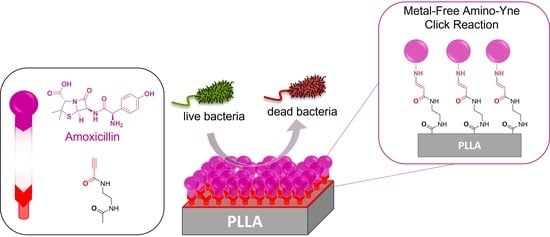
Catalyst-Free Amino-Yne Click Reaction: An Efficient Way for Immobilizing Amoxicillin onto Polymeric Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Preactivation and Drug Immobilization
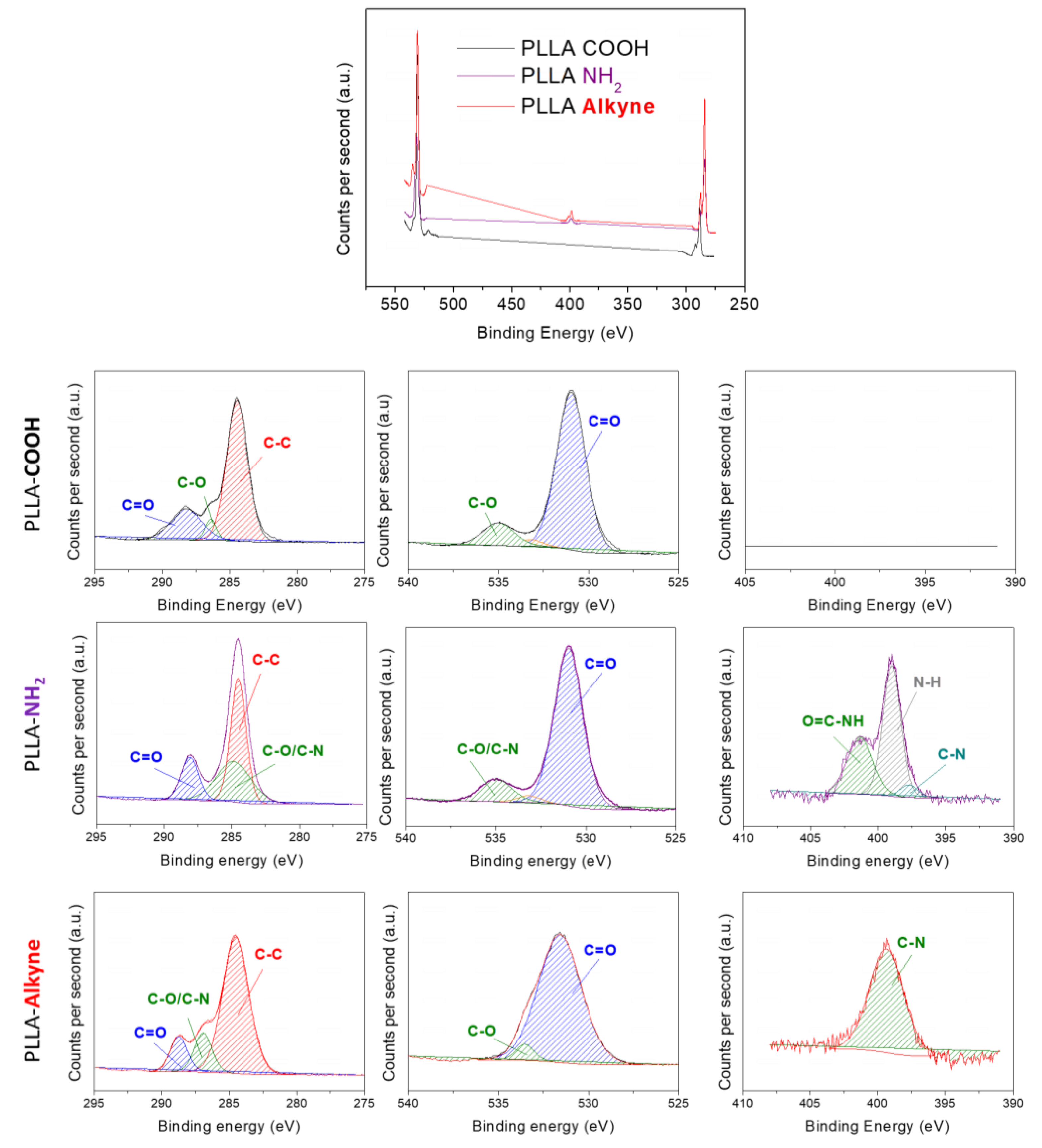
2.3. X-ray Photoelectron Spectroscopy (XPS)
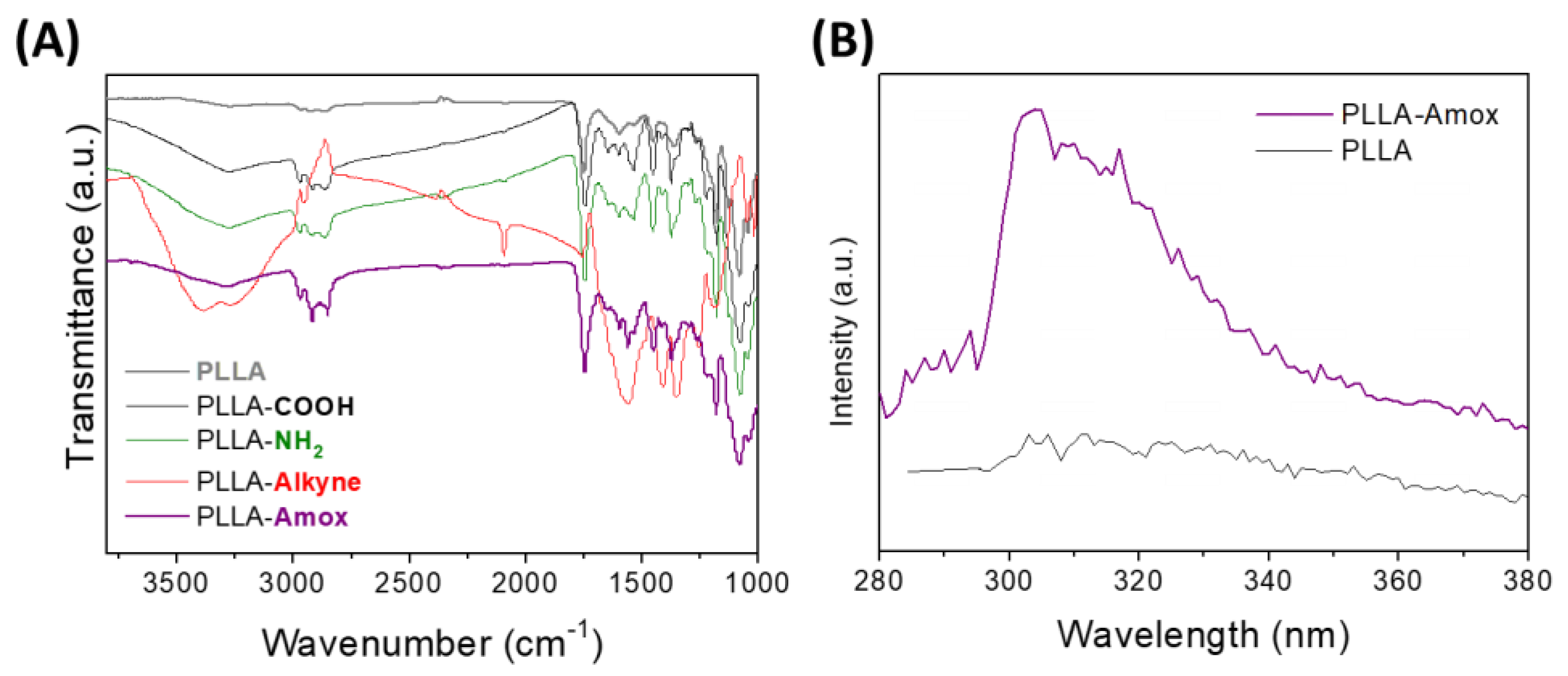
2.4. Attenuated Total Reflection Fourier-Transform Infrared (ATR-FTIR) Spectroscopy
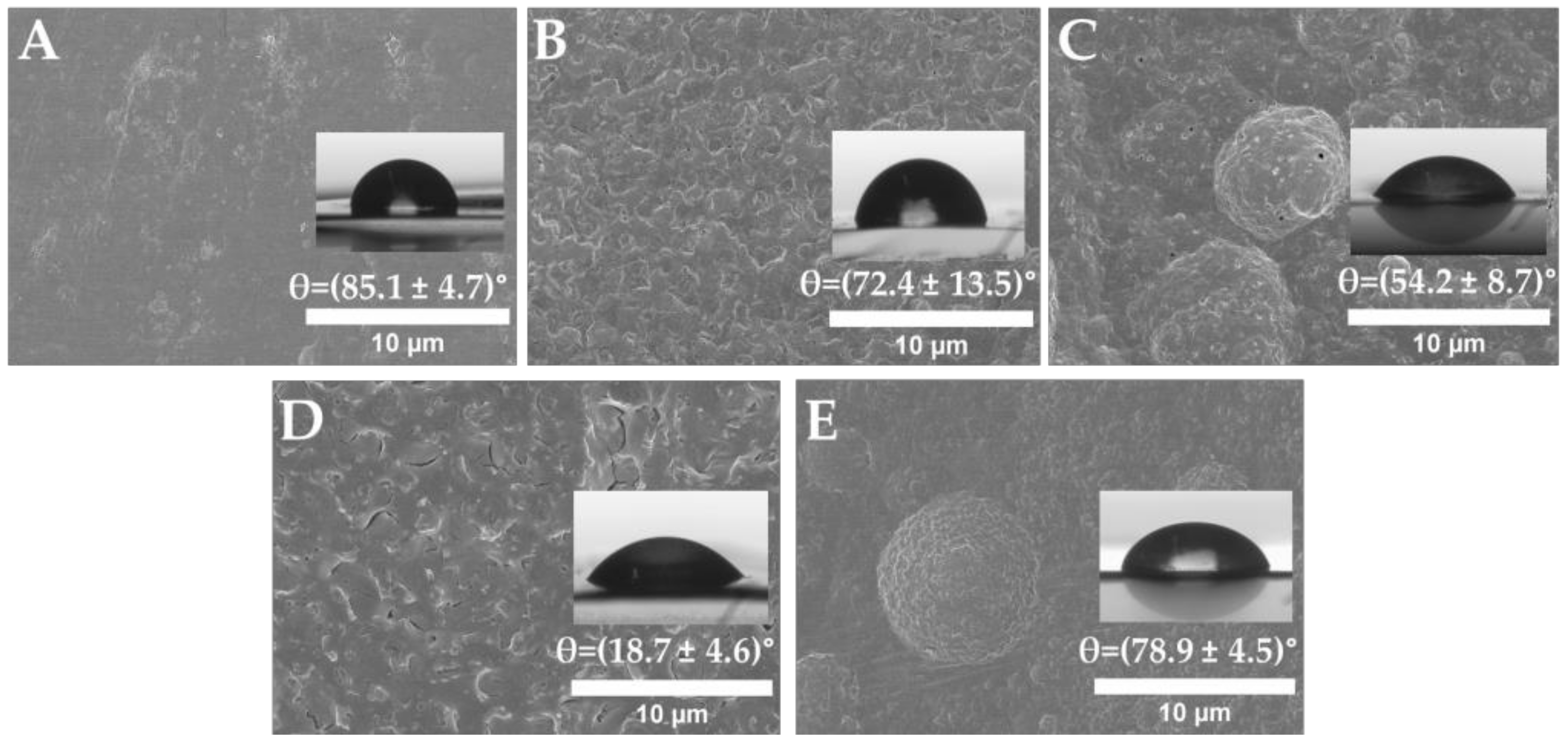
2.5. Scanning Electron Microscopy (SEM)
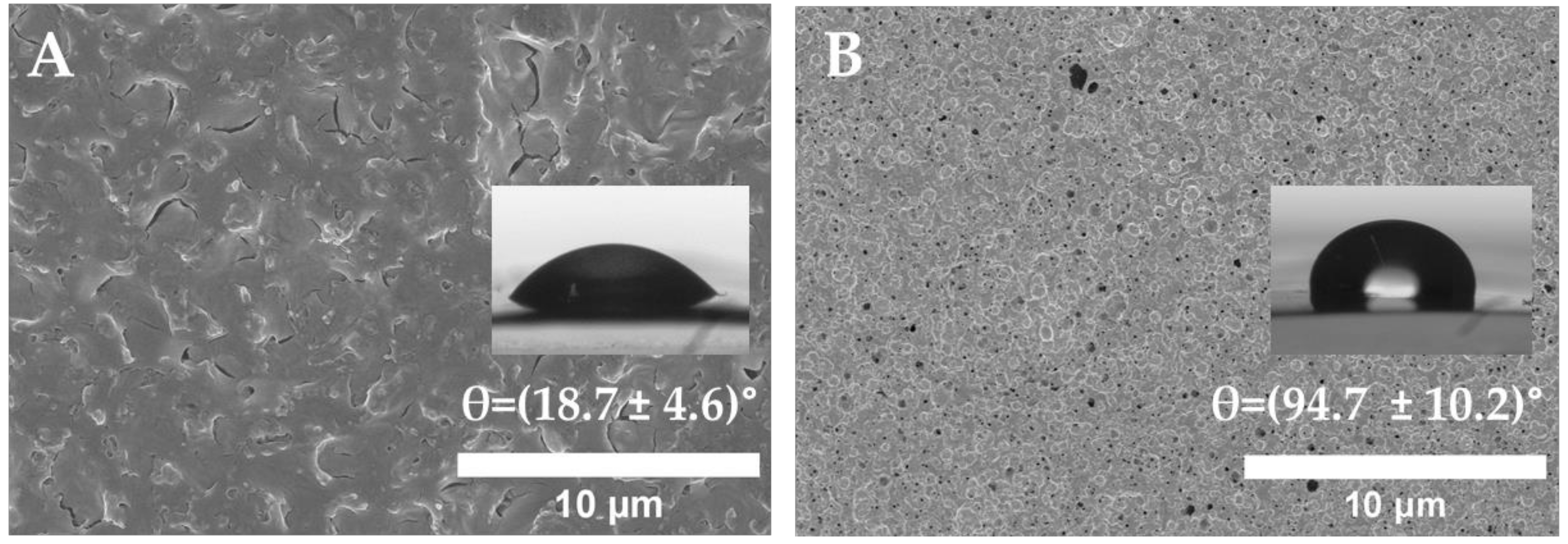
2.6. Water Contact Angle (WCA)
2.7. Confocal Fluorescence
2.8. Fluorescence Spectroscopy
3. Results
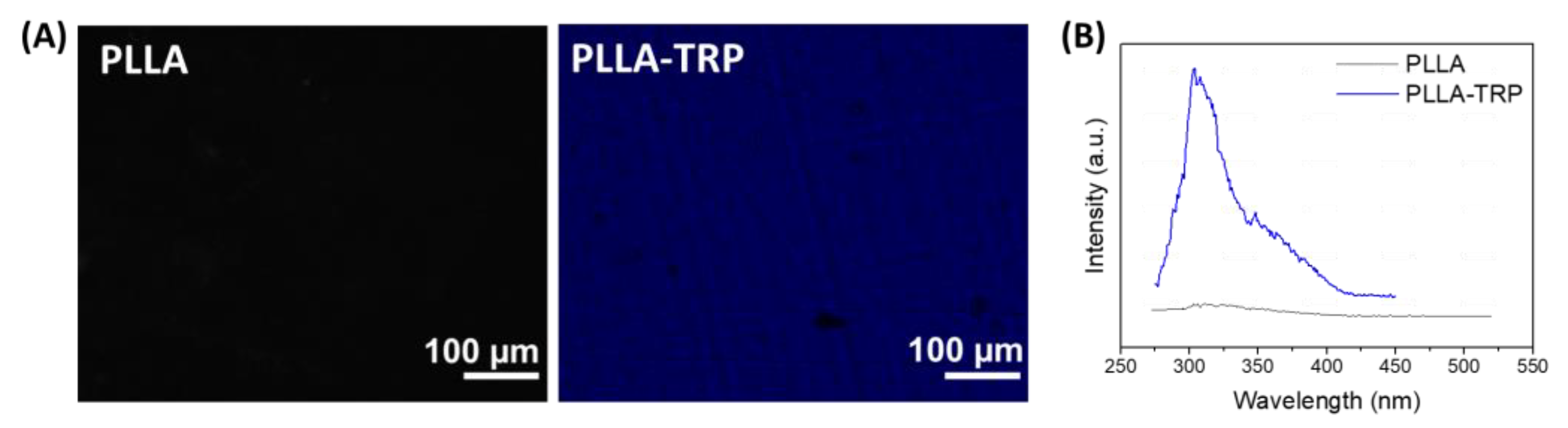
3.1. PLLA Surface Preactivation and Tryptophan Immobilization
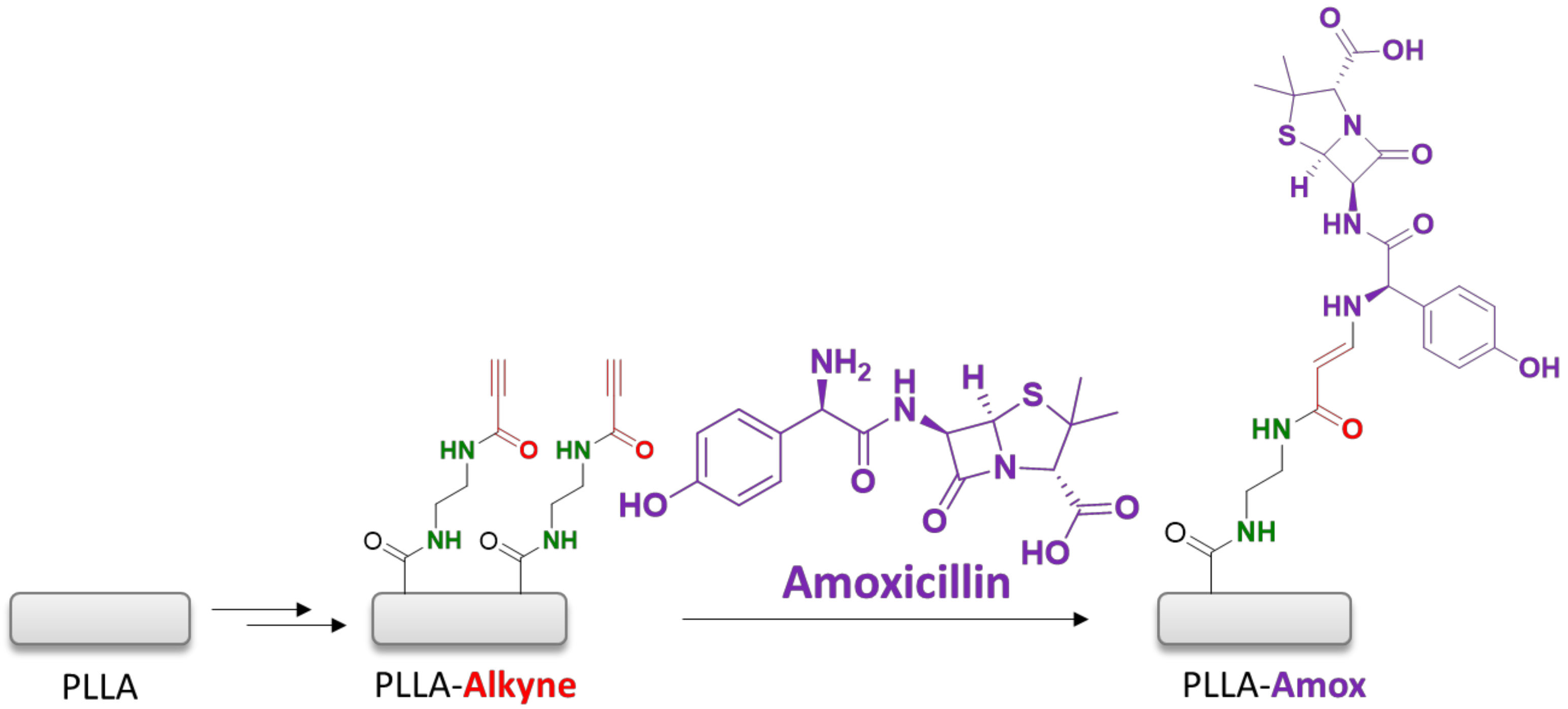
3.2. Amoxicillin Immobilization onto the PLLA Surfaces via a Copper-Free Amino-Yne Click Reaction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Taib, N.A.A.B.; Rahman, M.R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bakri, M.K.B.; Julaihi, M.R.M.B.; Khan, A. A Review on Poly Lactic Acid (PLA) as a Biodegradable Polymer; Springer: Berlin/Heidelberg, Germany, 2022; ISBN 0123456789. [Google Scholar]
- Garlotta, D. A Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2019, 9, 63–84. [Google Scholar] [CrossRef]
- Mehta, R.; Kumar, V.; Bhunia, H.; Upadhyay, S.N. Synthesis of poly(lactic acid): A review. J. Macromol. Sci. Polym. Rev. 2005, 45, 325–349. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Marino, A.; Nostro, A. Antimicrobial additives for poly(lactic acid) materials and their applications: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 7739–7756. [Google Scholar] [CrossRef] [PubMed]
