Active Cellulose-Based Food Packaging and Its Use on Foodstuff
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Functionalization of the Bleached Kraft Paper
2.3. Investigation Methods
2.3.1. ATR-FTIR Spectra
2.3.2. SEM—EDAX Analysis
2.3.3. Water Contact Angle Measurements
2.3.4. Differential Scanning Calorimetry (DSC)
2.3.5. DPPH Radical Scavenging Assay
2.3.6. In Vitro Antibacterial Activity
2.3.7. Microbiological Assessment on Food
3. Results and Discussion
3.1. ATR-FTIR Spectra Results
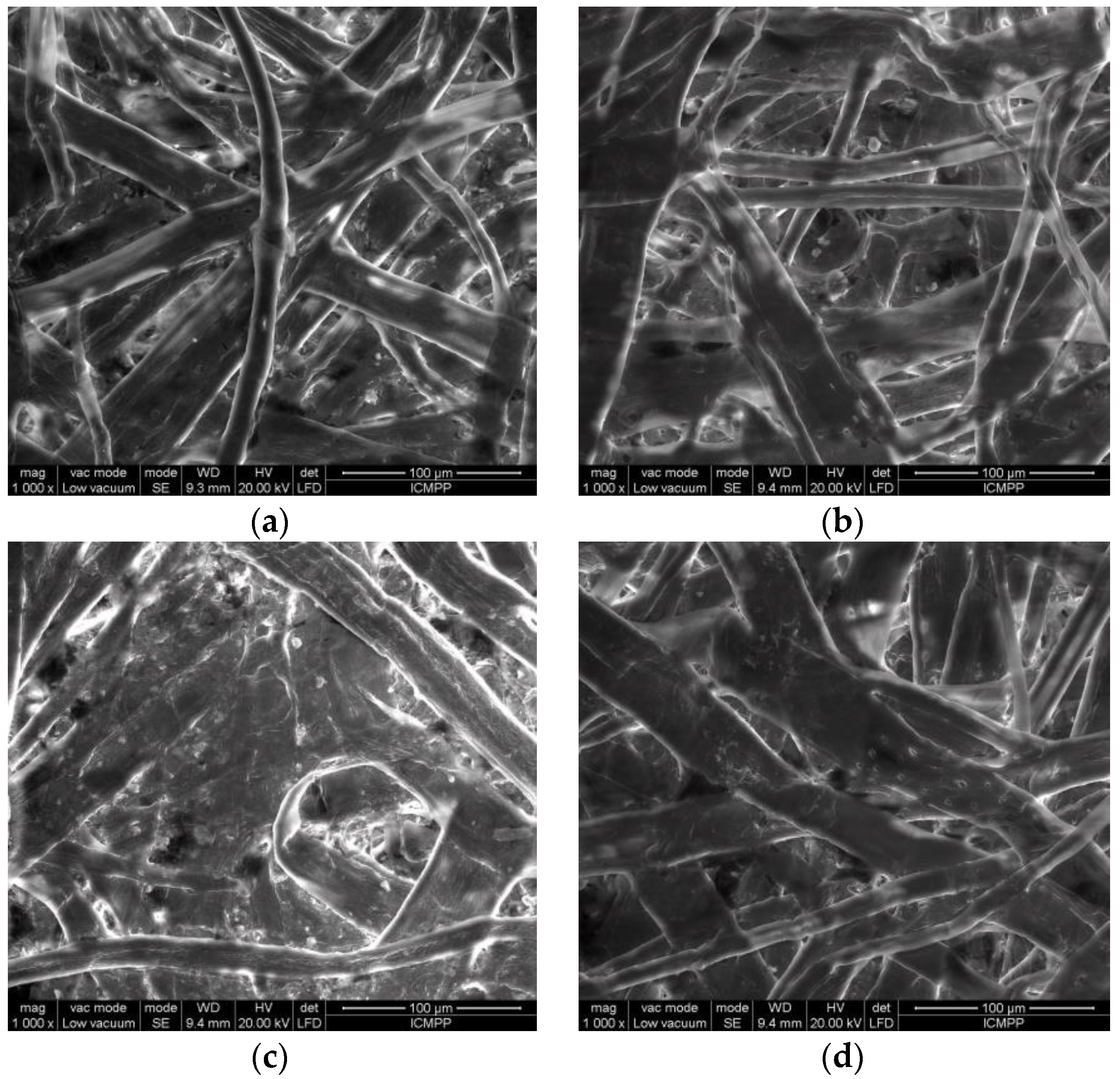
3.2. SEM Results
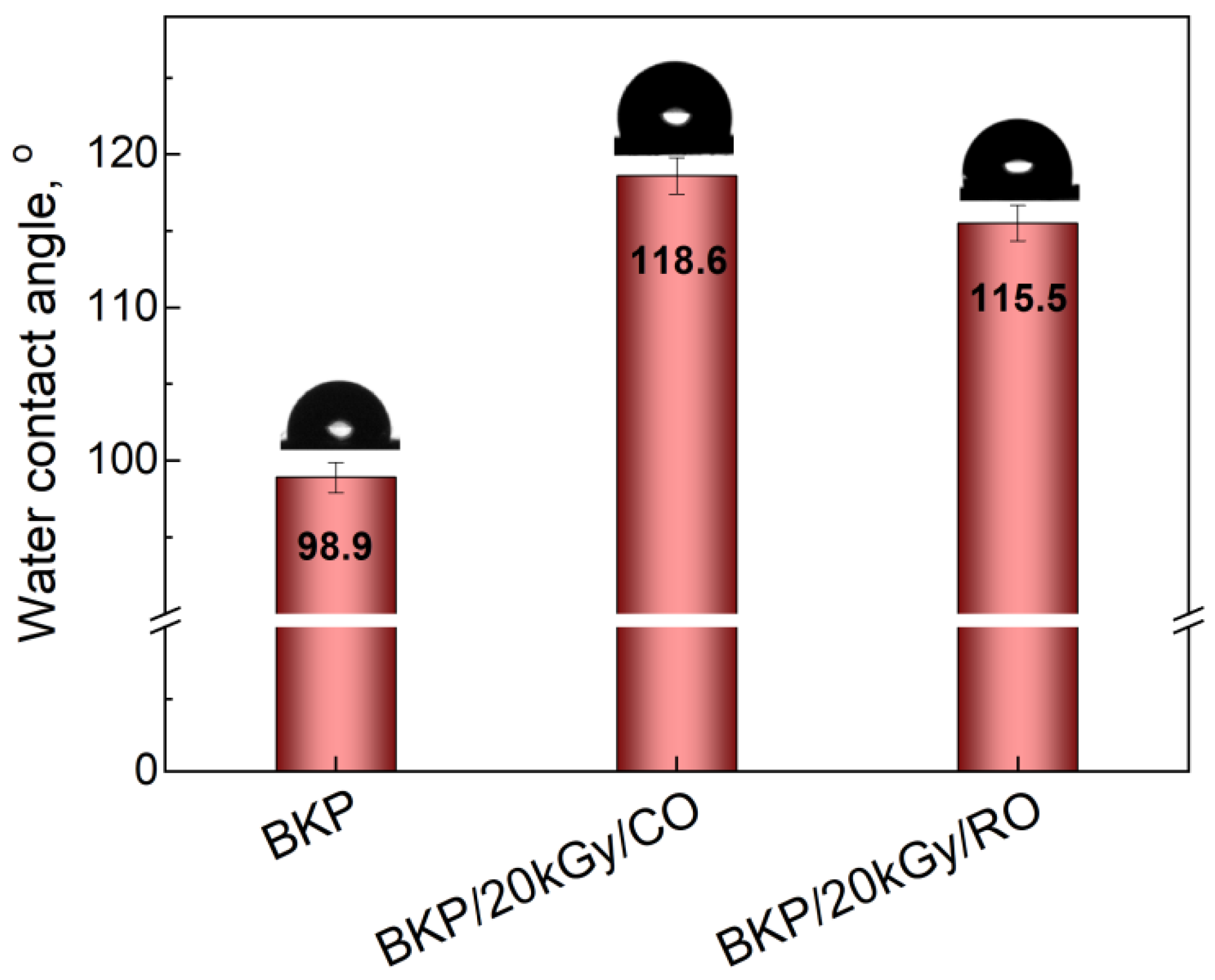
3.3. Water Contact Angle Measurements
3.4. DSC Results
3.5. DPPH Radical Scavenging Assay of Analyzed Samples
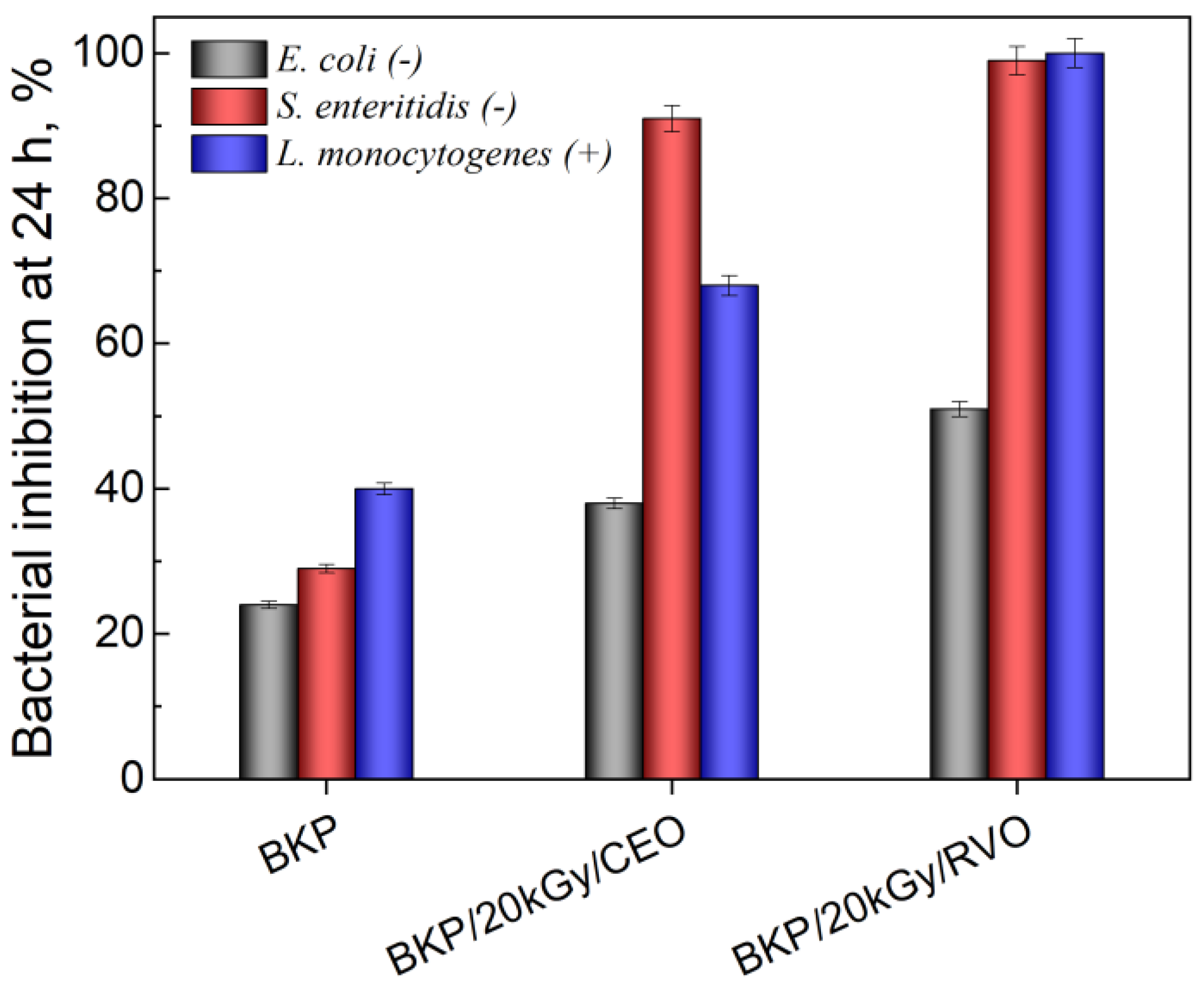
3.6. In Vitro Antibacterial Activity
3.7. Microbiological Evaluation on Fresh Food
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janjarasskul, T.; Suppakul, P. Active and intelligent packaging: The indication of quality and safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 808–831. [Google Scholar] [CrossRef]
- Soltani Firouz, M.; Mohi-Alden, K.; Omid, M. A critical review on intelligent and active packaging in the food industry: Research and development. Food Res. Int. 2021, 141, 110–113. [Google Scholar] [CrossRef]
