Collagen Matrix to Restore the Tympanic Membrane: Developing a Novel Platform to Treat Perforations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Creation of the Collagen Matrix
2.1.1. Preparation of a Collagen Suspension
2.1.2. Preparation of a Collagen Membrane by Semipermeable Barrier-Assisted Electrophoretic Deposition
2.1.3. Post-Treatment of the Collagen Membrane
2.2. Collagen Matrix Characterization
2.3. Tympanic Membrane Regeneration Study
2.3.1. Study Design
2.3.2. Surgical Procedures
2.4. Endo-Otoscopy
2.5. Morphology Study
2.6. Morphometric Analysis
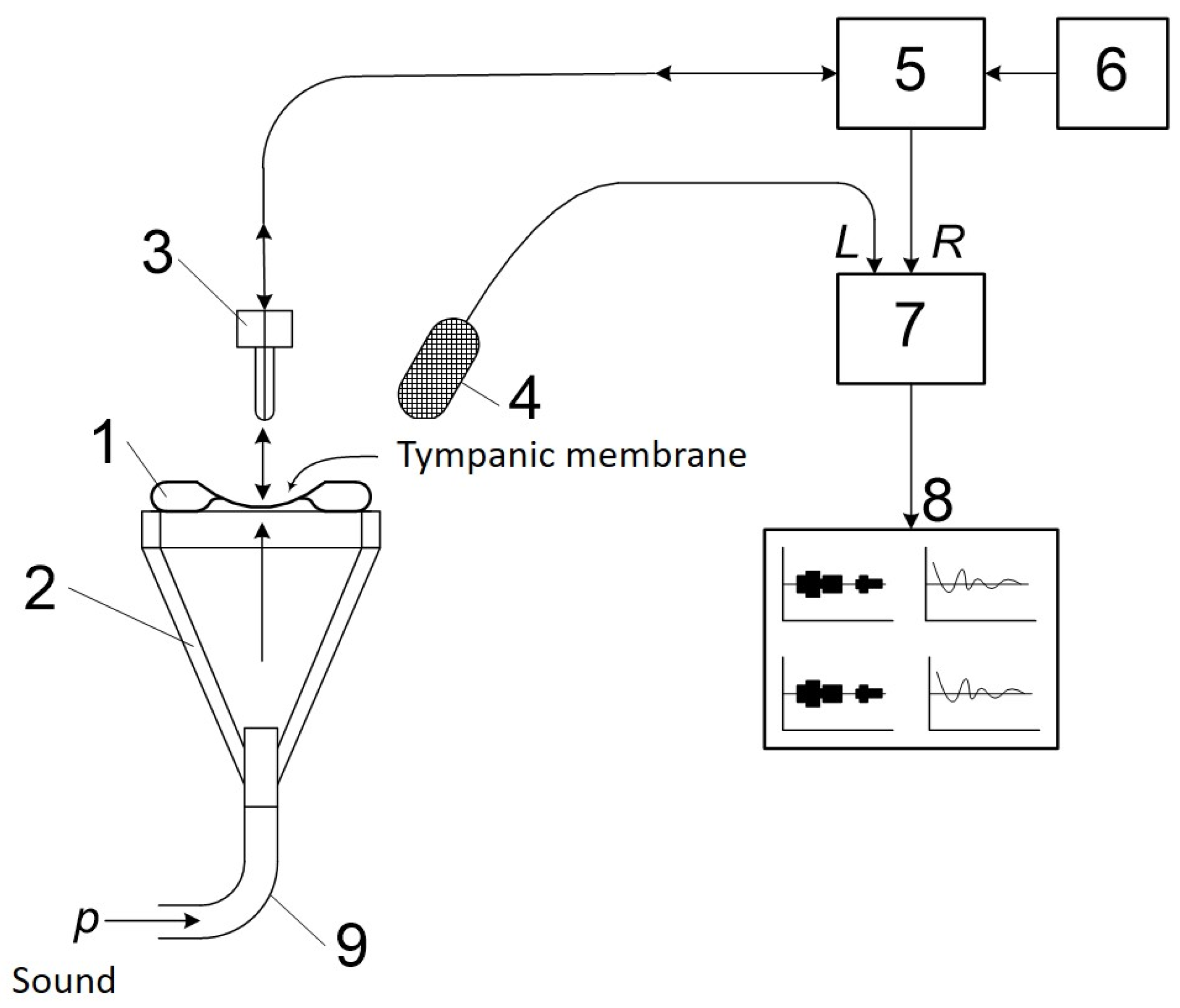
2.7. Study of Vibrational Properties and the Amplitude-Frequency Characteristic of the Tympanic Membrane
2.8. Statistical Analysis
3. Results
3.1. Collagen Matrix Characteristics
3.2. Endo-Otoscopy
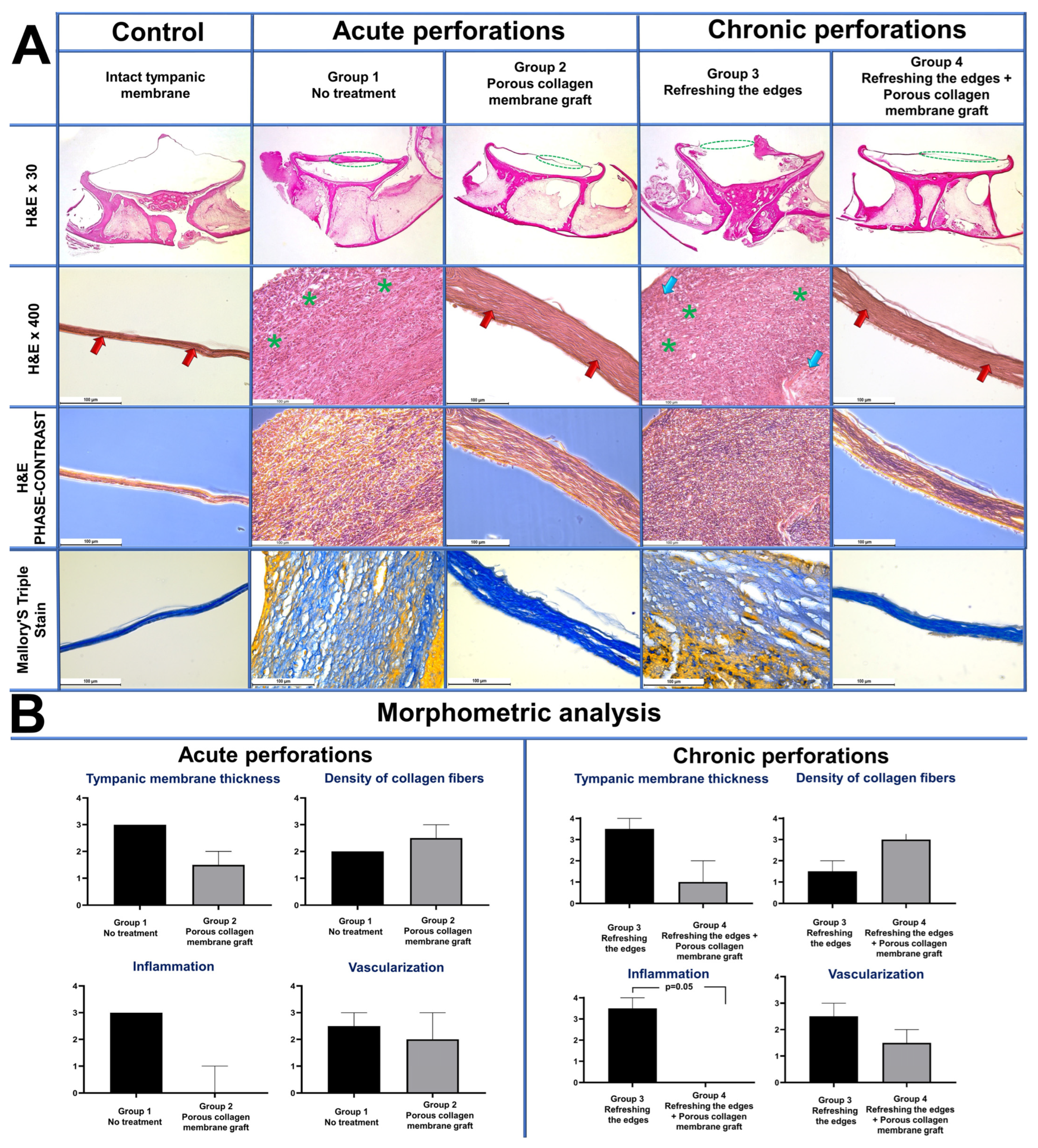
3.3. Morphology Study
3.4. The Study of the TM’s Vibrational Properties and Amplitude-Frequency Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


| Criteria | |
|---|---|
| Score | Tympanic Membrane Thickness |
| 0 | Absence of tympanic membrane |
| 1 | Very thin tympanic membrane (less than 29 мкм in the field of view at ×400 magnification) |
| 2 | Thin tympanic membrane (from 30 to 99 мкм in the field of view at ×400 magnification) |
| 3 | Thick tympanic membrane (from 100 to 499 мкм in the field of view at ×400 magnification) |
| 4 | Large tympanic membrane (more than 500 мкм in the field of view at ×400 magnification) |
| Criteria | |
|---|---|
| Score | Collagen Fiber Density |
| 0 | No signs of collagen fibers |
| 1 | Rare, thin and loosely arranged collagen fibers |
| 2 | An increase in the density of collagen fibers by less than 25% |
| 3 | An increase in the density of collagen fibers by 50% |
| 4 | Thick fibrous densely packed bundles of collagen fibers, forming scar tissue |
| Criteria | |
|---|---|
| Score | Inflammation |
| 0 | No immune cell (lymphocyte, macrophage and neutrophil) infiltration |
| 1 | Singular immune cells in the tissue (less than 4 in the field of view at ×400 magnification) |
| 2 | A low number of immune cells in the tissue (from 5 to 14 in the field of view at ×400 magnification) |
| 3 | A moderate number of immune cells in the tissue (from 15 to 29 in the field of view at ×400 magnification) |