- Seyednejad, H.; Ghassemi, A.H.; Van Nostrum, C.F.; Vermonden, T.; Hennink, W.E. Functional aliphatic polyesters for biomedical and pharmaceutical applications. J. Control. Release 2011, 152, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Chung, J.W.Y.; Yan, V.C.M.; Wong, T.K.S. Polylactic Acid-Based Biomaterials in Wound Healing: A Systematic Review. Adv. Ski. Wound Care 2023, 36, 1–8. [Google Scholar] [CrossRef]
- Narayanan, G.; Vernekar, V.N.; Kuyinu, E.L.; Laurencin, C.T. Poly (Lactic Acid)-Based Biomaterials for Orthopaedic Regenerative Engineering. Adv. Drug Deliv. Rev. 2016, 107, 247–276. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Mu, M.; Liu, S.; DeFlorio, W.; Hao, L.; Wang, X.; Salazar, K.S.; Taylor, M.; Castillo, A.; Cisneros-Zevallos, L.; Oh, J.K.; et al. Influence of Surface Roughness, Nanostructure, and Wetting on Bacterial Adhesion. Langmuir 2023, 39, 5426–5439. [Google Scholar] [CrossRef]
- Sánchez-Bodón, J.; Diaz-Galbarriatu, M.; Pérez-Álvarez, L.; Moreno-Benítez, I.; Vilas-Vilela, J.L. Strategies to Enhance Biomedical Device Performance and Safety: A Comprehensive Review. Coatings 2023, 13, 1981. [Google Scholar] [CrossRef]
- Olmo, J.A.D.; Ruiz-Rubio, L.; Pérez-Alvarez, L.; Sáez-Martínez, V.; Vilas-Vilela, J.L. Antibacterial coatings for improving the performance of biomaterials. Coatings 2020, 10, 139. [Google Scholar] [CrossRef]
- Pérez Davila, S.; González Rodríguez, L.; Chiussi, S.; Serra, J.; González, P. How to sterilize polylactic acid based medical devices? Polymers 2021, 13, 2115. [Google Scholar] [CrossRef] [PubMed]
