CrossRef]
- Jin, Y.; Lei, Z.; Taynton, P.; Huang, S.; Zhang, W. Malleable and Recyclable Thermosets: The Next Generation of Plastics. Matter 2019, 1, 1456–1493. [Google Scholar] [CrossRef]
- Morici, E.; Dintcheva, N.T. Recycling of Thermoset Materials and Thermoset-Based Composites: Challenge and Opportunity. Polymers 2022, 14, 4153. [Google Scholar] [CrossRef] [PubMed]
- Kloxin, C.J.; Bowman, C.N. Covalent adaptable networks: Smart, reconfigurable and responsive network systems. Chem. Soc. Rev. 2013, 42, 7161–7173. [Google Scholar] [CrossRef] [PubMed]
- Wojtecki, R.J.; Meador, M.A.; Rowan, S.J. Using the dynamic bond to access macroscopically responsive structurally dynamic polymers. Nat. Mater. 2011, 10, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Denissen, W.; Winne, J.M.; Du Prez, F.E. Vitrimers: Permanent organic networks with glass-like fluidity. Chem. Sci. 2016, 7, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Hammer, L.; Van Zee, N.J.; Nicolay, R. Dually Crosslinked Polymer Networks Incorporating Dynamic Covalent Bonds. Polymers 2021, 13, 396. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Chen, G.; Liao, Y.; Lu, X.; Hu, S.; Gan, T.; Handschuh-Wang, S.; Zhang, X. Self-Healable and Recyclable Dual-Shape Memory Liquid Metal-Elastomer Composites. Polymers 2022, 14, 2259. [Google Scholar] [CrossRef]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-Like Malleable Materials from Permanent Organic Networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef]
- Del Giudice, D.; Valentini, M.; Melchiorre, G.; Spatola, E.; Di Stefano, S. Dissipative Dynamic Covalent Chemistry (DDCvC) Based on the Transimination Reaction. Chemistry 2022, 28, e202200685. [Google Scholar] [CrossRef]
- Denissen, W.; Rivero, G.; Nicolaÿ, R.; Leibler, L.; Winne, J.M.; Du Prez, F.E. Vinylogous Urethane Vitrimers. Adv. Funct. Mater. 2015, 25, 2451–2457. [Google Scholar] [CrossRef]
- Cromwell, O.R.; Chung, J.; Guan, Z. Malleable and Self-Healing Covalent Polymer Networks through Tunable Dynamic Boronic Ester Bonds. J. Am. Chem. Soc. 2015, 137, 6492–6495. [Google Scholar] [CrossRef]
- Röttger, M.; Domenech, T.; van der Weegen, R.; Breuillac, A.; Nicolaÿ, R.; Leibler, L. High-performance vitrimers from commodity thermoplastics through dioxaborolane metathesis. Science 2017, 356, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xie, M.; Mai, L.; Zeng, E.Y. Biobased plastic: A plausible solution toward carbon neutrality in plastic industry? J. Hazard. Mater. 2022, 435, 129037. [Google Scholar] [CrossRef] [PubMed]
- Gu, S. Raw material recycled practices for carbon neutrality. Glob. Transit. 2023, 5, 14–20. [Google Scholar] [CrossRef]
- Gabrielli, P.; Gazzani, M.; Mazzotti, M. The Role of Carbon Capture and Utilization, Carbon Capture and Storage, and Biomass to Enable a Net-Zero-CO2 Emissions Chemical Industry. Ind. Eng. Chem. Res. 2020, 59, 7033–7045. [Google Scholar] [CrossRef]
- Mishra, K.; Siwal, S.S.; Nayaka, S.C.; Guan, Z.; Thakur, V.K. Waste-to-chemicals: Green solutions for bioeconomy markets. Sci. Total Environ. 2023, 887, 164006. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, X.-B. Current Challenges and Perspectives in CO2-Based Polymers. Macromolecules 2023, 56, 1759–1777. [Google Scholar] [CrossRef]