- Jem, K.J.; Tan, B. The development and challenges of poly (lactic acid) and poly (glycolic acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Irimia, A.; Ioanid, G.E.; Zaharescu, T.; Coroabă, A.; Doroftei, F.; Safrany, A.; Vasile, C. Comparative study on gamma irradiation and cold plasma pretreatment for a cellulosic substrate modification with phenolic compounds. Radiat. Phys. Chem. 2017, 130, 52–61. [Google Scholar] [CrossRef]
- Rahman, R.; Sood, M.; Gupta, N.; Bandral, J.D.; Hameed, F.; Ashraf, S. Bioplastics for food packaging: A review. Int. J. Curr. Microbiol. App. Sci. 2019, 8, 2311–2321. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R. Antimicrobial food packaging based on sustainable bio-based materials for reducing foodborne pathogens: A review. Food Chem. 2020, 310, 125915. [Google Scholar] [CrossRef]
- Sdrobis, A.; Darie, R.N.; Totolin, M.; Cazacu, G.; Vasile, C. Low density polyethylene composites containing cellulose pulp fibers. Compos. Part B 2012, 43, 1873–1880. [Google Scholar] [CrossRef]
- Irimia, A.; Stoleru, E.; Vasile, C.; Bele, A.; Brebu, M. Application of Vegetal Oils in Developing Bioactive Paper-Based Materials for Food Packaging. Coatings 2021, 11, 1211. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C. Natural food additives: Quo vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Becerril, R.; Nerín, C.; Silva, F. Encapsulation systems for antimicrobial food packaging components: An update. Molecules 2020, 25, 1134. [Google Scholar] [CrossRef]