| 4 | A large number of immune cells in the tissue (more than 30 in the field of view at ×400 magnification) |
| Criteria | |
|---|---|
| Score | Vascularization |
| 0 | No blood vessels in the tissue |
| 1 | Singular blood vessels in the tissue (less than 2 in the field of view at ×400 magnification) |
| 2 | A low number of blood vessels the tissue (from 3 to 5 in the field of view at ×400 magnification) |
| 3 | A moderate number of blood vessels in the tissue (from 6 to 9 in the field of view at ×400 magnification) |
| 4 | A large number of blood vessels in the tissue (more than 10 in the field of view at ×400 magnification) |
| Morphological Features | p Value Acute Perforation: Group 1 и Group 2 | p Value Chronic Perforations: Group 3 и Group 4 |
|---|---|---|
| Tympanic membrane thickness | 0.13 | 0.07 |
| Density of collagen fibers | 0.47 | 0.07 |
| Inflammation | 0.07 | * 0.05 |
| Vascularization | 0.99 | 0.99 |
| In total by all the criteria | * 0.01 | * 0.005 |
References
- Maria, P.L.S.; Atlas, M.D.; Ghassemifar, R. Chronic tympanic membrane perforation: A better animal model is needed. Wound Repair. Regen. 2007, 15, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Seonwoo, H.; Kim, S.W.; Kim, J.; Chunjie, T.; Lim, K.T.; Kim, Y.J.; Pandey, S.; Choung, P.H.; Choung, Y.H.; Chung, J.H. Regeneration of chronic tympanic membrane perforation using an EGF-releasing chitosan patch. Tissue Eng. Part A 2013, 19, 2097–2107. [Google Scholar] [CrossRef]
- Rana, A.K.; Upadhyay, D.; Yadav, A.; Prasad, S. Correlation of Tympanic Membrane Perforation with Hearing Loss and Its Parameters in Chronic Otitis Media: An Analytical Study. Indian J. Otolaryngol. Head Neck Surg. 2020, 72, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Pei, R. Necessities, opportunities, and challenges for tympanic membrane perforation scaffolding-based bioengineering. Biomed. Mater. 2021, 16, 032004. [Google Scholar] [CrossRef] [PubMed]
- Aleemardani, M.; Bagher, Z.; Farhadi, M.; Chahsetareh, H.; Najafi, R.; Eftekhari, B.; Seifalian, A.M. Can Tissue Engineering Bring Hope to the Development of Human Tympanic Membrane? Tissue Eng. Part B Rev. 2021, 27, 572–589. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, M.; Jiang, D.; Tucker, A.S. Developmental aspects of the tympanic membrane: Shedding light on function and disease. Genesis 2020, 58, e23348. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.P.; Yang, X.; Wang, Y.; Swift, B.; Adamson, R.; Zheng, Y.; Zhang, R.; Zhong, W.; Chen, F. High Resolution and Labeling Free Studying the 3D Microstructure of the Pars Tensa-Annulus Unit of Mice. Front. Cell Dev. Biol. 2021, 9, 720383. [Google Scholar] [CrossRef] [PubMed]