- Subbiahdoss, G.; Kuijer, R.; Grijpma, D.W.; van der Mei, H.C.; Busscher, H.J. Microbial biofilm growth vs. tissue integration: “The race for the surface” experimentally studied. Acta Biomater. 2009, 5, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, G.; O’Connor, W.; Grainge, I.; Palanisami, T. Understanding the Fundamental Basis for Biofilm Formation on Plastic Surfaces: Role of Conditioning Films. Front. Microbiol. 2021, 12, 687118. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Ramasamy, K.; Leena, M.; Pasricha, R.; Manivasagam, G.; Ramalingam, M. Surface Functionalization of Biomaterials; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128027561. [Google Scholar]
- Pujari, S.P.; Scheres, L.; Marcelis, A.T.M.; Zuilhof, H. Covalent surface modification of oxide surfaces. Angew. Chem. Int. Ed. 2014, 53, 6322–6356. [Google Scholar] [CrossRef]
- Zhang, L.C.; Chen, L.Y.; Wang, L. Surface Modification of Titanium and Titanium Alloys: Technologies, Developments, and Future Interests. Adv. Eng. Mater. 2020, 22, 1901258. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Shi, Y.; Tang, J.; Huang, D.; Yan, M.; Dargusch, M.S. Surface Modification of Biomedical Ti and Ti Alloys: A Review on Current Advances. Materials 2022, 15, 1749. [Google Scholar] [CrossRef]
- He, B.; Su, H.; Bai, T.; Wu, Y.; Li, S.; Gao, M.; Hu, R.; Zhao, Z.; Qin, A.; Ling, J.; et al. Spontaneous Amino-yne Click Polymerization: A Powerful Tool toward Regio- and Stereospecific Poly(β-aminoacrylate)s. J. Am. Chem. Soc. 2017, 139, 5437–5443. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Wang, J.; Zang, Q.; Sun, J.Z.; Tang, B.Z. Recent progress in the applications of amino-yne click chemistry. Polym. Chem. 2021, 12, 2978–2986. [Google Scholar] [CrossRef]
- Li, H.-C.; Sun, X.-M.; Huang, Y.-R.; Peng, Y.-H.; Liu, J.; Ren, L. Synthetic Crosslinker Based on Amino–yne Click to Enhance the Suture Tension of Collagen-Based Corneal Repair Materials. ACS Appl. Polym. Mater. 2022, 4, 4495–4507. [Google Scholar] [CrossRef]