- Seow, Y.X.; Yeo, C.R.; Chung, H.L.; Yuk, H.G. Plant essential oils as active antimicrobial agents. Crit. Rev. Food Sci. Nutr. 2014, 54, 625–644. [Google Scholar] [CrossRef]
- Vasile, C.; Sivertsvik, M.; Mitelut, A.C.; Brebu, M.A.; Stoleru, E.; Rosnes, J.T.; Tanase, E.E.; Khan, W.; Pamfil, D.; Cornea, C.P.; et al. Comparative Analysis of the Composition and Active Property Evaluation of Certain Essential Oils to Assess their Potential Applications in Active Food Packaging. Materials 2017, 10, 45. [Google Scholar] [CrossRef]
- Goñi, P.; López, P.; Sánchez, C.; Gómez-Lus, R.; Becerril, R.; Nerin, C. Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chem. 2009, 116, 982–989. [Google Scholar] [CrossRef]
- Hu, Q.; Zhou, M.; Wei, S. Progress on the Antimicrobial Activity Research of Clove Oil and Eugenol in the food antisepsis field. J. Food Sci. 2018, 83, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Musa, Ö. Nutrient composition of Rose (Rosa canina L.) seed and oils. J. Med. Food 2002, 5, 137–140. [Google Scholar]
- Grajzer, M.; Prescha, A.; Korzonek, K.; Wojakowska, A.; Dziadas, M.; Kulma, A.; Grajeta, H. Characteristics of rose hip (Rosa canina L.) cold-pressed oil and its oxidative stability studied by the differential scanning calorimetry method. Food Chem. 2015, 188, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Tarhan, I. A robust method for simultaneous quantification of eugenol, eugenyl acetate, and β-caryophyllene in clove essential oil by vibrational spectroscopy. Phytochem 2021, 91, 112928. [Google Scholar] [CrossRef] [PubMed]
