Research Advances in Superabsorbent Polymers
Abstract
:1. Introduction
2. Superabsorbent Polymers
2.1. Synthetic Superabsorbent Polymers
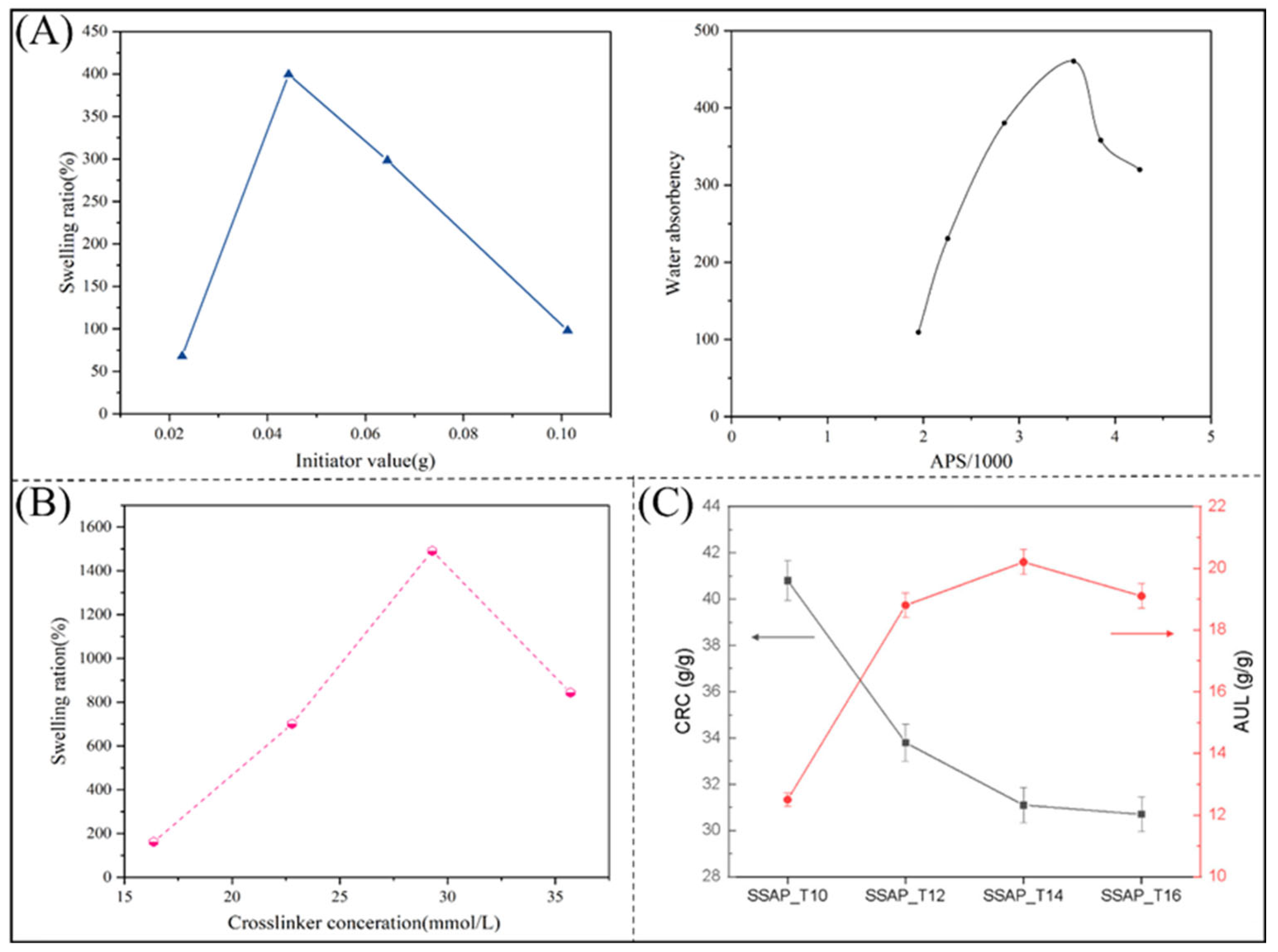
2.1.1. Polyacrylic Acids
2.1.2. Polyacrylonitrile
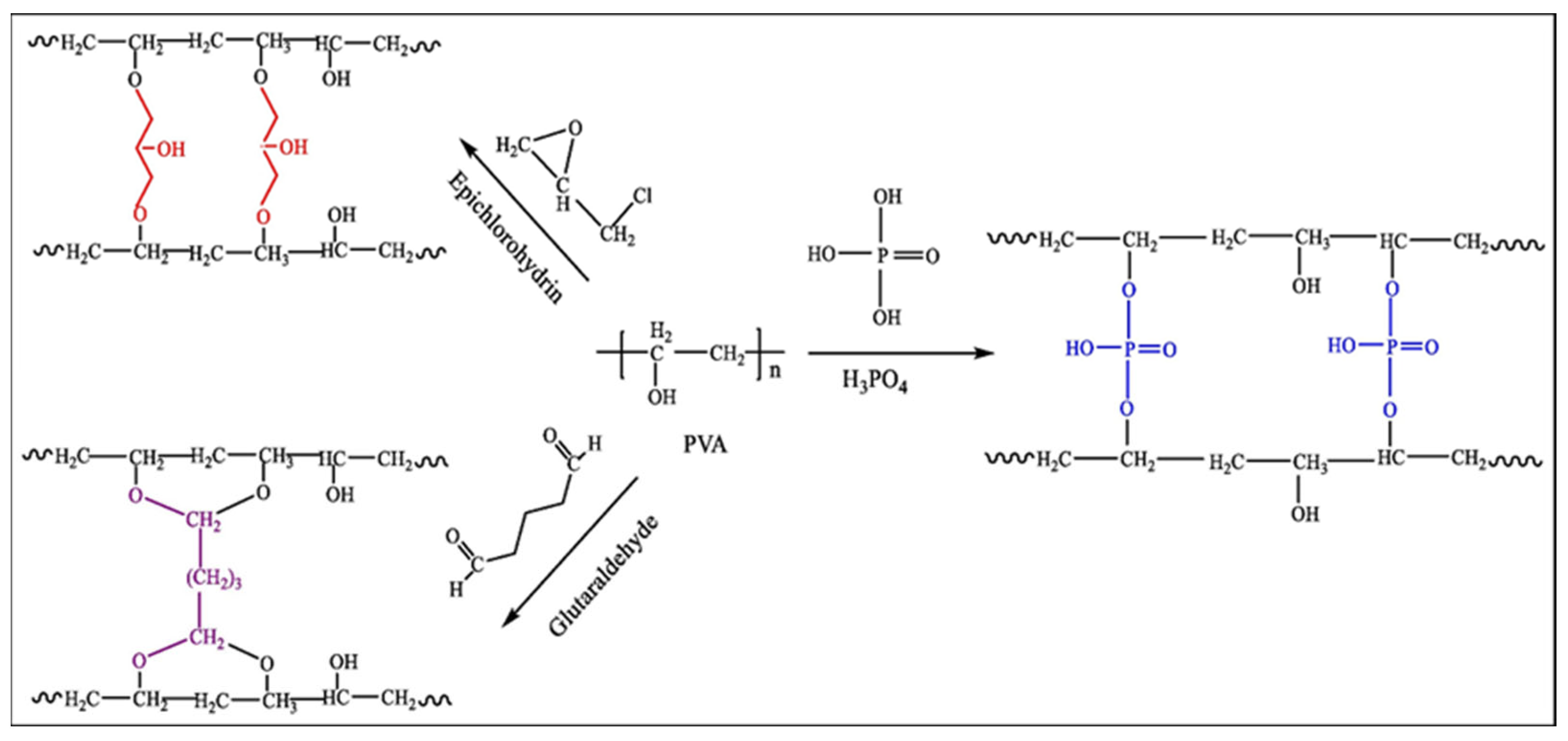
2.1.3. Polyvinyl Alcohol
2.2. Natural Superabsorbent Polymer
2.2.1. Common Natural Polymers
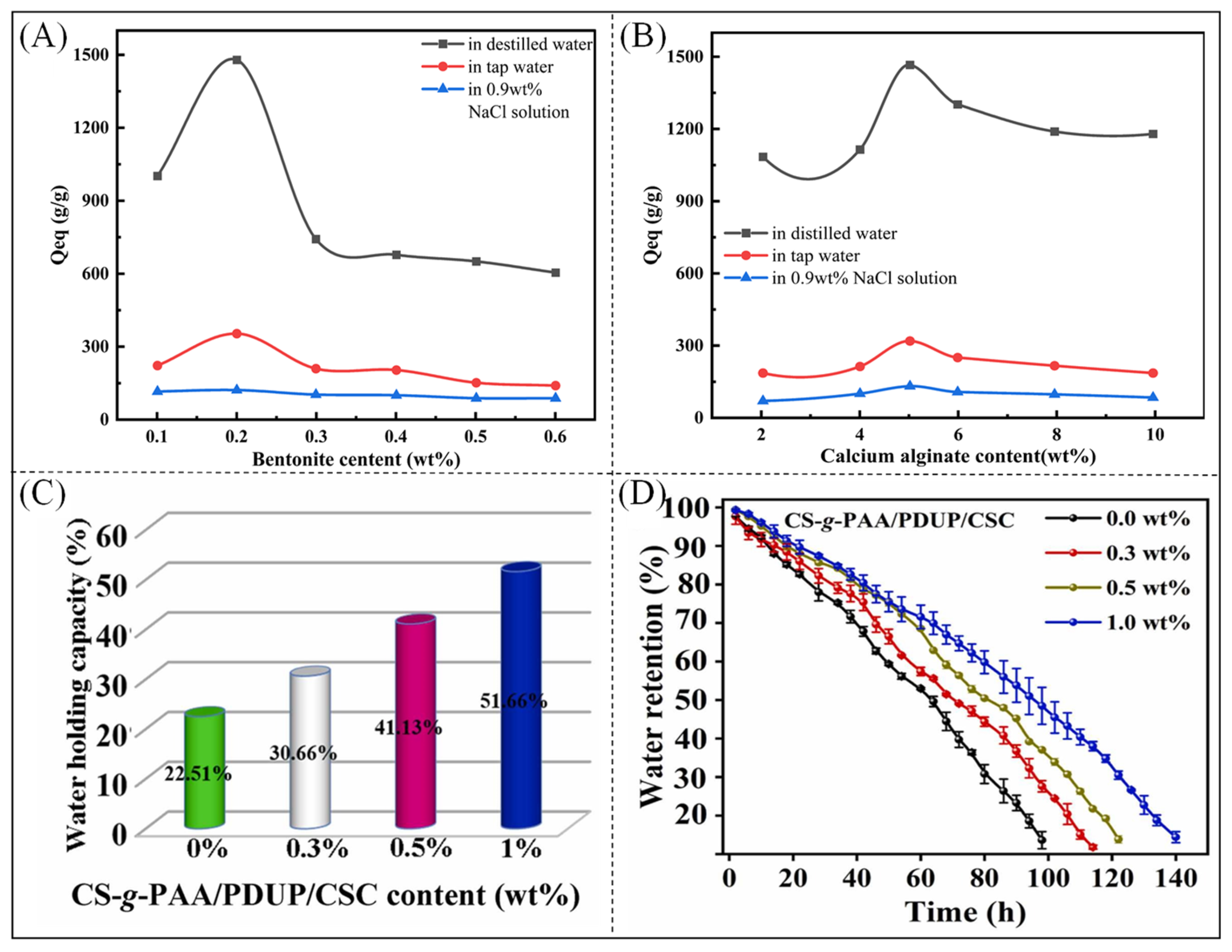
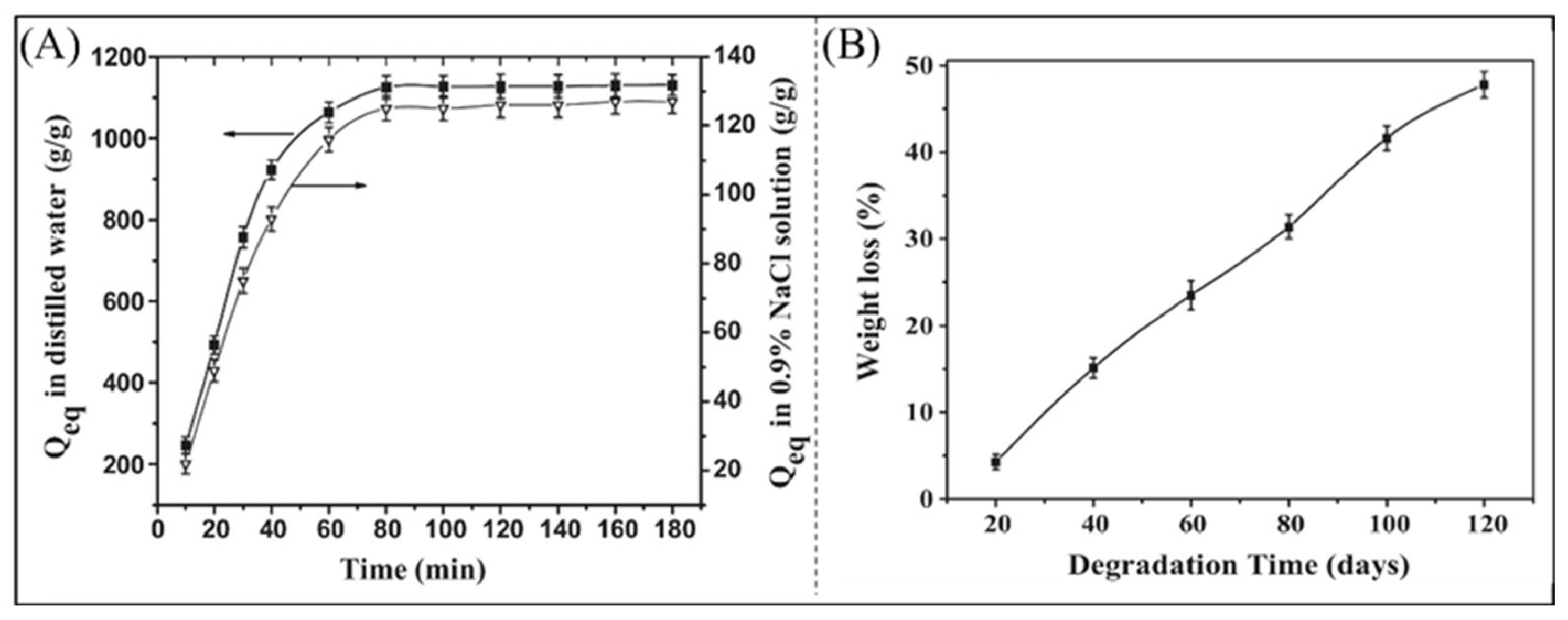
2.2.2. Modified Natural Superabsorbent Polymer
3. Forms of Superabsorbent Polymers
3.1. Superabsorbent Polymer Particles
3.2. Superabsorbent Polymer Fibers
3.3. Superabsorbent Polymer Gels
4. Applications of Superabsorbent Polymers
4.1. Industrial Applications
4.2. Agricultural Applications
4.3. Biomedical Applications
4.3.1. Drug Delivery
4.3.2. Wound Dressing
4.3.3. Tissue Engineering
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Buchholz, F.L.; Graham, A.T. Modern Superabsorbent Polymer Technology; Wiley-VCH: New York, NY, USA, 1998. [Google Scholar]
- Fanta, G.F.; Burr, R.C.; Russell, C.R.; Rist, C.E. Graft copolymers of starch. I. Copolymerization of gelatinized wheat starch with acrylonitrile. Fractionation of copolymer and effect of solvent on copolymer composition. J. Appl. Polym. Sci. 1966, 10, 929–937. [Google Scholar] [CrossRef]
- Fanta, G.F.; Burr, R.C.; Russell, C.R.; Rist, C.E. Graft copolymers of starch. II. Copolymerization of gelatinized wheat starch with acrylonitrile: Influence of reaction conditions on copolymer composition. J. Polym. Sci. Part B Polym. Lett. 1966, 4, 765–769. [Google Scholar] [CrossRef]
- Fanta, G.F.; Burr, R.C.; Russell, C.R.; Rist, C.E. Graft copolymers of starch. III. Copolymerization of gelatinized wheat starch with acrylonitrile. Influence of chain modifiers on copolymer composition. J. Appl. Polym. Sci. 1967, 11, 457–463. [Google Scholar] [CrossRef]
- Athawale, V.D.; Lele, V. Recent trends in hydrogels based on starch-graft-acrylic acid: A review. Starch 2001, 53, 7–13. [Google Scholar] [CrossRef]
- Zohuriaan-Mehr, M.J.; Kabiri, K. Superabsorbent polymer materials: A review. Iran. Polym. J. 2008, 17, 451–477. [Google Scholar]
- Gooch, J.W. (Ed.) Super absorbent fibers. In Encyclopedic Dictionary of Polymers; Springer: New York, NY, USA, 2011; pp. 712–714. [Google Scholar] [CrossRef]
- Fanta, G.F.; Burr, R.C.; Doane, W.M.; Russell, C.R. Absorbent polymers from starch and flour through graft polymerization of acrylonitrile and comonomer mixtures. Starch 1978, 30, 237–242. [Google Scholar] [CrossRef]
- Capezza, A.; Newson, W.R.; Olsson, R.T.; Hedenqvist, M.S.; Johansson, E. Advances in the use of protein-based materials: Toward sustainable naturally sourced absorbent materials. ACS Sustain. Chem. Eng. 2019, 7, 4532–4547. [Google Scholar] [CrossRef]
- Ma, J.; Li, X.; Bao, Y. Advances in cellulose-based superabsorbent hydrogels. RSC Adv. 2015, 5, 59745–59757. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Wang, A. Synthesis and characterization of chitosan-g-poly(acrylic acid)/attapulgite superabsorbent composites. Carbohydr. Polym. 2007, 68, 367–374. [Google Scholar] [CrossRef]
- Laftah, W.A.; Hashim, S.; Ibrahim, A.N. Polymer hydrogels: A review. Polym. Plast. Technol. Eng. 2011, 50, 1475–1486. [Google Scholar] [CrossRef]
- Zohuriaan-Mehr, M.J.; Omidian, H.; Doroudiani, S.; Kabiri, K. Advances in non-hygienic applications of superabsorbent hydrogel materials. J. Mater. Sci. 2010, 45, 5711–5735. [Google Scholar] [CrossRef]
- Cheng, B.; Pei, B.; Wang, Z.; Hu, Q. Advances in chitosan-based superabsorbent hydrogels. RSC Adv. 2017, 7, 42036–42046. [Google Scholar] [CrossRef]
- Zohuriaan-Mehr, M.J.; Pourjavadi, A.; Salimi, H.; Kurdtabar, M. Protein- and homo poly(amino acid)-based hydrogels with super-swelling properties. Polym. Adv. Technol. 2009, 20, 655–671. [Google Scholar] [CrossRef]
- Liu, Q.X.; Ding, Z.R.; Dong, Z. Swelling behaviors of acrylic-based superabsorbent fibers. Adv. Mater. Res. 2012, 476–478, 1331–1335. [Google Scholar] [CrossRef]
- Feng, D.; Bai, B.; Ding, C.; Wang, H.; Suo, Y. Synthesis and Swelling Behaviors of Yeast-g-poly(acrylic acid) Superabsorbent Co-polymer. Ind. Eng. Chem. Res. 2014, 53, 12760–12769. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Zohuriaan-Mehr, M.J.; Ghasempoori, S.N.; Hossienzadeh, H. Modified CMC. V. Synthesis and super-swelling behavior of hydrolyzed CMC-g-PAN hydrogel. J. Appl. Polym. Sci. 2007, 103, 877–883. [Google Scholar] [CrossRef]
- Mucientes, A.E.; Santiago, F.; Delgado, A.M. Effect of initial N,N′-methylene bisacrylamide concentration on the swelling behaviour of acrylic-based superabsorbent polymers. Pol. J. Chem. 2005, 79, 897–905. [Google Scholar]
- Fang, S.; Wang, G.; Xing, R.; Chen, X.; Liu, S.; Qin, Y.; Li, K.; Wang, X.; Li, R.; Li, P. Synthesis of superabsorbent polymers based on chitosan derivative graft acrylic acid-co-acrylamide and its property testing. Int. J. Biol. Macromol. 2019, 132, 575–584. [Google Scholar] [CrossRef]
- Utech, S.; Boccaccini, A.R. A review of hydrogel-based composites for biomedical applications: Enhancement of hydrogel properties by addition of rigid inorganic fillers. J. Mater. Sci. 2016, 51, 271–310. [Google Scholar] [CrossRef]
- Husain, M.S.B.; Gupta, A.; Alashwal, B.Y.; Sharma, S. Synthesis of PVA/PVP based hydrogel for biomedical applications: A review. Energy Sources Part A Recover. Util. Environ. Eff. 2018, 40, 2388–2393. [Google Scholar] [CrossRef]
- Shalla, A.H.; Yaseen, Z.; Bhat, M.A.; Rangreez, T.A.; Maswal, M. Recent review for removal of metal ions by hydrogels. Sep. Sci. Technol. 2019, 54, 89–100. [Google Scholar] [CrossRef]