- Stenfeldt, K.; Johansson, C.; Hellström, S. The Collagen Structure of the Tympanic Membrane: Collagen Types I, II, and III in the Healthy Tympanic Membrane, During Healing of a Perforation, and During Infection. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 293–298. [Google Scholar] [CrossRef]
- Volandri, G.; Di Puccio, F.; Forte, P.; Carmignani, C. Biomechanics of the tympanic membrane. J. Biomech. 2011, 44, 1219–1236. [Google Scholar] [CrossRef]
- Stomackin, G.; Kidd, S.; Jung, T.T.; Martin, G.K.; Dong, W. Effects of tympanic membrane perforation on middle ear transmission in gerbil. Hear. Res. 2019, 373, 48–58. [Google Scholar] [CrossRef]
- Martin, H.C.; Munro, K.J.; Lam, M.C. Perforation of the tympanic membrane and its effect on the real-ear-to-coupler difference acoustic transform function. Br. J. Audiol. 2001, 35, 259–264. [Google Scholar] [CrossRef]
- Mokoyan, Z.; Svistushkin, V.; Zolotova, A.; Svistushkin, M. Chronic tympanic membrane perforation: Histopathological evidence of the experimental model. Int. J. Pediatr. Otorhinolaryngol. 2021, 151, 110964. [Google Scholar] [CrossRef] [PubMed]
- Teh, B.M.; Marano, R.J.; Shen, Y.; Friedland, P.L.; Dilley, R.J.; Atlas, M.D. Tissue engineering of the tympanic membrane. Tissue Eng. Part B Rev. 2013, 19, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, J.; Tian, P.; Cui, X. The latest progress of tympanic membrane repair materials. Am. J. Otolaryngol. 2022, 43, 103408. [Google Scholar] [CrossRef] [PubMed]
- Ghanad, I.; Polanik, M.D.; Trakimas, D.R.; Knoll, R.M.; Castillo-Bustamante, M.; Black, N.L.; Kozin, E.D.; Remenschneider, A.K. A Systematic Review of Nonautologous Graft Materials Used in Human Tympanoplasty. Laryngoscope 2021, 131, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.D.; Strachan, D.R.; Raine, C.H. Factors affecting myringoplasty success. J. Laryngol. Otol. 2015, 129, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Illés, K.; Gergő, D.; Keresztély, Z.; Dembrovszky, F.; Fehérvári, P.; Bánvölgyi, A.; Csupor, D.; Hegyi, P.; Horváth, T. Factors influencing successful reconstruction of tympanic membrane perforations: A systematic review and meta-analysis. Eur. Arch. Otorhinolaryngol. 2023, 280, 2639–2652. [Google Scholar] [CrossRef]