- Poonia, M.; Küster, T.; Bothun, G.D. Organic Anion Detection with Functionalized SERS Substrates via Coupled Electrokinetic Preconcentration, Analyte Capture, and Charge Transfer. ACS Appl. Mater. Interfaces 2022, 14, 23964–23972. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yin, G.; Wang, J.; Li, L.; Liang, Q.; Zhao, X.; Chen, Y.; Zheng, X.; Zhao, X. An emerging paradigm to develop analytical methods based on immobilized transmembrane proteins and its applications in drug discovery. TrAC Trends Anal. Chem. 2022, 157, 116728. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, S.M.; Lee, I.S. Immobilizing bioactive molecules onto titanium implants to improve osseointegration. Surf. Coatings Technol. 2013, 228, S312–S317. [Google Scholar] [CrossRef]
- Sánchez-Bodón, J.; Andrade-Del Olmo, J.; Alonso, J.M.; Moreno-Benítez, I.; Vilas-Vilela, J.L.; Pérez-Alvarez, L. Bioactive Coatings on Titanium: A Review on Hydroxylation, Self-Assembled Monolayers (SAMs) and Surface Modification Strategies. Polymers 2022, 14, 165. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, J.; Hu, R.; Shi, X.; Hu, X.; He, B.; Qin, A.; Tang, B.Z. Fast surface immobilization of native proteins through catalyst-free amino-yne click bioconjugation. Chem. Sci. 2020, 11, 3931–3935. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Lee, D.; Lim, D.K.; Koo, H.; Kim, K. Copper-Free Click Chemistry: Applications in Drug Delivery, Cell Tracking, and Tissue Engineering. Adv. Mater. 2022, 34, 2107192. [Google Scholar] [CrossRef] [PubMed]
- Amna, B.; Ozturk, T. Click chemistry: A fascinating method of connecting organic groups. Org. Commun. 2021, 78, 97–120. [Google Scholar] [CrossRef]