- Atta, A.M.; Abdel-Rahman, A.A.-H.; El Aassy, I.E.; Ahmed, F.Y.; Hamza, M.F. Adsorption properties of uranium (VI) ions on reactive crosslinked Acrylamidoxime and acrylic acid copolymer resins. J. Dispers. Sci. Technol. 2010, 32, 84–94. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, H.; Liu, S.; Guo, J. Superabsorbent polymer with high swelling ratio, and temperature-sensitive and magnetic properties employed as an efficient dewatering medium of fine coal. Energy Fuels 2017, 31, 1825–1831. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, W.; Zhou, J.; Yu, D.-G.; Liu, H. The Applications of Ferulic-Acid-Loaded Fibrous Films for Fruit Preservation. Polymers 2022, 14, 4947. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Upadhyay, M.; Reddy Adena, S.; Vasant, B.; Muthu, M. Hydrogels: An introduction to a controlled drug delivery device, synthesis and application in drug delivery and tissue engineering. Austin J. Biomed. Eng. 2017, 4, 1037. [Google Scholar] [CrossRef]
- Wei, Y.; Zeng, Q.; Wang, M.; Huang, J.; Guo, X.; Wang, L. Near-infrared light-responsive electrochemical protein imprinting biosensor based on a shape memory conducting hydrogel. Biosens. Bioelectron. 2019, 131, 156–162. [Google Scholar] [CrossRef]
- Pontremoli, C.; Boffito, M.; Fiorilli, S.; Laurano, R.; Torchio, A.; Bari, A.; Tonda-Turo, C.; Ciardelli, G.; Vitale-Brovarone, C. Hybrid injectable platforms for the in situ delivery of therapeutic ions from mesoporous glasses. Chem. Eng. J. 2018, 340, 103–113. [Google Scholar] [CrossRef]
- Singh, A.; Vaishagya, K.; Verma, R.K.; Shukla, R. Temperature/pH-triggered PNIPAM-based smart nanogel system loaded with anastrozole delivery for application in cancer chemotherapy. Aaps Pharmscitech 2019, 20, 213. [Google Scholar] [CrossRef]
- Lin, X.; Miao, L.; Wang, X.; Tian, H. Design and evaluation of pH-responsive hydrogel for oral delivery of amifostine and study on its radioprotective effects. Colloids Surf. B Biointerfaces 2020, 195, 111200. [Google Scholar] [CrossRef]
- Spicer, C.D. Hydrogel scaffolds for tissue engineering: The importance of polymer choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Mannarino, M.M.; Bassett, M.; Donahue, D.T.; Biggins, J.F. Novel high-strength thromboresistant poly(vinyl alcohol)-based hydrogel for vascular access applications. J. Biomater. Sci. Polym. Ed. 2020, 31, 601–621. [Google Scholar] [CrossRef]
- Wang, M.; Hou, J.; Yu, D.-G.; Li, S.; Zhu, J.; Chen, Z. Electrospun tri-layer nanodepots for sustained release of acyclovir. J. Alloys Compd. 2020, 846, 156471. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, D.-G.; Liu, Y.; Liu, Y.-N. Progress of Electrospun Nanofibrous Carriers for Modifications to Drug Release Profiles. J. Funct. Biomater. 2022, 13, 289. [Google Scholar] [CrossRef]
- Du, J.; Gao, S.; Shi, P.; Fan, J.; Xu, Q.; Min, Y. Three-dimensional carbonaceous for potassium ion batteries anode to boost rate and cycle life performance. J. Power Sources 2020, 451, 227727. [Google Scholar] [CrossRef]
- Buckingham, M.A.; Zhang, S.; Liu, Y.; Chen, J.; Marken, F.; Aldous, L. Thermogalvanic and thermocapacitive behavior of superabsorbent hydrogels for combined low-temperature thermal energy conversion and harvesting. ACS Appl. Energy Mater. 2021, 4, 11204–11214. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Liu, W.; Shi, J.; Dong, G.; Zhang, H.; Li, D.; Lu, G. Polymer salt-derived carbon-based nanomaterials for high-performance hybrid Li-ion capacitors. J. Mater. Sci. 2019, 54, 7811–7822. [Google Scholar] [CrossRef]
- Mendoza, D.J.; Ayurini, M.; Raghuwanshi, V.S.; Simon, G.P.; Hooper, J.F.; Garnier, G. Synthesis of superabsorbent polyacrylic acid-grafted cellulose nanofibers via silver-promoted decarboxylative radical polymerization. Macromolecules 2023, 56, 3497–3506. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, J.; Zhang, X.; Gao, P. Fabrication and swelling properties of a novel superabsorbent composite derived from waste coal gangue. Polym. Polym. Compos. 2023, 31, 13. [Google Scholar] [CrossRef]
- Sadeghi, M.; Gudarzi, A.; Safari, S.; Shahsavari, H.; Sadeghi, H. Synthesis of superabsorbent hydrogels consisted of pectin and poly(methacrylonitrile) for drug delivery systems. Asian J. Chem. 2013, 25, 4847–4850. [Google Scholar] [CrossRef]
- de Oliveira, M.R.; Gonçalves, E.P. Addition of polyacrylonitrile superabsorbent polymer in cement mixture with variation in the amount of water. Rev. UniVap. 2022, 28, 12. [Google Scholar] [CrossRef]
- Ge, J.; Jia, Y.; Cheng, C.; Sun, K.; Peng, Y.; Tu, Y.; Qiang, Y.; Hua, Z.; Zheng, Z.; Ye, X.; et al. Polydimethylsiloxane-functionalized polyacrylonitrile nanofibrous aerogels for efficient oil absorption and oil/water separation. J. Appl. Polym. Sci. 2021, 138, 51339. [Google Scholar] [CrossRef]
- Ma, C.; Fan, F.; Chen, M.; Li, S.; Chen, Y.; Pan, Z.; Liu, R. Preparation of a novel superabsorbent fiber–cement composite and evaluation of its self-healing performance. Cem. Concr. Compos. 2022, 133, 104713. [Google Scholar] [CrossRef]
- Sun, J.; Sun, G.; Zhao, X.; Liu, X.; Zhao, H.; Xu, C.; Yan, L.; Jiang, X.; Cui, Y. Ultrafast and efficient removal of Pb(II) from acidic aqueous solution using a novel polyvinyl alcohol superabsorbent. Chemosphere 2021, 282, 131032. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, S.A.; Hameed, A.M.; Rasheed, R.T. Synthesis and study of magnesium complexes derived from polyacrylate and polyvinyl alcohol and their applications as superabsorbent polymers. J. Mech. Behav. Mater. 2022, 31, 462–472. [Google Scholar] [CrossRef]
- Sedighim, S.; Chen, Y.; Xu, C.; Mohindra, R.; Liu, H.; Agrawal, D.K.; Thankam, F.G. Carboxymethyl cellulose–alginate interpenetrating hydroxy ethyl methacrylate crosslinked polyvinyl alcohol reinforced hybrid hydrogel templates with improved biological performance for cardiac tissue engineering. Biotechnol. Bioeng. 2023, 120, 819–835. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, R.; Shi, X.; Lian, S.; Zhou, Q.; Chen, Y.; Liu, W.; Li, W. Synthesis of cellulose-based superabsorbent hydrogel with high salt tolerance for soil conditioning. Int. J. Biol. Macromol. 2022, 209, 1169–1178. [Google Scholar] [CrossRef]