- Delidovich, I.; Hausoul, P.J.; Deng, L.; Pfutzenreuter, R.; Rose, M.; Palkovits, R. Alternative Monomers Based on Lignocellulose and Their Use for Polymer Production. Chem. Rev. 2016, 116, 1540–1599. [Google Scholar] [CrossRef]
- Kumar, S.; Krishnan, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Itaconic acid used as a versatile building block for the synthesis of renewable resource-based resins and polyesters for future prospective: A review. Polym. Int. 2017, 66, 1349–1363. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Peng, Y.; Zhu, J.; Zhao, W.; Liu, X. Advances in sustainable thermosetting resins: From renewable feedstock to high performance and recyclability. Prog. Polym. Sci. 2021, 113, 101353. [Google Scholar] [CrossRef]
- Chernyshev, V.M.; Kravchenko, O.A.; Ananikov, V.P. Conversion of plant biomass to furan derivatives and sustainable access to the new generation of polymers, functional materials and fuels. Russ. Chem. Rev. 2017, 86, 357–387. [Google Scholar] [CrossRef]
- Oh, C.; Choi, E.H.; Choi, E.J.; Premkumar, T.; Song, C. Facile Solid-State Mechanochemical Synthesis of Eco-Friendly Thermoplastic Polyurethanes and Copolymers Using a Biomass-Derived Furan Diol. ACS Sustain. Chem. Eng. 2020, 8, 4400–4406. [Google Scholar] [CrossRef]
- Kim, H.; Cha, I.; Yoon, Y.; Cha, B.J.; Yang, J.; Kim, Y.D.; Song, C. Facile Mechanochemical Synthesis of Malleable Biomass-Derived Network Polyurethanes and Their Shape-Memory Applications. ACS Sustain. Chem. Eng. 2021, 9, 6952–6961. [Google Scholar] [CrossRef]
- Baek, S.; Lee, J.; Kim, H.; Cha, I.; Song, C. Self-Healable and Recyclable Biomass-Derived Polyurethane Networks through Carbon Dioxide Immobilization. Polymers 2021, 13, 4381. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.L.; Shi, L. Emerging challenges and materials for thermal management of electronics. Mater. Today 2014, 17, 163–174. [Google Scholar] [CrossRef]
- Khan, J.; Momin, S.A.; Mariatti, M. A review on advanced carbon-based thermal interface materials for electronic devices. Carbon 2020, 168, 65–112. [Google Scholar] [CrossRef]
- Razeeb, K.M.; Dalton, E.; Cross, G.L.W.; Robinson, A.J. Present and future thermal interface materials for electronic devices. Int. Mater. Rev. 2017, 63, 1–21. [Google Scholar] [CrossRef]
- Due, J.; Robinson, A.J. Reliability of thermal interface materials: A review. Appl. Therm. Eng. 2013, 50, 455–463. [Google Scholar] [CrossRef]
- Mehra, N.; Mu, L.; Ji, T.; Yang, X.; Kong, J.; Gu, J.; Zhu, J. Thermal transport in polymeric materials and across composite interfaces. Appl. Mater. Today 2018, 12, 92–130. [Google Scholar] [CrossRef]
- Akhtar, S.S. An Integrated Approach to Design and Develop High-Performance Polymer-Composite Thermal Interface Material. Polymers 2021, 13, 807. [Google Scholar] [CrossRef]
- Zhang, H.; He, Q.; Yu, H.; Qin, M.; Feng, Y.; Feng, W. A Bioinspired Polymer-Based Composite Displaying Both Strong Adhesion and Anisotropic Thermal Conductivity. Adv. Funct. Mater. 2023, 33, 2211985. [Google Scholar] [CrossRef]