- Chen, Y.; Tong, Z.-R. Click Chemistry: Approaches, Applications, and Challenges; Chen, Y., Tonfg, Z.-R., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2017; ISBN 978-1-53611-903-9. [Google Scholar]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Lutz, J. 1,3-Dipolar Cycloadditions of Azides and Alkynes: A Universal Ligation Tool in Polymer and Materials Science. Angew. Chem. Int. Ed. 2007, 46, 1018–1025. [Google Scholar] [CrossRef]
- Suárez, A. Reacciones de cicloadición 1,3-dipolares a alquinos catalizadas por cobre. An. Quím 2012, 108, 306–313. [Google Scholar]
- Binder, W.; Kluger, C. Azide/Alkyne-“Click” Reactions: Applications in Material Science and Organic Synthesis. Curr. Org. Chem. 2006, 10, 1791–1815. [Google Scholar] [CrossRef]
- Arslan, M.; Acik, G.; Tasdelen, M.A. The emerging applications of click chemistry reactions in the modification of industrial polymers. Polym. Chem. 2019, 10, 3806–3821. [Google Scholar] [CrossRef]
- Agrahari, A.K.; Bose, P.; Jaiswal, M.K.; Rajkhowa, S.; Singh, A.S.; Hotha, S.; Mishra, N.; Tiwari, V.K. Cu(I)-Catalyzed Click Chemistry in Glycoscience and Their Diverse Applications. Chem. Rev. 2021, 121, 7638–7956. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ikhlef, D.; Kahlal, S.; Saillard, J.Y.; Astruc, D. Metal-catalyzed azide-alkyne “click” reactions: Mechanistic overview and recent trends. Coord. Chem. Rev. 2016, 316, 1–20. [Google Scholar] [CrossRef]
- O’Hern, C.I.Z.; Djoko, K.Y. Copper Cytotoxicity: Cellular Casualties of Noncognate Coordination Chemistry. mBio 2022, 13, e0043422. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.M.; Bertozzi, C.R. Bioorthogonal click chemistry: Covalent labeling in living systems. QSAR Comb. Sci. 2007, 26, 1211–1219. [Google Scholar] [CrossRef]