- Tian, H.; Cheng, S.; Zhen, J.; Lei, Z. Superabsorbent polymer with excellent water/salt absorbency and water retention, and fast swelling properties for preventing soil water evaporation. J. Polym. Environ. 2023, 31, 812–824. [Google Scholar] [CrossRef]
- Gao, L.; Luo, H.; Wang, Q.; Hu, G.; Xiong, Y. Synergistic Effect of Hydrogen Bonds and Chemical Bonds to Construct a Starch-Based Water-Absorbing/Retaining Hydrogel Composite Reinforced with cellulose and poly(ethylene glycol). ACS Omega 2021, 6, 35039–35049. [Google Scholar] [CrossRef] [PubMed]
- Jayanudin; Lestari, R.S.D.; Barleany, D.R.; Pitaloka, A.B.; Yulvianti, M.; Prasetyo, D.; Anggoro, D.V.; Ruhiatna, A. Chitosan-Graft-Poly(acrylic acid) Superabsorbent’s Water Holding in Sandy Soils and Its Application in Agriculture. Polymers 2022, 14, 5175. [Google Scholar] [CrossRef]
- Akkaya, R.; Akkaya, B.; Çakıcı, G.T. Chitosan–poly(acrylamide-co-maleic acid) composite synthesis, characterization, and investigation of protein adsorption behavior. Polym. Bull. 2023, 80, 4153–4168. [Google Scholar] [CrossRef]
- Barleany, D.R.; Heriyanto, H.; Alwan, H.; Kurniawati, V.; Muyassaroh, A.; Erizal, E. Effect of starch and chitosan addition on swelling properties of neutralized poly(acrylic acid)-based superabsorbent hydrogels prepared by using γ-irradiation technique. At. Indones. 2022, 48, 99–106. [Google Scholar] [CrossRef]
- Yao, Y.; Shen, Y.; Hu, C.; Wu, H. Superabsorbent fabric: In situ polymerisation of macroporous starch–sodium alginate–polyacrylate hydrogel on fibre surface. Cellulose 2023, 30, 7113–7128. [Google Scholar] [CrossRef]
- Xiong, H.; Peng, H.; Ye, X.; Kong, Y.; Wang, N.; Yang, F.; Meni, B.-H.; Lei, Z. High salt tolerance hydrogel prepared of hydroxyethyl starch and its ability to increase soil water holding capacity and decrease water evaporation. Soil Tillage Res. 2022, 222, 105427. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, R.; Sui, S.; Liu, X.; Liu, J.; Shi, C.; Zhao, N.; Zhong, C.; Hu, W. Starch-Based superabsorbent hydrogel with high electrolyte retention capability and synergistic interface engineering for long-lifespan flexible zinc−air batteries. Angew. Chem. Int. Ed. 2023, 62, e202302640. [Google Scholar] [CrossRef]
- Jancar, J.; Skarpa, P.; Mohsen-Latiff, A. Fertilizer technology based on optimized nitrogen release from urea-loaded natural superabsorbent carriers. Soil Use Manag. 2023, 39, 1583–1599. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Bengoechea, C.; Felix, M.; Guerrero, A. Freeze-Drying versus Heat-Drying: Effect on Protein-Based Superabsorbent Material. Processes 2021, 9, 1076. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, L.; Liu, Q.; Yang, M.; Chen, J.; Lei, Z. Preparation and properties of a biodegradability superabsorbent composite based on flax cake protein-g-poly (acrylic acid)/kaolinite. J. Appl. Polym. Sci. 2022, 139, 51975. [Google Scholar] [CrossRef]
- Jahan, N.; Mahbub, S.I.; Lee, B.-T.; Bae, S.H. In Vivo and in vitro investigation of a novel gelatin/sodium polyacrylate composite hemostatic sponge for topical bleeding. J. Funct. Biomater. 2023, 14, 265. [Google Scholar] [CrossRef]
- Chen, W.; Mei, E.; Xie, X. Virus stabilization with enhanced porous superabsorbent polymer (PSAP) beads for diagnostics and surveillance. ACS ES&T Water 2022, 2, 2378–2387. [Google Scholar] [CrossRef]
- Meng, H.; Zhang, X.; Sun, S.; Tan, T.; Cao, H. Preparation of γ-aminopropyltriethoxysilane cross-linked poly(aspartic acid) superabsorbent hydrogels without organic solvent. J. Biomater. Sci. Polym. Ed. 2016, 27, 133–143. [Google Scholar] [CrossRef]
- Vakili, M.R.; Rahneshin, N. Synthesis and characterization of novel stimuli-responsive hydrogels based on starch and L-aspartic acid. Carbohydr. Polym. 2013, 98, 1624–1630. [Google Scholar] [CrossRef]
- Agnihotri, S.; Singhal, R. Effect of sodium alginate content in acrylic acid/sodium humate/sodium alginate superabsorbent hydrogel on removal capacity of MB and CV dye by adsorption. J. Polym. Environ. 2019, 27, 372–385. [Google Scholar] [CrossRef]
- Snoeck, D.; Moerkerke, B.; Mignon, A.; De Belie, N. In-Situ crosslinking of superabsorbent polymers as external curing layer compared to internal curing to mitigate plastic shrinkage. Constr. Build. Mater. 2020, 262, 120819. [Google Scholar] [CrossRef]
- Nita, L.E.; Chiriac, A.P.; Ghilan, A.; Rusu, A.G.; Tudorachi, N.; Timpu, D. Alginate enriched with phytic acid for hydrogels preparation. Int. J. Biol. Macromol. 2021, 181, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Yarimkaya, S.; Basan, H. Synthesis and Swelling behavior of acrylate-based hydrogels. J. Macromol. Sci. Part A 2007, 44, 699–706. [Google Scholar] [CrossRef]
- Tang, Q.; Wu, J.; Lin, J.; Li, Q.; Fan, S. Two-step synthesis of polyacrylamide/polyacrylate interpenetrating network hydrogels and its swelling/deswelling properties. J. Mater. Sci. 2008, 43, 5884–5890. [Google Scholar] [CrossRef]
- Hu, X.Y.; Xiao, C.F. Study on superabsorbent polyacrylonitrile-based fibre. Indian J. Fibre Text. Res. 2005, 30, 207–210. [Google Scholar]
- Bary, E.M.A.; Fekri, A.; Soliman, Y.A.; Harmal, A.N. Manufacturing of superabsorbent membranes of PVA and rice husk fibres reinforced with nanosilica for agricultural and horticultural applications. Int. J. Environ. Stud. 2019, 76, 150–167. [Google Scholar] [CrossRef]
- Ballard, N.; Asua, J.M. Radical polymerization of acrylic monomers: An overview. Prog. Polym. Sci. 2018, 79, 40–60. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, V.; Kaith, B.; Kumar, V.; Som, S.; Kalia, S.; Swart, H. Synthesis, characterization and water retention study of biodegradable Gum ghatti-poly(acrylic acid–aniline) hydrogels. Polym. Degrad. Stab. 2015, 111, 20–31. [Google Scholar] [CrossRef]
- Athawale, V.D.; Lele, V. Factors influencing absorbent properties of saponified starch-g-(acrylic acid-co-acrylamide). J. Appl. Polym. Sci. 2000, 77, 2480–2485. [Google Scholar] [CrossRef]
- Miyajima, T.; Matsubara, Y.; Komatsu, H.; Miyamoto, M.; Suzuki, K. Development of a superabsorbent polymer using iodine transfer polymerization. Polym. J. 2020, 52, 365–373. [Google Scholar] [CrossRef]