- Sagiv, D.; Chin, O.Y.; Diaz, R.C.; Brodie, H.A. State of the art regeneration of the tympanic membrane. Curr. Opin. Otolaryngol. Head Neck Surg. 2020, 28, 314–322. [Google Scholar] [CrossRef]
- Sainsbury, E.; Amaral, R.D.; Blayney, A.W.; Walsh, R.M.C.; O’Brien, F.J.; O’Leary, C. Tissue engineering and regenerative medicine strategies for the repair of tympanic membrane perforations. Biomater. Biosyst. 2022, 6, 100046. [Google Scholar] [CrossRef]
- Villar-Fernandez, M.A.; Lopez-Escamez, J.A. Outlook for Tissue Engineering of the Tympanic Membrane. Audiol. Res. 2015, 5, 117. [Google Scholar] [CrossRef]
- Hong, P.; Bance, M.; Gratzer, P.F. Repair of tympanic membrane perforation using novel adjuvant therapies: A contemporary review of experimental and tissue engineering studies. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 3–12. [Google Scholar] [CrossRef] [PubMed]
- McFeely, W.J.; Bojrab, D.I.; Kartush, J.M. Tympanic membrane perforation repair using AlloDerm. Otolaryngol. Head Neck Surg. 2000, 123 Pt 1, 17–21. [Google Scholar] [CrossRef]
- Farahani, F.; Yazdi, A.K.; Ghasemi, M.; Shariatpanahi, E.; Kajbafzadeh, A.-M.; Amanpour, S. Results of Acellular Dermis Matrix graft Used for Tympanoplasty in Guinea Pig Model. Iran. J. Otorhinolaryngol. 2015, 27, 95. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4409953/ (accessed on 23 July 2023). [PubMed]
- Deng, Z.; Wu, J.; Qiu, J.; Wang, J.; Tian, Y.; Li, Y.; Jin, Y. Comparison of porcine acellular dermis and dura mater as natural scaffolds for bioengineering tympanic membranes. Tissue Eng. Part A 2009, 15, 3729–3739. [Google Scholar] [CrossRef]
- Shen, Y.; Redmond, S.L.; Teh, B.M.; Yan, S.; Wang, Y.; Atlas, M.D.; Dilley, R.J.; Zheng, M.; Marano, R.J. Tympanic membrane repair using silk fibroin and acellular collagen scaffolds. Laryngoscope 2013, 123, 1976–1982. [Google Scholar] [CrossRef]
