Cation-Selective Actuator–Sensor Response of Microcrystalline Cellulose Multi-Walled Carbon Nanotubes of Different Electrolytes Using Propylene Carbonate Solvent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microcrystalline Cellulose MWCNT Formulation
2.2. Linear Actuation Measurements
2.3. Characterizations
3. Results and Discussion
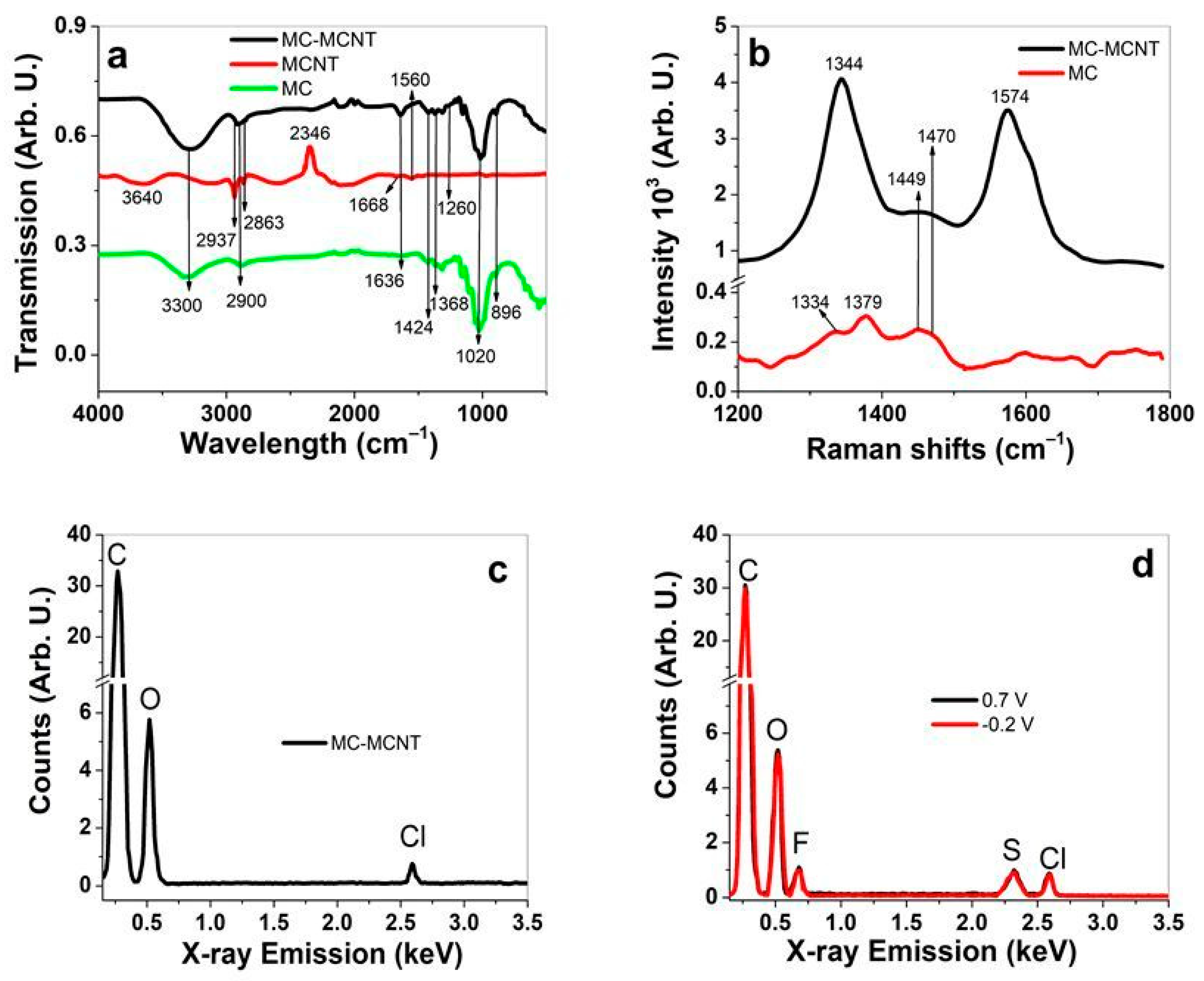
3.1. MC-MCNT Fiber Formulation and Characterization
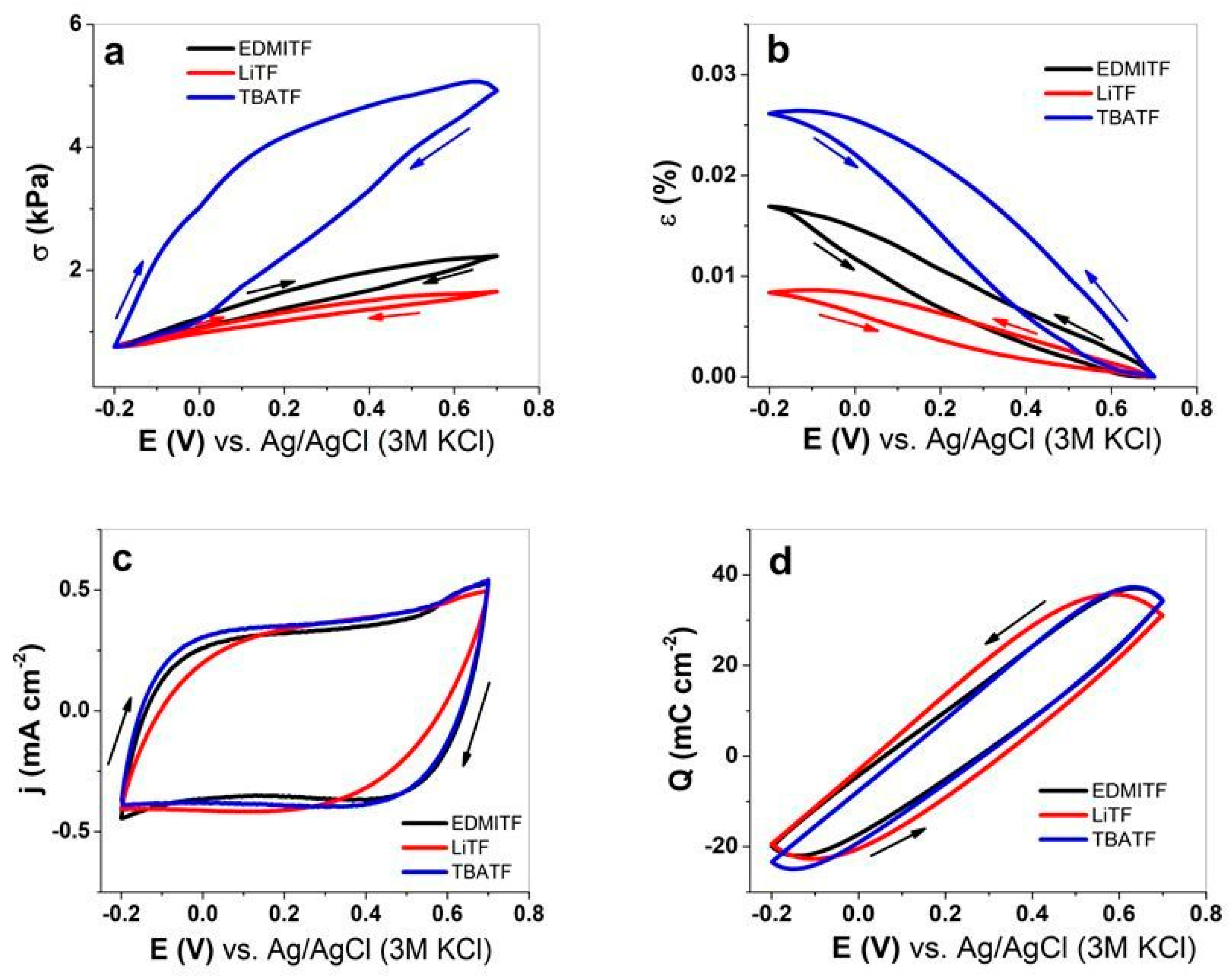
3.2. Linear Actuation of MC-MCNT
3.2.1. Cyclic Voltammetry
3.2.2. Square Wave Potential Steps of MC-MCNT Fiber
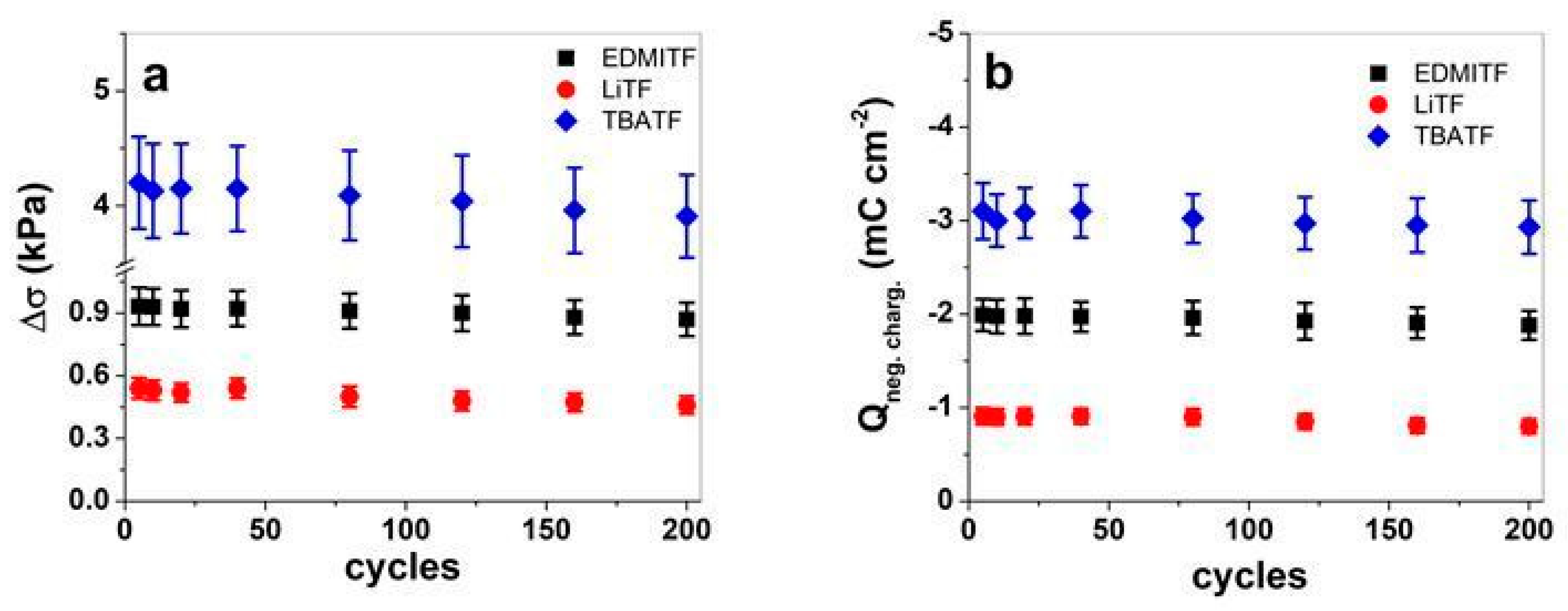
3.3. Sensor Properties over Chronopotentiometric Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, Z.; Yang, L.; Zhao, J.; Song, W. Natural Cellulose-Full-Hydrogels Bioinspired Electroactive Artificial Muscles: Highly Conductive Ionic Transportation Channels and Ultrafast Electromechanical Response. J. Electrochem. Soc. 2020, 167, 047515. [Google Scholar] [CrossRef]
- Liu, Y.; Shang, S.; Mo, S.; Wang, P.; Yin, B.; Wei, J. Soft Actuators Built from Cellulose Paper: A Review on Actuation, Material, Fabrication, and Applications. J. Sci. Adv. Mater. Devices 2021, 6, 321–337. [Google Scholar] [CrossRef]
- Miyashiro, D.; Hamano, R.; Umemura, K. A Review of Applications Using Mixed Materials of Cellulose, Nanocellulose and Carbon Nanotubes. Nanomaterials 2020, 186, 186. [Google Scholar] [CrossRef] [PubMed]
- Gui, Z.; Zhu, H.; Gillette, E.; Han, X.; Rubloff, G.W.; Hu, L.; Lee, S.B. Natural Cellulose Fiber as Substrate for Supercapacitor. ACS Nano 2013, 7, 6037–6046. [Google Scholar] [CrossRef] [PubMed]
- Ummartyotin, S.; Manuspiya, H. A Critical Review on Cellulose: From Fundamental to an Approach on Sensor Technology. Renew. Sustain. Energy Rev. 2015, 41, 402–412. [Google Scholar] [CrossRef]