- Agard, N.J.; Prescher, J.A.; Bertozzi, C.R. A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 2004, 126, 15046–15047. [Google Scholar] [CrossRef]
- Bach, R.D. Ring strain energy in the cyclooctyl system. The effect of strain energy on [3 + 2] cycloaddition reactions with azides. J. Am. Chem. Soc. 2009, 131, 5233–5243. [Google Scholar] [CrossRef]
- Sakata, Y.; Nabekura, R.; Hazama, Y.; Hanya, M.; Nishiyama, T.; Kii, I.; Hosoya, T. Synthesis of Functionalized Dibenzoazacyclooctynes by a Decomplexation Method for Dibenzo-Fused Cyclooctyne-Cobalt Complexes. Org. Lett. 2022, 25, 1051–1055. [Google Scholar] [CrossRef]
- Worch, J.C.; Stubbs, C.J.; Price, M.J.; Dove, A.P. Click Nucleophilic Conjugate Additions to Activated Alkynes: Exploring Thiol-yne, Amino-yne, and Hydroxyl-yne Reactions from (Bio)Organic to Polymer Chemistry. Chem. Rev. 2021, 121, 6744–6776. [Google Scholar] [CrossRef] [PubMed]
- Stump, B. Click Bioconjugation: Modifying Proteins Using Click-Like Chemistry. ChemBioChem 2022, 23, e202200016. [Google Scholar] [CrossRef]
- Bodey, G.P.; Nance, J. Amoxicillin: In Vitro and Pharmacological Studies. Antimicrob. Agents Chemother. 1972, 1, 358–362. [Google Scholar] [CrossRef] [PubMed]
- De Marco, B.A.; Natori, J.S.H.; Fanelli, S.; Tótoli, E.G.; Salgado, H.R.N. Characteristics, Properties and Analytical Methods of Amoxicillin: A Review with Green Approach. Crit. Rev. Anal. Chem. 2017, 47, 267–277. [Google Scholar] [CrossRef]
- Salvo, F.; De Sarro, A.; Caputi, A.P.; Polimeni, G. Amoxicillin and amoxicillin plus clavulanate: A safety review. Expert Opin. Drug Saf. 2009, 8, 111–118. [Google Scholar] [CrossRef]