- Jang, C.H.; Kim, W.; Moon, C.; Kim, G. Bioprinted Collagen-Based Cell-Laden Scaffold With Growth Factors for Tympanic Membrane Regeneration in Chronic Perforation Model. IEEE Trans. Nanobiosci. 2022, 21, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Seo, Y.J.; Shim, D.B.; Lee, H.J.; Kim, S.H. Surgical outcomes of tympanoplasty using a sterile acellular dermal allograft: A prospective randomised controlled study. Acta Otorhinolaryngol. Ital. 2018, 38, 554–562. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, X.; Chen, X.; Huang, Y.; Fang, L.; Li, X.; Zhang, Y.; Jia, M. Comparison of type I tympanoplasty with acellular dermal allograft and cartilage perichondrium. Acta Otolaryngol. 2019, 139, 833–836. [Google Scholar] [CrossRef]
- Spiegel, J.H.; Kessler, J.L. Tympanic membrane perforation repair with acellular porcine submucosa. Otol. Neurotol. 2005, 26, 563–566. [Google Scholar] [CrossRef]
- D’Eredità, R. Porcine small intestinal submucosa (SIS) myringoplasty in children: A randomized controlled study. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 1085–1089. [Google Scholar] [CrossRef]
- Basonbul, R.A.; Cohen, M.S. Use of porcine small intestinal submucosa for pediatric endoscopic tympanic membrane repair. World J. Otorhinolaryngol. Head Neck Surg. 2017, 3, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Antoshin, A.; Dubinin, O.; Miao, L.; Istranova, E.; Bikmulina, P.; Fayzullin, A.; Magdanov, A.; Kravchik, M.; Kosheleva, N.; Solovieva, A.; et al. Semipermeable barrier-assisted electrophoretic deposition of robust collagen membranes. JMatS 2023, 58, 9675–9697. [Google Scholar] [CrossRef]
- Chan, B.P.; So, K.-F. Photochemical crosslinking improves the physicochemical properties of collagen scaffolds. J. Biomed. Mater. Res. A 2005, 75A, 689–701. [Google Scholar] [CrossRef]
- Angele, P.; Abke, J.; Kujat, R.; Faltermeier, H.; Schumann, D.; Nerlich, M.; Kinner, B.; Englert, C.; Ruszczak, Z.; Mehrl, R.; et al. Influence of different collagen species on physico-chemical properties of crosslinked collagen matrices. Biomaterials 2004, 25, 2831–2841. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.E.; Semaan, M.T.; Wasman, J.K.; Beane, R.; Bonassar, L.J.; Megerian, C.A. Tissue-engineered calcium alginate patches in the repair of chronic chinchilla tympanic membrane perforations. Laryngoscope 2006, 116, 700–704. [Google Scholar] [CrossRef] [PubMed]
- GOST 33216-2014; Guidelines for Accommodation and Care of Animals. Species-Specific Provisions for Laboratory Rodents and Rab-Bits. RussianGost: Moscow, Russia, 2014.