- Xiong, C.; Yang, Q.; Dang, W.; Zhou, Q.; Jiang, X.; Sun, X.; Wang, Z.; An, M.; Ni, Y. A Multifunctional Paper-Based Supercapacitor with Excellent Temperature Adaptability, Plasticity, Tensile Strength, Self-Healing, and High Thermoelectric Effects. J. Mater. Chem. A 2023, 11, 4769–4779. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, Y.; Chen, Q.; Yu, Z.; Wang, C.; Jin, S.; Ding, Y.; Wu, G. Dissolution of Cellulose with Ionic Liquids and Its Application: A Mini-Review. Green Chem. 2006, 8, 325–327. [Google Scholar] [CrossRef]
- Jatoi, A.W.; Ogasawara, H.; Kim, I.S.; Ni, Q.Q. Cellulose Acetate/Multi-Wall Carbon Nanotube/Ag Nanofiber Composite for Antibacterial Applications. Mater. Sci. Eng. C 2020, 110, 110679. [Google Scholar] [CrossRef] [PubMed]
- Elhi, F.; Aid, T.; Koel, M. Ionic Liquids as Solvents for Making Composite Materials from Cellulose. Proc. Est. Acad. Sci. 2016, 65, 255–266. [Google Scholar] [CrossRef]
- Kiefer, R.; Elhi, F.; Peikolainen, A.-L.; Puust, L.; Tamm, T. The Importance of Potential Range Choice on the Electromechanical Response of Cellulose-Carbon Nanotube Fibers. Synth. Met. 2022, 283, 116966. [Google Scholar] [CrossRef]
- Kiefer, R.; Elhi, F.; Peikolainen, A.-L.; Tamm, T. Wider Potential Windows of Cellulose Multiwall Carbon Nanotube Fibers Leading to Qualitative Multifunctional Changes in an Organic Electrolyte. Polymers 2021, 13, 4439. [Google Scholar] [CrossRef] [PubMed]
- Kosidlo, U.; Omastova, M.; Micusik, M.; Ciric-Marjanovic, G.; Randriamahazaka, H.; Wallmersperger, T.; Aabloo, A.; Kolaric, I.; Bauernhansl, T. Nanocarbon Based Ionic Actuators-a Review. Smart Mater. Struct. 2013, 22, 104022. [Google Scholar] [CrossRef]
- Baughman, R.H.; Cui, C.; Zakhidov, A.A.; Iqbal, Z.; Barisci, J.N.; Spinks, G.M.; Wallace, G.G.; Mazzoldi, A.; De Rossi, D.; Rinzler, A.G.; et al. Carbon Nanotube Actuators. Science 1999, 284, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Elhi, F.; Puust, L.; Kiefer, R.; Tamm, T. Electrolyte Contribution to the Multifunctional Response of Cellulose Carbon Nanotube Fibers. React. Funct. Polym. 2023, 182, 105480. [Google Scholar] [CrossRef]