- Baloch, F.E.; Afzali, D.; Fathirad, F. Design of acrylic acid/nanoclay grafted polysaccharide hydrogels as superabsorbent for controlled release of chlorpyrifos. Appl. Clay Sci. 2021, 211, 106194. [Google Scholar] [CrossRef]
- Raju, K.M.; Raju, M.P.; Mohan, Y.M. Synthesis and water absorbency of crosslinked superabsorbent polymers. J. Appl. Polym. Sci. 2002, 85, 1795–1801. [Google Scholar] [CrossRef]
- Ma, S.; Liu, M.; Chen, Z. Preparation and properties of a salt-resistant superabsorbent polymer. J. Appl. Polym. Sci. 2004, 93, 2532–2541. [Google Scholar] [CrossRef]
- Singh, J.; Dhaliwal, A. Synthesis, characterization and swelling behavior of silver nanoparticles containing superabsorbent based on grafted copolymer of polyacrylic acid/Guar gum. Vacuum 2018, 157, 51–60. [Google Scholar] [CrossRef]
- Kwon, Y.-R.; Kim, J.-S.; Kim, D.-H. Effective enhancement of water absorbency of itaconic acid based-superabsorbent polymer via tunable surface—Crosslinking. Polymers 2021, 13, 2782. [Google Scholar] [CrossRef]
- Kwon, Y.R.; Lim, S.H.; Kim, H.C.; Kim, J.S.; Chang, Y.W.; Choi, J.; Kim, D.H. Superabsorbent polymer with improved permeability and absorption rate using hollow glass microspheres. J. Polym. Sci. 2021, 59, 462–470. [Google Scholar] [CrossRef]
- Stahl, J.D.; Cameron, M.D.; Haselbach, J.; Aust, S.D. Biodegradation of superabsorbent polymers in soil. Environ. Sci. Pollut. Res. 2000, 7, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Hebeish, A.; Hashem, M.; El-Hady, M.A.; Sharaf, S. Development of CMC hydrogels loaded with silver nano-particles for medical applications. Carbohydr. Polym. 2013, 92, 407–413. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Suchithra, P.S.; Senan, P.; Tharun, A.R. Kinetic and Equilibrium Profiles of Adsorptive Recovery of thorium(IV) from Aqueous Solutions Using poly(methacrylic acid) Grafted cellulose/bentonite Superabsorbent Composite. Ind. Eng. Chem. Res. 2012, 51, 4825–4836. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Wang, Y.; Mu, B.; Wang, X.; Wang, A. Eco-friendly superabsorbent composites based on calcined semicoke and polydimethylourea phosphate: Synthesis, swelling behavior, degradability and their impact on cabbage growth. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 648, 129439. [Google Scholar] [CrossRef]
- Soleimani, F.; Sadeghi, M.; Shasevari, H.; Soleimani, A.; Sadeghi, H. Effective parameters onto swelling capacity of biosuperabsorbent based on acrylonitrile-sucrose. Asian J. Chem. 2013, 25, 2259–2261. [Google Scholar] [CrossRef]
- Wang, K.Y.; Cen, R.F.; Shu, W.W. Preparation and performance of super-absorbent resin using polyacrylonitrile fiber wastes. Adv. Mater. Res. 2015, 1120–1121, 498–501. [Google Scholar] [CrossRef]
- Trivedi, J.; Chourasia, A. Sodium salt of partially carboxymethylated sodium alginate-g-poly(acrylonitrile): I. Photo-induced synthesis, characterization, and alkaline hydrolysis. Gels 2023, 9, 410. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.S.; Mishra, A.; Pal, D.; Ghosh, A.K.; Ghosh, A.; Banerjee, S.; Sen, K.K. Synthesis and Characterization of poly(acrylic acid)/poly(vinyl alcohol)-xanthan gum Interpenetrating Network (IPN) Superabsorbent Polymeric Composites. Polym.-Plast. Technol. Eng. 2012, 51, 878–884. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Recent advances in composites based on cellulose derivatives for biomedical applications. Carbohydr. Polym. 2020, 247, 116683. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Wang, F.; Meng, W.; Yang, X.; Li, P.; Jiang, J.; Tan, H.; Zheng, Y. Preparation of porous carboxymethyl chitosan grafted poly (acrylic acid) superabsorbent by solvent precipitation and its application as a hemostatic wound dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 63, 18–29. [Google Scholar] [CrossRef]
- Dragan, E.S.; Apopei, D.F. Synthesis and swelling behavior of pH-sensitive semi-interpenetrating polymer network composite hydrogels based on native and modified potatoes starch as potential sorbent for cationic dyes. Chem. Eng. J. 2011, 178, 252–263. [Google Scholar] [CrossRef]
- Sharma, S.; Dua, A.; Malik, A. Polyaspartic acid based superabsorbent polymers. Eur. Polym. J. 2014, 59, 363–376. [Google Scholar] [CrossRef]
- Zhao, Y.; Kang, J.; Tan, T. Salt-, pH- and temperature-responsive semi-interpenetrating polymer network hydrogel based on poly(aspartic acid) and poly(acrylic acid). Polymer 2006, 47, 7702–7710. [Google Scholar] [CrossRef]
- Zhang, Z.; Abidi, N.; Lucia, L.; Chabi, S.; Denny, C.T.; Parajuli, P.; Rumi, S.S. Cellulose/nanocellulose superabsorbent hydrogels as a sustainable platform for materials applications: A mini-review and perspective. Carbohydr. Polym. 2023, 299, 120140. [Google Scholar] [CrossRef]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel superabsorbent cellulose-based hydrogels crosslinked with citric acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Hazmi, A.T.; Ahmad, F.B.; Akmal, M.M.; Ralib, A.A.M.; Ali, F.B. Fungal chitosan for potential application in piezoelectric energy harvesting: Review on experimental procedure of chitosan extraction. Alex. Eng. J. 2023, 67, 105–116. [Google Scholar] [CrossRef]
- Jolayemi, O.L.; Malik, A.H.; Ekblad, T.; Fredlund, K.; Olsson, M.E.; Johansson, E. Protein-based biostimulants to enhance plant growth—State-of-the-art and future direction with sugar beet as an example. Agronomy 2022, 12, 3211. [Google Scholar] [CrossRef]
- Sajilata, M.; Singhal, R.S.; Kulkarni, P.R. Resistant starch–A review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch—Composition, fine structure and architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Primarini, D.; Ohta, Y. Some Enzyme Properties of Raw Starch Digesting Amylases from Streptomyces sp. No. 4. Starch 2000, 52, 28–32. [Google Scholar] [CrossRef]