- Neu, H.C. Antimicrobial Activity and Human Pharmacology of Amoxicillin. J. Infect. Dis. 1974, 129, S123–S131. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, E.C.; Citron, D.M. Comparative In Vitro Activities of Amoxicillin-Clavulanic Acid and Imipenem against Anaerobic Bacteria Isolated from Community Hospitals. Antimicrob. Agents Chemother. 1986, 29, 158–160. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Feng, X.; Jin, S.; Wang, M.; Pang, Q.; Liu, C.; Liu, R.; Wang, Y.; Yang, H.; Liu, F.; Liu, Y. The Critical Role of Tryptophan in the Antimicrobial Activity and Cell Toxicity of the Duck Antimicrobial Peptide DCATH. Front. Microbiol. 2020, 11, 1146. [Google Scholar] [CrossRef]
- Palego, L.; Betti, L.; Rossi, A.; Giannaccini, G. Tryptophan Biochemistry: Structural, Nutritional, Metabolic, and Medical Aspects in Humans. J. Amino Acids 2016, 2016, 8952520. [Google Scholar] [CrossRef]
- Friedman, M. Analysis, Nutrition, and Health Benefits of Tryptophan. Int. J. Tryptophan Res. 2018, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.N.; Singh, R.B.; Buttar, H.S. Role of tryptophan in health and disease: Systematic review of the anti-oxidant, anti-inflammation, and nutritional aspects of tryptophan and its metabolites. World Heart J. 2019, 11, 161–178. [Google Scholar]
- Sánchez-Bodón, J.; Ruiz-Rubio, L.; Hernáez-Laviña, E.; Vilas-Vilela, J.L.; Moreno-Benítez, M.I. Poly(L-lactide)-based anti-inflammatory responsive surfaces for surgical implants. Polymers 2021, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Renò, F.; D’Angelo, D.; Gottardi, G.; Rizzi, M.; Aragno, D.; Piacenza, G.; Cartasegna, F.; Biasizzo, M.; Trotta, F.; Cannas, M. Atmospheric pressure plasma surface modification of poly(D, L-lactic acid) increases fibroblast, osteoblast and keratinocyte adhesion and proliferation. Plasma Process. Polym. 2012, 9, 491–502. [Google Scholar] [CrossRef]