- European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes. Available online: https://rm.coe.int/168007a67b (accessed on 29 December 2023).
- Grover, C.N.; Cameron, R.E.; Best, S.M. Investigating the morphological, mechanical and degradation properties of scaffolds comprising collagen, gelatin and elastin for use in soft tissue engineering. J. Mech. Behav. Biomed. Mater. 2012, 10, 62–74. [Google Scholar] [CrossRef]
- Yawn, R.J.; Dedmon, M.M.; O’Connell, B.P.; Virgin, F.W.; Rivas, A. Tympanic Membrane Perforation Repair Using Porcine Small Intestinal Submucosal Grafting. Otol. Neurotol. 2018, 39, e332–e335. [Google Scholar] [CrossRef] [PubMed]
- Ghassemifar, R.; Redmond, S.; Zainuddin; Chirila, T.V. Advancing towards a tissue-engineered tympanic membrane: Silk fibroin as a substratum for growing human eardrum keratinocytes. J. Biomater. Appl. 2010, 24, 591–606. [Google Scholar] [CrossRef]
- Levin, B.; Redmond, S.L.; Rajkhowa, R.; Eikelboom, R.H.; Marano, R.J.; Atlas, M.D. Preliminary results of the application of a silk fibroin scaffold to otology. Otolaryngol. Head Neck Surg. 2010, 142 (Suppl. 1), S33–S35. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.-K.; Park, H.S.; Jeong, J.Y.; Yeon, Y.K.; Kumar, V.; Bae, S.H.; Lee, J.M.; Moon, B.M.; Park, C.H. A prospective cohort study of the silk fibroin patch in chronic tympanic membrane perforation. Laryngoscope 2016, 126, 2798–2803. [Google Scholar] [CrossRef]
- Kind, G.M.; Bines, S.D.; Staren, E.D.; Templeton, A.J.; Economou, S.G. Chitosan: Evaluation of a new hemostatic agent. Curr. Surg. 1990, 47, 37–39. [Google Scholar] [PubMed]
- Kakehata, S.; Hirose, Y.; Kitani, R.; Futai, K.; Maruya, S.-I.; Ishii, K.; Shinkawa, H. Autologous serum eardrops therapy with a chitin membrane for closing tympanic membrane perforations. Otol. Neurotol. 2008, 29, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Bae, J.; Lim, K.T.; Choung, P.; Park, J.; Choi, S.J.; Im, A.L.; Lee, E.T.; Choung, Y.; Chung, J.H. Development of water-insoluble chitosan patch scaffold to repair traumatic tympanic membrane perforations. J. Biomed. Mater. Res. A 2009, 90, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choi, S.J.; Park, J.-S.; Lim, K.T.; Choung, P.-H.; Kim, S.W.; Bin Lee, J.; Chung, J.H.; Choung, Y.-H. Tympanic membrane regeneration using a water-soluble chitosan patch. Tissue Eng. Part A 2010, 16, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Choi, S.J.; Lim, K.T.; Bin Lee, J.; Seonwoo, H.; Choung, P.-H.; Park, K.; Cho, C.-S.; Choung, Y.-H.; Chung, J.H.; et al. A healing method of tympanic membrane perforations using three-dimensional porous chitosan scaffolds. Tissue Eng. Part A 2011, 17, 2763–2772. [Google Scholar] [CrossRef] [PubMed]