- Harish Prashanth, K.V.; Tharanathan, R.N. Chitin/chitosan: Modifications and their unlimited application potential—An overview. Trends Food Sci. Technol. 2007, 18, 117–131. [Google Scholar] [CrossRef]
- Tang, W.J.; Fernandez, J.G.; Sohn, J.J.; Amemiya, C.T. Chitin is endogenously produced in vertebrates. Curr. Biol. 2015, 25, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Y.-F.; Tan, H.-M.; Jiang, J.-X. Synthesis and characterization of a novel superabsorbent polymer of N,O-carboxymethyl chitosan graft copolymerized with vinyl monomers. Carbohydr. Polym. 2009, 75, 287–292. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Chitin and chitosan: Sources, production and medical applications. In Renewable Resources for Functional Polymers and Biomaterials; Williams, P., Ed.; The Royal Society of Chemistry: London, UK, 2011; Chapter 10; pp. 292–318. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Q.; Yue, Q.; Gao, B.; Xu, X.; Zhong, Q. Synthesis and characterization of a novel super-absorbent based on wheat straw. Bioresour. Technol. 2011, 102, 2853–2858. [Google Scholar] [CrossRef]
- Jia, Z.; Shen, D.; Xu, W. Synthesis and antibacterial activities of quaternary ammonium salt of chitosan. Carbohydr. Res. 2001, 333, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.; Malik, A.H.; Hussain, A.; Rasheed, F.; Newson, W.R.; Plivelic, T.; Hedenqvist, M.S.; Gällstedt, M.; Kuktaite, R. Wheat gluten polymer structures: The impact of genotype, environment, and processing on their functionality in various applications. Cereal Chem. 2013, 90, 367–376. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhang, Y.; Wang, L.; Yang, Z. A supramolecular hydrogel to boost the production of antibodies for phosphorylated proteins. Chem. Commun. 2019, 55, 12388–12391. [Google Scholar] [CrossRef]
- Jonker, A.M.; Löwik, D.W.P.M.; van Hest, J.C.M. Peptide- and protein-based hydrogels. Chem. Mater. 2012, 24, 759–773. [Google Scholar] [CrossRef]
- Min, S.K.; Kim, J.H.; Chung, D.J. Swelling behavior of biodegradable crosslinked gel based on poly(aspartic acid) and PEG-diepoxide. Korea Polym. J. 2001, 9, 143–149. Available online: https://api.semanticscholar.org/CorpusID:101405488 (accessed on 4 February 2024).
- Kunioka, M. Biodegradable water absorbent synthesized from Bacterial poly(amino acid)s. Macromol. Biosci. 2004, 4, 324–329. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Qin, Y. The gel swelling properties of alginate fibers and their applications in wound management. Polym. Adv. Technol. 2008, 19, 6–14. [Google Scholar] [CrossRef]
- Manjula, B.; Varaprasad, K.; Sadiku, R.; Raju, K.M. Preparation and characterization of sodium alginate–based hydrogels and their in vitro release studies. Adv. Polym. Technol. 2013, 32, 21340. [Google Scholar] [CrossRef]
- Hua, S.; Wang, A. Synthesis, characterization and swelling behaviors of sodium alginate-g-poly(acrylic acid)/sodium humate superabsorbent. Carbohydr. Polym. 2009, 75, 79–84. [Google Scholar] [CrossRef]
- Phang, Y.-N.; Chee, S.-Y.; Lee, C.-O.; Teh, Y.-L. Thermal and microbial degradation of alginate-based superabsorbent polymer. Polym. Degrad. Stab. 2011, 96, 1653–1661. [Google Scholar] [CrossRef]
- Sang, Y.; Zhao, J.R. Reduction of water absorption capacity of cellulose fibres for its application in cementitious materials. J. Compos. Mater. 2015, 49, 2757–2763. [Google Scholar] [CrossRef]
- Meimoun, J.; Wiatz, V.; Saint-Loup, R.; Parcq, J.; Favrelle, A.; Bonnet, F.; Zinck, P. Modification of starch by graft copolymerization. Starch 2018, 70, 1600351. [Google Scholar] [CrossRef]
- Sawut, A.; Yimit, M.; Sun, W.; Nurulla, I. Photopolymerisation and characterization of maleylatedcellulose-g-poly(acrylic acid) superabsorbent polymer. Carbohydr. Polym. 2014, 101, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, A.; Dhamodharan, R. Super water-absorbing new material from chitosan, EDTA and urea. Carbohydr. Polym. 2015, 134, 337–343. [Google Scholar] [CrossRef]
- Athawale, V.; Lele, V. Graft copolymerization onto starch. II. Grafting of acrylic acid and preparation of it’s hydrogels. Carbohydr. Polym. 1998, 35, 21–27. [Google Scholar] [CrossRef]
- Qiao, D.; Liu, H.; Yu, L.; Bao, X.; Simon, G.P.; Petinakis, E.; Chen, L. Preparation and characterization of slow-release fertilizer encapsulated by starch-based superabsorbent polymer. Carbohydr. Polym. 2016, 147, 146–154. [Google Scholar] [CrossRef]
- Narmani, A.; Jafari, S.M. Chitosan-based nanodelivery systems for cancer therapy: Recent advances. Carbohydr. Polym. 2021, 272, 118464. [Google Scholar] [CrossRef] [PubMed]
- Nge, T.T.; Hori, N.; Takemura, A.; Ono, H. Swelling behavior of chitosan/poly(acrylic acid) complex. J. Appl. Polym. Sci. 2004, 92, 2930–2940. [Google Scholar] [CrossRef]
- Wang, X.; Lou, T.; Zhao, W.; Song, G. Preparation of pure chitosan film using ternary solvents and its super absorbency. Carbohydr. Polym. 2016, 153, 253–257. [Google Scholar] [CrossRef]
- Raju, M.P.; Raju, K.M. Design and synthesis of superabsorbent polymers. J. Appl. Polym. Sci. 2001, 80, 2635–2639. [Google Scholar] [CrossRef]
- Tang, H.; Chen, H.; Duan, B.; Lu, A.; Zhang, L. Swelling behaviors of superabsorbent chitin/carboxymethylcellulose hydrogels. J. Mater. Sci. 2014, 49, 2235–2242. [Google Scholar] [CrossRef]
- Hanani, Z.N.; Roos, Y.; Kerry, J. Use and application of gelatin as potential biodegradable packaging materials for food products. Int. J. Biol. Macromol. 2014, 71, 94–102. [Google Scholar] [CrossRef]
- Daniele, M.A.; Adams, A.A.; Naciri, J.; North, S.H.; Ligler, F.S. Interpenetrating networks based on gelatin methacrylamide and PEG formed using concurrent thiol click chemistries for hydrogel tissue engineering scaffolds. Biomaterials 2014, 35, 1845–1856. [Google Scholar] [CrossRef] [PubMed]
- Bagheri Marandi, G.; Beheshti Rouzbahani, G.; Kurdtabar, M. Synthesis and swelling behavior of gelatin-based hydrogel nanocomposites. J. Appl. Chem. Res. 2014, 8, 63–80. Available online: https://api.semanticscholar.org/CorpusID:4984958 (accessed on 4 February 2024).