- Clegg, D.W.; Collyer, A. Irradiation Effects on Polymers; Elsevier: London, UK, 1991. [Google Scholar]
- de Santana, F.B.; Gontijo, L.C.; Mitsutake, H.; Mazivila, S.J.; de Souza, L.M.; Borges Neto, W. Non-destructive fraud detection in rosehip oil by MIR spectroscopy and chemometrics. Food Chem. 2016, 209, 228–233. [Google Scholar] [CrossRef]
- Yasser, F.H.A.; Amr El-Hag, A.; Ghada, F.E.M.; Reda, R. Gamma irradiation effect on the thermal stability, optical and electrical properties of acrylic acid/methyl methacrylate copolymer films. World J. Condens. Matter Phys. 2011, 1, 12. [Google Scholar]
- Kim, D.Y.; Nishiyama, Y.; Wada, M.; Kuga, S.; Okano, T. Thermal decomposition of cellulose crystallites in wood. Holzforschung 2001, 55, 521–524. [Google Scholar] [CrossRef]
- Nishiyama, Y. Structure and properties of the cellulose microfibril. J. Wood Sci. 2009, 55, 241–249. [Google Scholar] [CrossRef]
- Coppola, F.; Fiorillo, F.; Modelli, A.; Montanari, M.; Vandini, M. Effects of γ-ray treatment on paper. Polym. Degrad. Stabil. 2018, 150, 25–30. [Google Scholar] [CrossRef]
- Adamo, M.; Brizzi, M.; Magaudda, G.; Martinelli, G.; Plossi-Zappalà, M.; Rocchetti, F.; Savagnone, F. Gamma radiation treatment of paper in different environmental conditions: Chemical, physical and microbiological analysis. Restaurator 2001, 22, 107–131. [Google Scholar] [CrossRef]