- Kanemaru, S.I.; Kanai, R.; Omori, K.; Yamamoto, N.; Okano, T.; Kishimoto, I.; Ogawa, K.; Kanzaki, S.; Fujioka, M.; Oishi, N.; et al. Multicenter phase III trial of regenerative treatment for chronic tympanic membrane perforation. Auris Nasus Larynx 2021, 48, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Omae, K.; Kanemaru, S.-I.; Nakatani, E.; Kaneda, H.; Nishimura, T.; Tona, R.; Naito, Y.; Kawamoto, A.; Fukushima, M. Regenerative treatment for tympanic membrane perforation using gelatin sponge with basic fibroblast growth factor. Auris Nasus Larynx 2017, 44, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.C.; He, J.G. A randomised controlled trial comparing spontaneous healing, gelfoam patching and edge-approximation plus gelfoam patching in traumatic tympanic membrane perforation with inverted or everted edges. Clin. Otolaryngol. 2011, 36, 221–226. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Zhang, Y. Repair of large traumatic tympanic membrane perforation using ofloxacin otic solution and gelatin sponge. Braz. J. Otorhinolaryngol. 2022, 88, 9–14. [Google Scholar] [CrossRef]
- Azimi, B.; Milazzo, M.; Danti, S. Cellulose-Based Fibrous Materials From Bacteria to Repair Tympanic Membrane Perforations. Front. Bioeng. Biotechnol. 2021, 9, 669863. [Google Scholar] [CrossRef]
- Wieland, A.M.; Sundback, C.A.; Hart, A.; Kulig, K.; Masiakos, P.T.; Hartnick, C.J. Poly(glycerol sebacate)-engineered plugs to repair chronic tympanic membrane perforations in a chinchilla model. Otolaryngol. Head Neck Surg. 2010, 143, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Park, A.H.; Hughes, C.W.; Jackson, A.; Hunter, L.; McGill, L.; Simonsen, S.E.; Alder, S.C.; Shu, X.Z.; Prestwich, G.D. Crosslinked hydrogels for tympanic membrane repair. Otolaryngol. Head Neck Surg. 2006, 135, 877–883. [Google Scholar] [CrossRef]
- Farhadi, M.; Mirzadeh, H.; Solouk, A.; Asghari, A.; Jalessi, M.; Ghanbari, H.; Yazdanifard, P. Collagen-immobilized patch for repairing small tympanic membrane perforations: In vitro and in vivo assays. J. Biomed. Mater. Res. A 2012, 100, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jeong, J.Y.; Park, H.J.; Moon, B.M.; Park, Y.R.; Lee, O.J.; Sultan, T.; Kim, D.-K.; Park, H.S.; Lee, J.H.; et al. Application of a Collagen Patch Derived from Duck Feet in Acute Tympanic Membrane Perforation. Tissue Eng. Regen. Med. 2017, 14, 233–241. [Google Scholar] [CrossRef]
- Jang, C.H.; Cho, Y.B.; Yeo, M.; Lee, H.; Min, E.J.; Lee, B.H.; Kim, G.H. Regeneration of chronic tympanic membrane perforation using 3D collagen with topical umbilical cord serum. Int. J. Biol. Macromol. 2013, 62, 232–240. [Google Scholar] [CrossRef]
- Ghomi, E.R.; Nourbakhsh, N.; Kenari, M.A.; Zare, M.; Ramakrishna, S. Collagen-based biomaterials for biomedical applications. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- Glowacki, J.; Mizuno, S. Collagen scaffolds for tissue engineering. Biopolymers 2008, 89, 338–344. [Google Scholar] [CrossRef]
- Shekhter, A.B.; Fayzullin, A.L.; Vukolova, M.N.; Rudenko, T.G.; Osipycheva, V.D.; Litvitsky, P.F. Medical Applications of Collagen and Collagen-Based Materials. Curr. Med. Chem. 2019, 26, 506–516. [Google Scholar] [CrossRef]
- Anand, S.; Danti, S.; Moroni, L.; Mota, C. Regenerative therapies for tympanic membrane. Prog. Mater. Sci. 2022, 127, 100942. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, Z.; Sun, P.; Huang, H.; Zhang, Y.; Dai, J.; Liu, J.; Shi, Q. Acceleration of Healing of Traumatic Tympanic Membrane Perforation in Rats by Implanted Collagen Membrane Integrated with Collagen-Binding Basic Fibroblast Growth Factor. Tissue Eng. Part A 2017, 23, 20–29. [Google Scholar] [CrossRef]