- Rathna, G.V.N. Hydrogels of modified ethylenediaminetetraacetic dianhydride gelatin conjugated with poly(ethylene glycol) dialdehyde as a drug-release matrix. J. Appl. Polym. Sci. 2004, 91, 1059–1067. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Salimi, H. New Protein-based hydrogel with superabsorbing properties: Effect of monomer ratio on swelling behavior and kinetics. Ind. Eng. Chem. Res. 2008, 47, 9206–9213. [Google Scholar] [CrossRef]
- Song, W.; Zhang, Y.; Tran, C.H.; Choi, H.K.; Yu, D.-G.; Kim, I. Porous organic polymers with defined morphologies: Synthesis, assembly, and emerging applications. Prog. Polym. Sci. 2023, 142, 101691. [Google Scholar] [CrossRef]
- Zhou, J.; Yi, T.; Zhang, Z.; Yu, D.-G.; Liu, P.; Wang, L.; Zhu, Y. Electrospun Janus core (ethyl cellulose//polyethylene oxide) @ shell (hydroxypropyl methyl cellulose acetate succinate) hybrids for an enhanced colon-targeted prolonged drug absorbance. Adv. Compos. Hybrid Mater. 2023, 6, 189. [Google Scholar] [CrossRef]
- Yu, D.-G.; Zhou, J. How can Electrospinning Further Service Well for Pharmaceutical Researches? J. Pharm. Sci. 2023, 112, 2719–2723. [Google Scholar] [CrossRef]
- Fujita, S.; Tazawa, T.; Kono, H. Preparation and enzyme degradability of spherical and water-absorbent gels from sodium carboxymethyl cellulose. Gels 2022, 8, 321. [Google Scholar] [CrossRef] [PubMed]
- Maijan, P.; Chantarak, S. Synthesis and characterization of highly durable and reusable superabsorbent core–shell particles. Polym. Eng. Sci. 2020, 60, 306–313. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, M.; Yang, H.; Yan, W.; Jiang, F. Effect of aggregate size on liquid absorption characteristics of konjac glucomannan superabsorbent. J. Appl. Polym. Sci. 2017, 134, 45416. [Google Scholar] [CrossRef]
- Sand, A.; Shin, N.-J.; Nam, H.-G.; Kwark, Y.-J. Effects of Reaction Parameters on water Absorption of poly(itaconic acid) Superabsorbent Particles Synthesized by Inverse Suspension Polymerization. Fibers Polym. 2021, 22, 898–903. [Google Scholar] [CrossRef]
- Soly, S.J.; Nosrati, A.; Skinner, W.; Addai-Mensah, J. Superabsorbent-mediated dewaterability of fine hydrophobic sulphide mineral slurries. Sep. Sci. Technol. 2019, 54, 3055–3069. [Google Scholar] [CrossRef]
- Fernández, P.; Kraemer, F.B.; Sabatté, L.; Guiroy, J.; Boem, F.G. Superabsorbent polyacrylamide Effects on Hydrophysical Soil Properties and Plant Biomass in a Sandy Loam soil. Commun. Soil Sci. Plant Anal. 2022, 53, 2892–2906. [Google Scholar] [CrossRef]
- Lee, K.M.; Min, J.H.; Oh, S.; Lee, H.; Koh, W.-G. Preparation and characterization of superabsorbent polymers (SAPs) surface-crosslinked with polycations. React. Funct. Polym. 2020, 157, 104774. [Google Scholar] [CrossRef]
- Ramazani-Harandi, M.; Zohuriaan-Mehr, M.; Yousefi, A.; Ershad-Langroudi, A.; Kabiri, K. Rheological determination of the swollen gel strength of superabsorbent polymer hydrogels. Polym. Test. 2006, 25, 470–474. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Yoon, K.-J.; Ko, S.-W. Preparation and properties of alginate superabsorbent filament fibers crosslinked with glutaraldehyde. J. Appl. Polym. Sci. 2000, 78, 1797–1804. [Google Scholar] [CrossRef]
- Petroudy, S.R.D.; Kahagh, S.A.; Vatankhah, E. Environmentally friendly superabsorbent fibers based on electrospun cellulose nanofibers extracted from wheat straw. Carbohydr. Polym. 2021, 251, 117087. [Google Scholar] [CrossRef]
- Angel, N.; Li, S.; Yan, F.; Kong, L. Recent advances in electrospinning of nanofibers from bio-based carbohydrate polymers and their applications. Trends Food Sci. Technol. 2022, 120, 308–324. [Google Scholar] [CrossRef]
- Yu, D.-G.; Zhao, P. The Key Elements for Biomolecules to Biomaterials and to Bioapplications. Biomolecules 2022, 12, 1234. [Google Scholar] [CrossRef]
- Song, W.; Tang, Y.; Qian, C.; Kim, B.J.; Liao, Y.; Yu, D.-G. Electrospinning spinneret: A bridge between the visible world and the invisible nanostructures. Innovation 2023, 4, 100381. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Zhu, X.Z.; Yang, H.X. Effect of the pore fractal dimensions on absorption ability in superabsorbent fibers. Appl. Mech. Mater. 2013, 345, 205–208. [Google Scholar] [CrossRef]
- Vasilyev, G.; Vilensky, R.; Zussman, E. The ternary system amylose-amylopectin-formic acid as precursor for electrospun fibers with tunable mechanical properties. Carbohydr. Polym. 2019, 214, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Güler, B.; Çallioğlu, F.C. Comparative analysis of superabsorbent properties of PVP and PAA nanofibres. Ind. Textila 2021, 72, 460–466. [Google Scholar] [CrossRef]
- Liu, W.; Lin, H.; Wang, J.; Han, Q.; Liu, F. Polytetrafluoroethylene (PTFE) hollow fibers modified by hydrophilic crosslinking network (HCN) for robust resistance to fouling and harsh chemical cleaning. J. Membr. Sci. 2021, 630, 119301. [Google Scholar] [CrossRef]
- Jia, E.; Mou, H.; Liu, Z.; Wang, J.; Zeng, L.; Yang, X.; Liu, P. Surface Hydrophilic Modification of Polypropylene Fibers and Their Application in Fiber-Reinforced Cement-Based Materials. J. Macromol. Sci. Part B Phys. 2020, 60, 286–298. [Google Scholar] [CrossRef]
- Lu, L.; Yuan, S.; Wang, J.; Shen, Y.; Deng, S.; Xie, L.; Yang, Q. The formation mechanism of hydrogels. Curr. Stem Cell Res. Ther. 2018, 13, 490–496. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Q.; Guo, L.; Wang, P.; Liu, S.; Chen, J.; Lei, Z. White Cabbage (Brassica oleracea L.) waste, as biowaste for the preparation of novel superabsorbent polymer gel. J. Environ. Chem. Eng. 2021, 9, 106689. [Google Scholar] [CrossRef]
- Zhai, N.; Wang, B. Preparation of fast-swelling porous superabsorbent hydrogels with high saline water absorbency under pressure by foaming and post surface crosslinking. Sci. Rep. 2023, 13, 13815. [Google Scholar] [CrossRef]
- Olad, A.; Doustdar, F.; Gharekhani, H. Fabrication and characterization of a starch-based superabsorbent hydrogel composite reinforced with cellulose nanocrystals from potato peel waste. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 601, 124962. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Adnan, A.; Yahya, E.B.; Olaiya, N.; Safrida, S.; Hossain, M.S.; Balakrishnan, V.; Gopakumar, D.A.; Abdullah, C.; Oyekanmi, A.; et al. A review on plant cellulose nanofibre-based aerogels for biomedical applications. Polymers 2020, 12, 1759. [Google Scholar] [CrossRef]