- Jiang, R.; Liu, N.; Gao, S.; Mamat, X.; Su, Y.; Wagberg, T.; Li, Y.; Hu, X.; Hu, G. A Facile Electrochemical Sensor Based on PyTS-CNTs for Simultaneous Determination of Cadmium and Lead Ions. Sensors 2018, 18, 1567. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Kim, B.; Li, J.; Meyyappan, M. A Carbon Nanotube Based Ammonia Sensor on Cellulose Paper. RSC Adv. 2014, 4, 549–553. [Google Scholar] [CrossRef]
- Teodoro, K.B.R.; Shimizu, F.M.; Scagion, V.P.; Correa, D.S. Ternary Nanocomposites Based on Cellulose Nanowhiskers, Silver Nanoparticles and Electrospun Nanofibers: Use in an Electronic Tongue for Heavy Metal Detection. Sens. Actuators B Chem. 2019, 290, 387–395. [Google Scholar] [CrossRef]
- Wardak, C.; Pietrzak, K.; Morawska, K.; Grabarczyk, M. Ion-Selective Electrodes with Solid Contact Based on Composite Materials: A Review. Sensors 2023, 23, 5839. [Google Scholar] [CrossRef]
- Lin, Z.; Liang, R.; Qin, W. Towards Potentiometric Detection in Non-aqueous Media: Evaluation of the Impacts of Organic Solvents on Polymeric Membrane Ion-Selective Electrodes. Talanta 2022, 241, 123238. [Google Scholar] [CrossRef]
- Harjo, M.; Tamm, T.; Anbarjafari, G.; Kiefer, R. Hardware and Software Development for Isotonic Strain and Isometric Stress Measurements of Linear Ionic Actuators. Polymers 2019, 11, 1054. [Google Scholar] [CrossRef]
- Suárez, I.J.; Otero, T.F.; Márquez, M. Diffusion Coefficients in Swelling Polypyrrole: ESCR and Cottrell Models. J. Phys. Chem. B 2005, 109, 1723–1729. [Google Scholar] [CrossRef]
- Otero, T.F.; Martinez, J.G. Activation Energy for Polypyrrole Oxidation: Film Thickness Influence. J. Solid State Electrochem. 2011, 15, 1169–1178. [Google Scholar] [CrossRef]
- Dehghanpour, H.; Yilmaz, K. The Relationship between Resistances Measured by Two-Probe, Wenner Probe and C1760-12 ASTM Methods in Electrically Conductive Concretes. SN Appl. Sci. 2020, 2, 10. [Google Scholar] [CrossRef]