- Sourkouni, G.; Kalogirou, C.; Moritz, P.; Gödde, A.; Pandis, P.K.; Höfft, O.; Vouyiouka, S.; Zorpas, A.A.; Argirusis, C. Study on the influence of advanced treatment processes on the surface properties of polylactic acid for a bio-based circular economy for plastics. Ultrason. Sonochem. 2021, 76, 105627. [Google Scholar] [CrossRef]
- De Geyter, N.; Morent, R.; Desmet, T.; Trentesaux, M.; Gengembre, L.; Dubruel, P.; Leys, C.; Payen, E. Plasma modification of polylactic acid in a medium pressure DBD. Surf. Coatings Technol. 2010, 204, 3272–3279. [Google Scholar] [CrossRef]
- Ubuo, E.E.; Udoetok, I.A.; Tyowua, A.T.; Ekwere, I.O.; Al-Shehri, H.S. The direct cause of amplified wettability: Roughness or surface chemistry? J. Compos. Sci. 2021, 5, 213. [Google Scholar] [CrossRef]
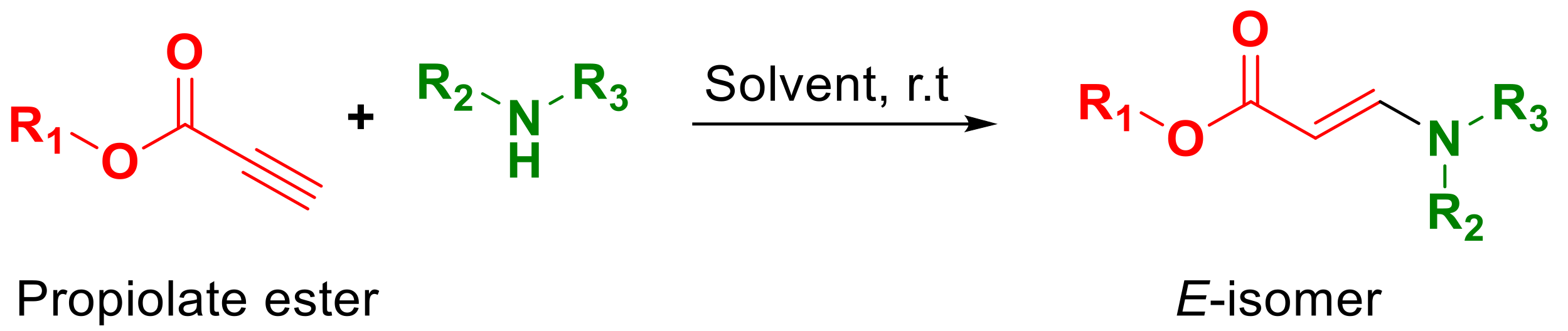


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Bodón, J.; Diaz-Galbarriatu, M.; Sola-Llano, R.; Ruiz-Rubio, L.; Vilas-Vilela, J.L.; Moreno-Benitez, I. Catalyst-Free Amino-Yne Click Reaction: An Efficient Way for Immobilizing Amoxicillin onto Polymeric Surfaces. Polymers 2024, 16, 246. https://doi.org/10.3390/polym16020246
Sánchez-Bodón J, Diaz-Galbarriatu M, Sola-Llano R, Ruiz-Rubio L, Vilas-Vilela JL, Moreno-Benitez I. Catalyst-Free Amino-Yne Click Reaction: An Efficient Way for Immobilizing Amoxicillin onto Polymeric Surfaces. Polymers. 2024; 16(2):246. https://doi.org/10.3390/polym16020246
Chicago/Turabian StyleSánchez-Bodón, Julia, Maria Diaz-Galbarriatu, Rebeca Sola-Llano, Leire Ruiz-Rubio, José Luis Vilas-Vilela, and Isabel Moreno-Benitez. 2024. "Catalyst-Free Amino-Yne Click Reaction: An Efficient Way for Immobilizing Amoxicillin onto Polymeric Surfaces" Polymers 16, no. 2: 246. https://doi.org/10.3390/polym16020246