- De Belie, N.; Gruyaert, E.; Al-Tabbaa, A.; Antonaci, P.; Baera, C.; Bajare, D.; Darquennes, A.; Davies, R.; Ferrara, L.; Jefferson, T.; et al. A review of self-healing concrete for damage management of structures. Adv. Mater. Interfaces 2018, 5, 1800074. [Google Scholar] [CrossRef]
- Liu, J.; Wang, M.; Liu, N.; Teng, L.; Wang, Y.; Chen, Z.; Shi, C. Development of ultra-fine SAP powder for lower-shrinkage and higher-strength cement pastes made with ultra-low water-to-binder ratio. Compos. Part B Eng. 2023, 262, 110810. [Google Scholar] [CrossRef]
- Feng, D.; Bai, B.; Wang, H.; Suo, Y. Novel fabrication of PAA/PVA/Yeast superabsorbent with interpenetrating polymer network for pH-dependent selective adsorption of dyes. J. Polym. Environ. 2018, 26, 567–588. [Google Scholar] [CrossRef]
- Zhang, Z.; Abidi, N.; Lucia, L. Smart superabsorbent alginate/carboxymethyl chitosan composite hydrogel beads as efficient biosorbents for methylene blue dye removal. J. Mater. Sci. Technol. 2023, 159, 81–90. [Google Scholar] [CrossRef]
- Zhang, C.; Meza, J.V.G.; Zhou, K.; Liu, J.; Song, S.; Zhang, M.; Meng, D.; Chen, J.; Xia, L.; Xiheng, H. Superabsorbent polymer used for saline-alkali soil water retention. J. Taiwan Inst. Chem. Eng. 2023, 145, 104830. [Google Scholar] [CrossRef]
- El Idrissi, A.; Dardari, O.; Metomo, F.N.N.N.; Essamlali, Y.; Akil, A.; Amadine, O.; Aboulhrouz, S.; Zahouily, M. Effect of sodium alginate-based superabsorbent hydrogel on tomato growth under different water deficit conditions. Int. J. Biol. Macromol. 2023, 253, 127229. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, Y.; Liu, M.; Liu, Z.; Zhou, T.; Liu, Y.; Cheng, D. Network interpenetrating slow-release nitrogen fertilizer based on carrageenan and urea: A new low-cost water and fertilizer regulation carrier. Int. J. Biol. Macromol. 2023, 242, 124858. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and their applications in targeted drug delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [PubMed]
- Vashist, A.; Vashist, A.; Gupta, Y.K.; Ahmad, S. Recent advances in hydrogel based drug delivery systems for the human body. J. Mater. Chem. B 2014, 2, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, L.; Zhu, Y.; Wang, L.; Yu, D.-G.; Liu, L.-Y. Tri-Layer Core–Shell Fibers from Coaxial Electrospinning for a Modified Release of Metronidazole. Pharmaceutics 2023, 15, 2561. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Hosseinzadeh, H.; Pashaei, S. Fabrication of nanocellulose loaded poly(AA-co-HEMA) hydrogels for ceftriaxone controlled delivery and crystal violet adsorption. Polym. Compos. 2019, 40, E559–E569. [Google Scholar] [CrossRef]
- Shin, Y.; Kim, D.; Hu, Y.; Kim, Y.; Hong, I.K.; Kim, M.S.; Jung, S. pH-responsive succinoglycan-carboxymethyl cellulose hydrogels with highly improved mechanical strength for controlled drug delivery systems. Polymers 2021, 13, 3197. [Google Scholar] [CrossRef]
- Bakravi, A.; Ahamadian, Y.; Hashemi, H.; Namazi, H. Synthesis of gelatin-based biodegradable hydrogel nanocomposite and their application as drug delivery agent. Adv. Polym. Technol. 2018, 37, 2625–2635. [Google Scholar] [CrossRef]
- Kapahi, H.; Khan, N.M.; Bhardwaj, A.; Mishra, N. Implication of nanofibers in oral drug delivery. Curr. Pharm. Des. 2015, 21, 2021–2036. [Google Scholar] [CrossRef]
- Zhou, J.; Dai, Y.; Fu, J.; Yan, C.; Yu, D.-G.; Yi, T. Dual-Step Controlled Release of Berberine Hydrochloride from the Trans-Scale Hybrids of Nanofibers and Microparticles. Biomolecules 2023, 13, 1011. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.; Gong, W.; Wang, B.; Yu, D.-G.; Zhu, Y. Integrating Chinese Herbs and Western Medicine for New Wound Dressings through Handheld Electrospinning. Biomedicines 2023, 11, 2146. [Google Scholar] [CrossRef]
- Schneider, A.; Wang, X.; Kaplan, D.; Garlick, J.; Egles, C. Biofunctionalized electrospun silk mats as a topical bioactive dressing for accelerated wound healing. Acta Biomater. 2009, 5, 2570–2578. [Google Scholar] [CrossRef]
- Wu, S.; Liu, J.; Cai, J.; Zhao, J.; Duan, B.; Chen, S. Combining electrospinning with hot drawing process to fabricate high performance poly (L-lactic acid) nanofiber yarns for advanced nanostructured bio-textiles. Biofabrication 2021, 13, 045018. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, L.; Wang, J.; Jin, M.; Tang, Q.; Chen, Z.; Cheng, Y.; Yang, R.; Zhao, G. Electrospun nanofibers promote wound healing: Theories, techniques, and perspectives. J. Mater. Chem. B 2021, 9, 3106–3130. [Google Scholar] [CrossRef]
- Memic, A.; Abudula, T.; Mohammed, H.S.; Joshi Navare, K.; Colombani, T.; Bencherif, S.A. Latest progress in electrospun nanofibers for wound healing applications. ACS Appl. Bio Mater. 2019, 2, 952–969. [Google Scholar] [CrossRef]
- Liu, Y.; Li, T.; Han, Y.; Li, F.; Liu, Y. Recent development of electrospun wound dressing. Curr. Opin. Biomed. Eng. 2021, 17, 100247. [Google Scholar] [CrossRef]
- Abrigo, M.; McArthur, S.L.; Kingshott, P. Electrospun nanofibers as dressings for chronic wound care: Advances, challenges, and future prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Sun, L.; Fu, X.; Lin, Z.; Xie, W.; Zhang, W.; Zhao, F.; Chen, X. Enhanced diabetic wound healing by electrospun core–sheath fibers loaded with dimethyloxalylglycine. J. Mater. Chem. B 2018, 6, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Leong, K.W.; Yoo, H.S. In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF). Biomaterials 2008, 29, 587–596. [Google Scholar] [CrossRef]
- Lalani, R.; Liu, L. Electrospun Zwitterionic poly(sulfobetaine methacrylate) for nonadherent, superabsorbent, and antimicrobial wound dressing applications. Biomacromolecules 2012, 13, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Gaydhane, M.K.; Kanuganti, J.S.; Sharma, C.S. Honey and curcumin loaded multilayered polyvinylalcohol/cellulose acetate electrospun nanofibrous mat for wound healing. J. Mater. Res. 2020, 35, 600–609. [Google Scholar] [CrossRef]