- Drábková, K.; Ďurovič, M.; Kučerová, I. Influence of gamma radiation on properties of paper and textile fibres during disinfection. Rad. Phys. Chem. 2018, 152, 75–80. [Google Scholar] [CrossRef]
- Rojas, J.; Cabrera, S.; Benavides, J.; Lopera, Y.; Yarce, C. Lipidic matrixes containing clove essential oil: Biological activity, microstructural and textural studies. Molecules 2021, 26, 2425. [Google Scholar] [CrossRef]
- Mannozzi, C.; Foligni, R.; Scalise, A.; Mozzon, M. Characterization of lipid substances of rose hip seeds as a potential source of functional components: A review. Ital. J. Food Sci. 2020, 32, 721–733. [Google Scholar]
- Rossi, D.; Pittia, P.; Realdon, N. Contact Angle Measurements and Applications in Pharmaceuticals and Foods: A Critical Review. Rev. Adhes. Adhes. 2019, 6, 202–252. [Google Scholar] [CrossRef]
- Irimia, A.; Popescu, C.M. Bioactive Paper Packaging for Extended Food Shelf Life. Coatings 2023, 13, 1658. [Google Scholar] [CrossRef]
- SR ISO 4833/2014; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms. International Organization for Standardization: Geneva, Switzerland, 2014.
- SR EN ISO 7218/2014; Microbiology of Food and Animal Feeding Stuffs–General Requirements and Guidance for Microbiological Examinations. International Organization for Standardization: Geneva, Switzerland, 2014.