- Foroughi, J.; Spinks, G. Carbon Nanotube and Graphene Fiber Artificial Muscles. Nanoscale Adv. 2019, 1, 4592–4614. [Google Scholar] [CrossRef] [PubMed]
- Khuyen, N.Q.; Elhi, F.; Le, Q.B.; Kiefer, R. Sustainability of Multiwall Carbon Nanotube Fibers and Their Cellulose Composite. Sustainability 2023, 15, 9227. [Google Scholar] [CrossRef]
- Jyothibasu, J.P.; Wang, R.-H.; Ong, K.; Ong, J.H.L.; Lee, R.-H. Cellulose/Carbon Nanotube/MnO2 Composite Electrodes with High Mass Loadings for Symmetric Supercapacitors. Cellulose 2021, 28, 3549–3567. [Google Scholar] [CrossRef]
- El Seoud, O.A.; Da Silva, V.C.; Possidonio, S.; Casarano, R.; Arêas, E.P.G.; Gimenes, P. Microwave-Assisted Derivatization of Cellulose, 2-The Surprising Effect of the Structure of Ionic Liquids on the Dissolution and Acylation of the Biopolymer. Macromol. Chem. Phys. 2011, 212, 2541–2550. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic Liquid Processing of Cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef]
- Elhi, F.; Peikolainen, A.L.; Kiefer, R.; Tamm, T. Cellulose-Multiwall Carbon Nanotube Fiber Actuator Behavior in Aqueous and Organic Electrolyte. Materials 2020, 13, 3213. [Google Scholar] [CrossRef]
- Liu, Y.; Kumar, S. Polymer/Carbon Nanotube Nano Composite Fibers-A Review. ACS Appl. Mater. Interfaces 2014, 6, 6069–6087. [Google Scholar] [CrossRef]
- Qi, H.; Schulz, B.; Vad, T.; Liu, J.; Mäder, E.; Seide, G.; Gries, T. Novel Carbon Nanotube/Cellulose Composite Fibers As Multifunctional Materials. ACS Appl. Mater. Interfaces 2015, 7, 22404–22412. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Han, M.; Lee, S.; Gyu, Y. Electrically Conductive and Strong Cellulose-Based Composite Fibers Reinforced with Multiwalled Carbon Nanotube Containing Multiple Hydrogen Bonding Moiety. Compos. Sci. Technol. 2016, 123, 57–64. [Google Scholar] [CrossRef]
- Yang, L.; Sun, Z.; Li, F.; Du, S.; Song, W. Performance Enhancement of Cellulose Based Biocomposite Ionic Actuator by Doping with MWCNT. Appl. Phys. A 2019, 125, 547. [Google Scholar] [CrossRef]