- Knutsson, J.; Von Unge, M.; Rask-Andersen, H. Localization of progenitor/stem cells in the human tympanic membrane. Audiol. Neurootol. 2011, 16, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kim, J.; Seonwoo, H.; Jang, K.-J.; Kim, Y.J.; Lim, H.J.; Lim, K.-T.; Tian, C.; Chung, J.H.; Choung, Y.-H. Latent progenitor cells as potential regulators for tympanic membrane regeneration. Sci. Rep. 2015, 5, 11542. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Z.; Tian, J. Epidermal stem cells in the tympanic membrane. Chin. J. Otorhinolaryngol. Head Neck Surg. 2004, 39, 712–716. [Google Scholar]
- Hill, M.J.; Taylor, C.L.; Scott, G.B.D. Chondromatous metaplasia in the human larynx. Histopathology 1980, 4, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, R. Application of cartilage in tympanoplasty. J. Clin. Otorhinolaryngol. Head Neck Surg. 2023, 37, 157–160. [Google Scholar] [CrossRef]
- Lyons, S.A.; Su, T.; Vissers, L.E.T.; Peters, J.P.M.; Smit, A.L.; Grolman, W. Fascia compared to one-piece composite cartilage-perichondrium grafting for tympanoplasty. Laryngoscope 2016, 126, 1662–1670. [Google Scholar] [CrossRef]
- Fay, J.; Puria, S.; Decraemer, W.F.; Steele, C. Three approaches for estimating the elastic modulus of the tympanic membrane. J. Biomech. 2005, 38, 1807–1815. [Google Scholar] [CrossRef]
- Allardyce, B.J.; Rajkhowa, R.; Dilley, R.J.; Xie, Z.; Campbell, L.; Keating, A.; Atlas, M.D.; von Unge, M.; Wang, X. Comparative acoustic performance and mechanical properties of silk membranes for the repair of chronic tympanic membrane perforations. J. Mech. Behav. Biomed. Mater. 2016, 64, 65–74. [Google Scholar] [CrossRef]



| Matrix | Dry Thickness, µm | Swelling, % | Shrinkage Temperature, °C |
|---|---|---|---|
| Initial SBA-EPD collagen membrane | 250 ± 30 | 410 ± 50 | 55 ± 1 |
| Porous collagen membrane | 440 ± 20 | 860 ± 70 | 55 ± 1 |
| Matrix | Young’s Modulus, MPa | Strain at Failure, % |
|---|---|---|
| Initial SBA-EPD collagen membrane | 15 ± 2 | 67 ± 4 |
| Porous collagen membrane | 3 ± 1 | 53 ± 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svistushkin, M.; Kotova, S.; Zolotova, A.; Fayzullin, A.; Antoshin, A.; Serejnikova, N.; Shekhter, A.; Voloshin, S.; Giliazova, A.; Istranova, E.; et al. Collagen Matrix to Restore the Tympanic Membrane: Developing a Novel Platform to Treat Perforations. Polymers 2024, 16, 248. https://doi.org/10.3390/polym16020248
Svistushkin M, Kotova S, Zolotova A, Fayzullin A, Antoshin A, Serejnikova N, Shekhter A, Voloshin S, Giliazova A, Istranova E, et al. Collagen Matrix to Restore the Tympanic Membrane: Developing a Novel Platform to Treat Perforations. Polymers. 2024; 16(2):248. https://doi.org/10.3390/polym16020248
Chicago/Turabian StyleSvistushkin, Mikhail, Svetlana Kotova, Anna Zolotova, Alexey Fayzullin, Artem Antoshin, Natalia Serejnikova, Anatoly Shekhter, Sergei Voloshin, Aliia Giliazova, Elena Istranova, and et al. 2024. "Collagen Matrix to Restore the Tympanic Membrane: Developing a Novel Platform to Treat Perforations" Polymers 16, no. 2: 248. https://doi.org/10.3390/polym16020248