- Robles, E.; Izaguirre, N.; Dogaru, B.-I.; Popescu, C.-M.; Barandiaran, I.; Labidi, J. Sonochemical production of nanoscaled crystalline cellulose using organic acids. Green Chem. 2020, 22, 4627. [Google Scholar] [CrossRef]
- Hongfang, G.; Hui, Y.; Chuang, W. Controllable preparation and mechanism of nano-silver mediated by the microemulsion system of the clove oil. Results Phys. 2017, 7, 3130–3136. [Google Scholar]
- Jiang, J.; Watowita, P.S.M.S.L.; Chen, R.; Shi, Y.; Geng, J.-T.; Takahashi, K.; Li, L.; Osako, K. Multilayer gelatin/myofibrillar films containing clove essential oil: Properties, protein-phenolic interactions, and migration of active compounds. Food Packag. Shelf Life 2022, 32, 100842. [Google Scholar] [CrossRef]
- Mahak, M.; Simran, A.; Anita, Y.; Neeraj, K.A. Development of poly(hydroxybutyrate) film incorporated with nano silica and clove essential oil intended for active packaging of brown bread. Int. J. Biol. Macromol. 2023, 233, 123512. [Google Scholar]
- Babaoglu, H.; Bayrak, A.; Özdemir, N.; Ozgun, N. Encapsulation of clove essential oil in hydroxypropyl beta-cyclodextrin for characterization, controlled release, and antioxidant activity. J. Food Process Preserv. 2017, 41, e13202. [Google Scholar] [CrossRef]
- El-Maati, M.F.A.; Mahgoub, S.; Labib, S.M.; Al-Gaby, A.M.; Ramadan, M.F. Phenolic extracts of clove (Syzygium aromaticum) with novel antioxidant and antibacterial activities. Eur. J. Integr. Med. 2016, 8, 494–504. [Google Scholar] [CrossRef]
- Irimia, A.; Csiszar, E.; Coroabă, A.; Varganici, C.D.; Vasile, C. Mercerization Mediated Modification of Cellulosic Fibers with NIPAAm and Some Compounds with Antioxidant Activity. Fibers Polym. 2016, 17, 1569–1578. [Google Scholar] [CrossRef]
- Koczka, N.; Stefanovits-Bányai, E.; Ombódi, A. Total polyphenol content and antioxidant capacity of rosehips of some rosa species. Medicines 2018, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Güney, M. Determination of fatty acid profile and antioxidant activity of Rosehip seeds from Turkey. Int. J. Agric. Environ. Food Sci. 2020, 4, 81–86. [Google Scholar] [CrossRef]
- Jakovljevic, M.; Moslavac, T.; Bilic, M.; Aladic, K.; Bakula, F.; Jokic, S. Supercritical CO2 extraction of oil from rose hips (Rosa canina L.) and cornelian cherry (Cornus mas L.) seeds. Croat. J. Food Sci. Technol. 2018, 10, 197–205. [Google Scholar] [CrossRef]




| Sample | C | O | N | O/C | |||
|---|---|---|---|---|---|---|---|
| Wt% | At% | Wt% | At% | Wt% | At% | At% | |
| BKP | 57.12 | 65.51 | 41.78 | 33.72 | 0.50 | 0.48 | 0.51 |
| BKP/20 kGy | 54.17 | 61.20 | 44.19 | 37.63 | 0.75 | 0.73 | 0.61 |
| BKP/20 kGy/CO | 54.60 | 62.20 | 42.08 | 35.53 | 1.99 | 1.76 | 0.57 |
| BKP/20 kGy/RO | 55.39 | 63.75 | 41.83 | 34.38 | 2.42 | 1.48 | 0.54 |
| Tmax (°C) | BKP | BKP/20 kGy/CO | BKP/20 kGy/RO |
|---|---|---|---|
| Endotherm | 340 | 348.1 | 346.4 |
| Exotherm | 355.6 | 360.4 | 358.4 |
| Sample | IC50, mg/mL |
|---|---|
| BKP | - |
| BKP/20 kGy/CO | 0.205 |
| BKP/20 kGy/RO | 95.289 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irimia, A.; Grigoraș, V.C.; Popescu, C.-M. Active Cellulose-Based Food Packaging and Its Use on Foodstuff. Polymers 2024, 16, 389. https://doi.org/10.3390/polym16030389
Irimia A, Grigoraș VC, Popescu C-M. Active Cellulose-Based Food Packaging and Its Use on Foodstuff. Polymers. 2024; 16(3):389. https://doi.org/10.3390/polym16030389
Chicago/Turabian StyleIrimia, Anamaria, Vasile Cristian Grigoraș, and Carmen-Mihaela Popescu. 2024. "Active Cellulose-Based Food Packaging and Its Use on Foodstuff" Polymers 16, no. 3: 389. https://doi.org/10.3390/polym16030389