- Tohamy, H.A.S.; El-Sakhawy, M.; Kamel, S. Carbon Nanotubes from Agricultural Wastes: Effective Environmental Adsorbent. Egypt. J. Chem. 2022, 65, 437–446. [Google Scholar] [CrossRef]
- Wulan, P.P.D.K.; Ulwani, S.H.; Wulandari, H.; Purwanto, W.W.; Mulia, K. The Effect of Hydrochloric Acid Addition to Increase Carbon Nanotubes Dispersibility as Drug Delivery System by Covalent Functionalization. IOP Conf. Ser. Mater. Sci. Eng. 2018, 316, 012013. [Google Scholar] [CrossRef]
- Ouyang, W.; Sun, J.; Memon, J.; Wang, C.; Geng, J.; Huang, Y. Scalable Preparation of Three-Dimensional Porous Structures of Reduced Graphene Oxide/Cellulose Composites and Their Application in Supercapacitors. Carbon N. Y. 2013, 62, 501–509. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Salleh, W.W.N.; Jaafar, J.; Ismail, A.F.; Mutalib, M.A.; Sani, N.A.A.; Asri, S.E.A.M.; Ong, C.S. Physicochemical Characteristic of Regenerated Cellulose/N-Doped TiO2 Nanocomposite Membrane Fabricated from Recycled Newspaper with Photocatalytic Activity under UV and Visible Light Irradiation. Chem. Eng. J. 2016, 284, 202–215. [Google Scholar] [CrossRef]
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of Cellulosic Fibers by FTIR Spectroscopy for Their Further Implementation to Building Materials. Am. J. Anal. Chem. 2018, 09, 303–310. [Google Scholar] [CrossRef]
- Baskaran, D.; Mays, J.W.; Bratcher, M.S. Noncovalent and Nonspecific Molecular Interactions of Polymers with Multiwalled Carbon Nanotubes. Chem. Mater. 2005, 17, 3389–3397. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Atalla, R.H. Raman Spectroscopy. In Surface Analysis of Paper; Conners, T.E., Banerjee, S., Eds.; CRC Press: Boca Raton, FL, USA, 1995; pp. 152–181. ISBN 9780429279997. [Google Scholar]
- Lucas, M.; Wagner, G.L.; Nishiyama, Y.; Hanson, L.; Samayam, I.P.; Schall, C.A.; Langan, P.; Rector, K.D. Reversible Swelling of the Cell Wall of Poplar Biomass by Ionic Liquid at Room Temperature. Bioresour. Technol. 2011, 102, 4518–4523. [Google Scholar] [CrossRef]