- Huang, C.; Qian, X.; Yang, R. Thermal conductivity of polymers and polymer nanocomposites. Mater. Sci. Eng. R Rep. 2018, 132, 1–22. [Google Scholar] [CrossRef]
- Si, P.; Zhao, B. Water-based polyurethanes for sustainable advanced manufacture. Can. J. Chem. Eng. 2021, 99, 1851–1869. [Google Scholar] [CrossRef]
- Seydibeyoğlu, M. Excellent Adhesion of Carbon Fibers to Polyurethane Matrix and Substantial Improvement of the Mechanical Properties of Polyurethane. Chem. Eng. 2011, 24, 501–506. [Google Scholar]
- Vallat, M.F.; Bessaha, N.; Schultz, J.; Maucourt, J.; Combette, C. Adhesive behavior of polyurethane-based materials. J. Appl. Polym. Sci. 2000, 76, 665–671. [Google Scholar] [CrossRef]
- Kwiecień, A. Shear bond of composites-to-brick applied with highly deformable, in relation to resin epoxy, interface materials. Mater. Struct. 2014, 47, 2005–2020. [Google Scholar] [CrossRef]
- Choi, J.H.; Song, H.J.; Jung, J.; Yu, J.W.; You, N.H.; Goh, M. Effect of crosslink density on thermal conductivity of epoxy/carbon nanotube nanocomposites. J. Appl. Polym. Sci. 2017, 134, 44253. [Google Scholar] [CrossRef]
- Cha, I.; Kim, T.; Kim, K.-s.; Baik, S.; Song, C. Ultralow Contact Resistance of Thermal Interface Materials Enabled by the Vitrimer Chemistry of a β-Hydroxy Phosphate Ester. Chem. Mater. 2023, 35, 7491–7499. [Google Scholar] [CrossRef]
- Elizalde, F.; Aguirresarobe, R.H.; Gonzalez, A.; Sardon, H. Dynamic polyurethane thermosets: Tuning associative/dissociative behavior by catalyst selection. Polym. Chem. 2020, 11, 5386–5396. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Wei, T.; Venerus, D.C.; Torkelson, J.M. Reprocessable Polyhydroxyurethane Network Composites: Effect of Filler Surface Functionality on Cross-link Density Recovery and Stress Relaxation. ACS Appl. Mater. Interfaces 2019, 11, 2398–2407. [Google Scholar] [CrossRef]
- Zheng, N.; Fang, Z.; Zou, W.; Zhao, Q.; Xie, T. Thermoset Shape-Memory Polyurethane with Intrinsic Plasticity Enabled by Transcarbamoylation. Angew. Chem. Int. Ed. Engl. 2016, 55, 11421–11425. [Google Scholar] [CrossRef]
- Wang, H.; Ihms, D.W.; Brandenburg, S.D.; Salvador, J.R. Thermal Conductivity of Thermal Interface Materials Evaluated by a Transient Plane Source Method. J. Electron. Mater. 2019, 48, 4697–4705. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.W.; Cha, I.; Choi, J.; Han, J.; Hwang, J.Y.; Cho, I.G.; Son, S.U.; Kang, E.J.; Song, C. Biomass- and Carbon Dioxide-Derived Polyurethane Networks for Thermal Interface Material Applications. Polymers 2024, 16, 177. https://doi.org/10.3390/polym16020177
Jang JW, Cha I, Choi J, Han J, Hwang JY, Cho IG, Son SU, Kang EJ, Song C. Biomass- and Carbon Dioxide-Derived Polyurethane Networks for Thermal Interface Material Applications. Polymers. 2024; 16(2):177. https://doi.org/10.3390/polym16020177
Chicago/Turabian StyleJang, Ji Won, Inhwan Cha, Junhyeon Choi, Jungwoo Han, Joon Young Hwang, Il Gyu Cho, Seung Uk Son, Eun Joo Kang, and Changsik Song. 2024. "Biomass- and Carbon Dioxide-Derived Polyurethane Networks for Thermal Interface Material Applications" Polymers 16, no. 2: 177. https://doi.org/10.3390/polym16020177