- Plaado, M.; Kaasik, F.; Valner, R.; Lust, E.; Saar, R.; Saal, K.; Peikolainen, A.-L.; Aabloo, A.; Kiefer, R. Electrochemical Actuation of Multiwall Carbon Nanotube Fiber with Embedded Carbide-Derived Carbon Particles. Carbon N. Y. 2015, 94, 911–918. [Google Scholar] [CrossRef]
- Ruch, P.W.; Kötz, R.; Wokaun, A. Electrochemical Characterization of Single-Walled Carbon Nanotubes for Electrochemical Double Layer Capacitors Using Non-Aqueous Electrolyte. Electrochim. Acta 2009, 54, 4451–4458. [Google Scholar] [CrossRef]
- Chaban, V. Solvation of the Fluorine Containing Anions and Their Lithium Salts in Propylene Carbonate and Dimethoxyethane. J. Mol. Model. 2015, 21, 172. [Google Scholar] [CrossRef]
- Marque, P.; Roncali, J.; Garnier, F. Electrolyte Effect on the Electrochemical Properties of Poly(3-Methylthiophene) Thin Films. J. Electroanal. Chem. 1987, 218, 107–118. [Google Scholar] [CrossRef]
- Ue, M.; Murakami, A.; Nakamura, S. A Convenient Method to Estimate Ion Size for Electrolyte Materials Design. J. Electrochem. Soc. 2002, 149, A1385–A1388. [Google Scholar] [CrossRef]
- Põldsalu, I.; Rohtlaid, K.; Plesse, C.; Vidal, F.; Nguyen, T.N.; Anna-Liisa, P.; Tarmo, T.; Rudolf, K. Printed PEDOT: PSS Trilayer: Mechanism Evaluation and Application in Energy Storage. Materials 2020, 13, 491. [Google Scholar] [CrossRef]
- Torop, J.; Arulepp, M.; Sugino, T.; Asaka, K.; Ja, A.; Lust, E.; Aabloo, A. Microporous and Mesoporous Carbide-Derived Carbons for Strain Modification of Electromechanical Actuators. Langmuir 2014, 30, 2583–2587. [Google Scholar] [CrossRef] [PubMed]
- Barisci, J.N.; Spinks, G.M.; Wallace, G.G.; Madden, J.D.; Baughman, R.H. Increased Actuation Rate of Electromechanical Carbon Nanotube Actuators Using Potential Pulses with Resistance Compensation. Smart Mater. Struct. 2003, 12, 549–555. [Google Scholar] [CrossRef]
- Otero, T.F.; Martinez, J.G.; Asaka, K. Faradaic and Capacitive Components of the CNT Electrochemical Responses. Front. Mater. 2016, 3, 3. [Google Scholar] [CrossRef]
- Rojas, A.; San-Roman, M.L.; Zicovich-Wilson, C.M.; Camblor, M.A. Host-Guest Stabilization of a Zeolite Strained Framework: In Situ Transformation of Zeolite MTW into the Less Dense and More Strained ITW. Chem. Mater. 2013, 25, 729–738. [Google Scholar] [CrossRef]
- Poli, I.; Eslava, S.; Cameron, P. Tetrabutylammonium Cations for Moisture-Resistant and Semitransparent Perovskite Solar Cells. J. Mater. Chem. A 2017, 5, 22325–22333. [Google Scholar] [CrossRef]
- Michardière, A.S.; Mateo-Mateo, C.; Derré, A.; Correa-Duarte, M.A.; Mano, N.; Poulin, P. Carbon Nanotube Microfiber Actuators with Reduced Stress Relaxation. J. Phys. Chem. C 2016, 120, 6851–6858. [Google Scholar] [CrossRef]
- Otero, T.F.; Martinez, J.G. Physical and Chemical Awareness from Sensing Polymeric Artificial Muscles. Experiments and Modeling. Prog. Polym. Sci. 2015, 44, 62–78. [Google Scholar] [CrossRef]
- Martínez, J.G.; Sugino, T.; Asaka, K.; Otero, T.F. Electrochemistry of Carbon Nanotubes: Reactive Processes, Dual Sensing-Actuating Properties and Devices. ChemPhysChem 2012, 13, 2108–2114. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Mao, H.; Zhang, Z.; Gao, J.; Han, X.; Wang, W.-J.; Lim, K.H.; Yang, X. Cellulosic Nanocomposite Filaments for an Ionic Strength Sensor with Ultrahigh Precision and Sensitivity. J. Mater. Chem. A 2023, 11, 20665–20675. [Google Scholar] [CrossRef]
- Qi, H.; Mäder, E.; Liu, J. Unique Water Sensors Based on Carbon Nanotube-Cellulose Composites. Sens. Actuators B Chem. 2013, 185, 225–230. [Google Scholar] [CrossRef]
- Yun, S.; Kim, J. Multi-Walled Carbon Nanotubes-Cellulose Paper for a Chemical Vapor Sensor. Sens. Actuators B Chem. 2010, 150, 308–313. [Google Scholar] [CrossRef]
- Qi, H.; Liu, J.; Pionteck, J.; Pötschke, P.; Mäder, E. Carbon Nanotube-Cellulose Composite Aerogels for Vapour Sensing. Sens. Actuators B Chem. 2015, 213, 20–26. [Google Scholar] [CrossRef]
- Khani, H.; Rofouei, M.K.; Arab, P.; Gupta, V.K.; Vafaei, Z. Multi-Walled Carbon Nanotubes-Ionic Liquid-Carbon Paste Electrode as a Super Selectivity Sensor: Application to Potentiometric Monitoring of Mercury Ion(II). J. Hazard. Mater. 2010, 183, 402–409. [Google Scholar] [CrossRef]



| Cations | MC-MCNT Linear Actuation | Cation Size + Solvation Number | ||
|---|---|---|---|---|
| Stress σ (kPa) | Strain ε (%) | V.D.W.V. (Å3) [46] | n | |
| EDMI+ | 1.49 ± 0.12 | 0.0170 ± 0.001 | 133 | n.A. |
| Li+ | 0.9 ± 0.07 | 0.0084 ± 5 × 10−4 | 1.84 | 3–4 [44] |
| TBA+ | 4.17 ± 0.31 | 0.026 ± 0.0022 | 293 | 0 |
| Cations | Ue (J g−1) | −Eneg.charg. (V) | Epos.charg. (V) | σ (kPa) |
|---|---|---|---|---|
| EDMI+ | 0.63–0.36·j (A g−1) | −0.21–0.58·j (A g−1) | 0.48–0.49·j (A g−1) | 1.65 + 0.01·j (A g−1) |
| Li+ | 0.39–0.40·j (A g−1) | −0.23–0.82·j (A g−1) | 0.41–0.71·j (A g−1) | 1.02 + 0.03·j (A g−1) |
| TBA+ | 0.8–1.13·j (A g−1) | −0.19–0.93·j (A g−1) | 0.52–0.66·j (A g−1) | 4.72 + 0.01·j (A g−1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhi, F.; Le, Q.B.; Kiefer, R. Cation-Selective Actuator–Sensor Response of Microcrystalline Cellulose Multi-Walled Carbon Nanotubes of Different Electrolytes Using Propylene Carbonate Solvent. Polymers 2024, 16, 339. https://doi.org/10.3390/polym16030339
Elhi F, Le QB, Kiefer R. Cation-Selective Actuator–Sensor Response of Microcrystalline Cellulose Multi-Walled Carbon Nanotubes of Different Electrolytes Using Propylene Carbonate Solvent. Polymers. 2024; 16(3):339. https://doi.org/10.3390/polym16030339
Chicago/Turabian StyleElhi, Fred, Quoc Bao Le, and Rudolf Kiefer. 2024. "Cation-Selective Actuator–Sensor Response of Microcrystalline Cellulose Multi-Walled Carbon Nanotubes of Different Electrolytes Using Propylene Carbonate Solvent" Polymers 16, no. 3: 339. https://doi.org/10.3390/polym16030339