- Varshney, N.; Sahi, A.K.; Poddar, S.; Vishwakarma, N.K.; Kavimandan, G.; Prakash, A.; Mahto, S.K. Freeze–thaw-induced physically cross-linked superabsorbent polyvinyl alcohol/soy protein isolate hydrogels for skin wound dressing: In Vitro and in vivo characterization. ACS Appl. Mater. Interfaces 2022, 14, 14033–14048. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-G.; Huang, C. Electrospun Biomolecule-Based Drug Delivery Systems. Biomolecules 2023, 13, 1152. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Abel, M.; Ruth, P.; Hipler, U.C. Superabsorbent polymer-containing wound dressings have a beneficial effect on wound healing by reducing PMN elastase concentration and inhibiting microbial growth. J. Mater. Sci. Mater. Med. 2011, 22, 2583–2590. [Google Scholar] [CrossRef] [PubMed]
- Tarlton, J.F.; Munro, H.S. Use of modified superabsorbent polymer dressings for protease modulation in improved chronic wound care. Wounds 2013, 25, 51–57. Available online: https://pubmed.ncbi.nlm.nih.gov/25867807/ (accessed on 4 February 2024). [PubMed]
- Cipriano, B.H.; Banik, S.J.; Sharma, R.; Rumore, D.; Hwang, W.; Briber, R.M.; Raghavan, S.R. Superabsorbent hydrogels that are robust and highly stretchable. Macromolecules 2014, 47, 4445–4452. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Garg, T.; Singh, O.; Arora, S.; Murthy, R.S.R. Scaffold: A novel carrier for cell and drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 2012, 29, 1–63. [Google Scholar] [CrossRef]
- Wojcik, M.; Kazimierczak, P.; Benko, A.; Palka, K.; Vivcharenko, V.; Przekora, A. Superabsorbent curdlan-based foam dressings with typical hydrocolloids properties for highly exuding wound management. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 124, 112068. [Google Scholar] [CrossRef]
- Chen, W.; Ma, J.; Zhu, L.; Morsi, Y.; Ei-Hamshary, H.A.; Al-Deyab, S.S.; Mo, X. Superelastic, superabsorbent and 3D nanofiber-assembled scaffold for tissue engineering. Colloids Surfaces B Biointerfaces 2016, 142, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, D.; Zhu, X.; Nie, J.; Ma, G. Electrospun and photocrosslinked gelatin/dextran–maleic anhydride composite fibers for tissue engineering. Eur. Polym. J. 2019, 113, 142–147. [Google Scholar] [CrossRef]
- Sartore, L.; Pandini, S.; Baldi, F.; Bignotti, F.; Di Landro, L. Biocomposites based on poly(lactic acid) and superabsorbent sodium polyacrylate. J. Appl. Polym. Sci. 2017, 134, 45655. [Google Scholar] [CrossRef]
- Mahmoodzadeh, A.; Moghaddas, J.; Jarolmasjed, S.; Kalan, A.E.; Edalati, M.; Salehi, R. Biodegradable cellulose-based superabsorbent as potent hemostatic agent. Chem. Eng. J. 2021, 418, 129252. [Google Scholar] [CrossRef]
- Chen, W.; Chen, S.; Morsi, Y.; El-Hamshary, H.; El-Newhy, M.; Fan, C.; Mo, X. Superabsorbent 3D scaffold based on electrospun nanofibers for cartilage tissue engineering. ACS Appl. Mater. Interfaces 2016, 8, 24415–24425. [Google Scholar] [CrossRef]
- Rnjak-Kovacina, J.; Weiss, A.S. Increasing the pore size of electrospun scaffolds. Tissue Eng. Part B Rev. 2011, 17, 365–372. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; McCarthy, A.; Yan, Z.; Kim, H.J.; Carlson, M.A.; Xia, Y.; Xie, J. Three-dimensional objects consisting of hierarchically assembled nanofibers with controlled alignments for regenerative medicine. Nano Lett. 2019, 19, 2059–2065. [Google Scholar] [CrossRef]
- Fei, J.; Tang, T.; Zhou, L.; He, H.; Ma, M.; Shi, Y.; Chen, S.; Wang, X. High-Toughness and Biodegradable Superabsorbent Hydrogels Based on Dual Functional Crosslinkers. ACS Appl. Polym. Mater. 2023, 5, 3686–3697. [Google Scholar] [CrossRef]
- Duan, H.; Chen, H.; Qi, C.; Lv, F.; Wang, J.; Liu, Y.; Liu, Z.; Liu, Y. A novel electrospun nanofiber system with PEGylated paclitaxel nanocrystals enhancing the transmucus permeability and in situ retention for an efficient cervicovaginal cancer therapy. Int. J. Pharm. 2024, 650, 123660. [Google Scholar] [CrossRef]
- Kang, S.; Hou, S.; Chen, X.; Yu, D.-G.; Wang, L.; Li, X.; Williams, G.R. Energy-Saving Electrospinning with a Concentric Teflon-Core Rod Spinneret to Create Medicated Nanofibers. Polymers 2020, 12, 2421. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, J.; Fang, B.; Ying, Y.; Yu, D.; He, H. Three EHDA Processes from a Detachable Spinneret for Fabricating Drug Fast Dissolution Composites. Macromol. Mater. Eng. 2023, 2300361. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Liu, P. Electrospun Core–Sheath Nanofibers with a Cellulose Acetate Coating for the Synergistic Release of Zinc Ion and Drugs. Mol. Pharm. 2023, 21, 173–182. [Google Scholar] [CrossRef]
- Qian, C.; Liu, Y.; Chen, S.; Zhang, C.; Chen, X.; Liu, Y.; Liu, P. Electrospun core–sheath PCL nanofibers loaded with nHA and simvastatin and their potential bone regeneration applications. Front. Bioeng. Biotechnol. 2023, 11, 1205252. [Google Scholar] [CrossRef]
- Cao, X.; Chen, W.; Zhao, P.; Yang, Y.; Yu, D.-G. Electrospun Porous Nanofibers: Pore−Forming Mechanisms and Applications for Photocatalytic Degradation of Organic Pollutants in Wastewater. Polymers 2022, 14, 3990. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Liu, Y.; Lv, H.; Shi, H.; Zhou, W.; Liu, Y.; Yu, D.-G. Processes of Electrospun Polyvinylidene Fluoride-Based Nanofibers, Their Piezoelectric Properties, and Several Fantastic Applications. Polymers 2022, 14, 4311. [Google Scholar] [CrossRef]
- Lv, Q.; Ma, X.; Zhang, C.; Han, J.; He, S.; Liu, K.; Jiang, S. Nanocellulose-based nanogenerators for sensor applications: A review. Int. J. Biol. Macromol. 2024, 259, 129268. [Google Scholar] [CrossRef]
- Yu, D.G.; Zhou, J. Electrospun Multi-Chamber Nanostructures for Sustainable Biobased Chemical Nanofibers. Next Mater. 2024, 2, 100119. [Google Scholar] [CrossRef]
- Li, J.; Du, Q.; Wan, J.; Yu, D.-G.; Tan, F.; Yang, X. Improved synergistic anticancer action of quercetin and tamoxifen citrate supported by an electrospun complex nanostructure. Mater. Des. 2024, 238, 112657. [Google Scholar] [CrossRef]




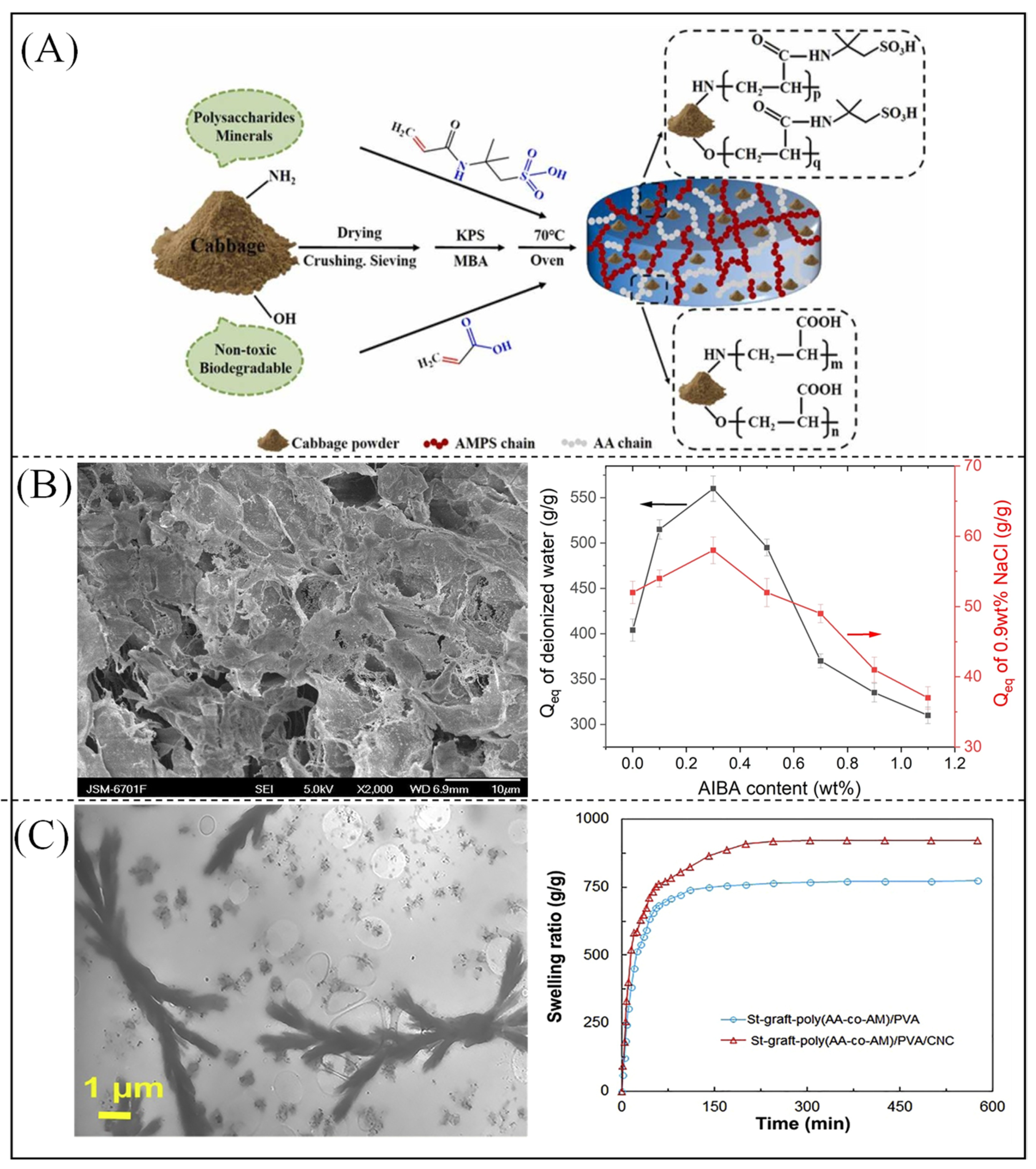
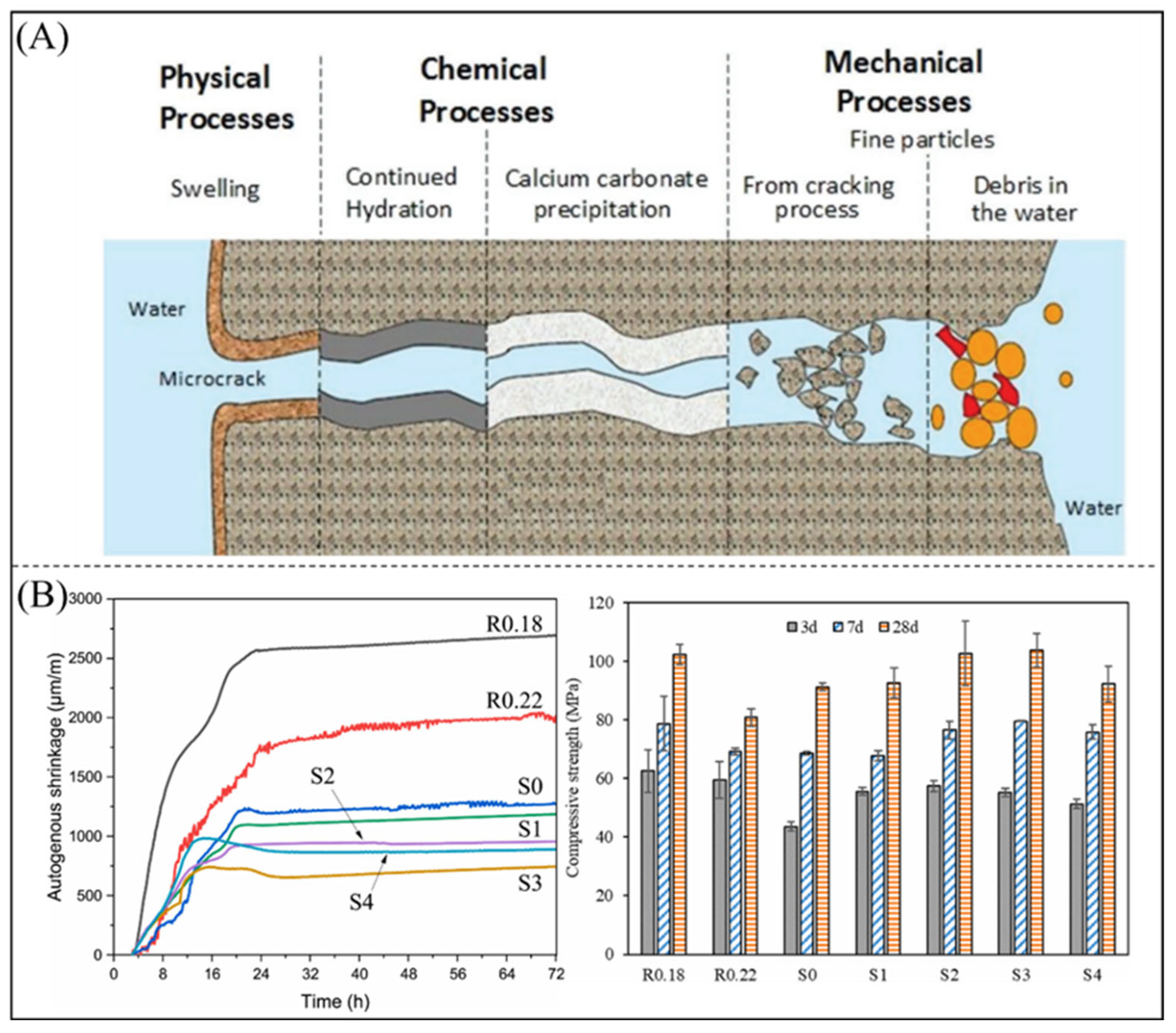


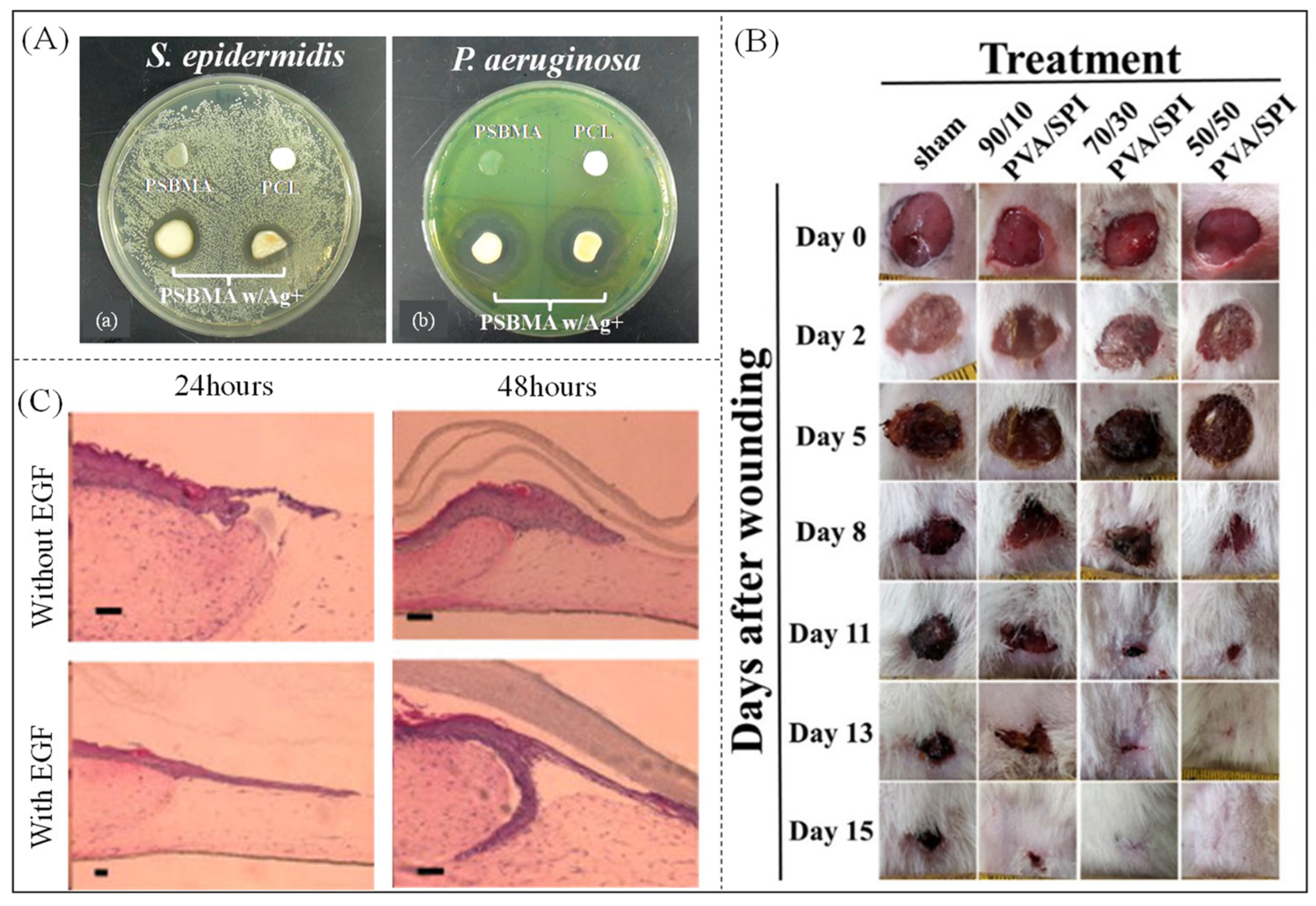
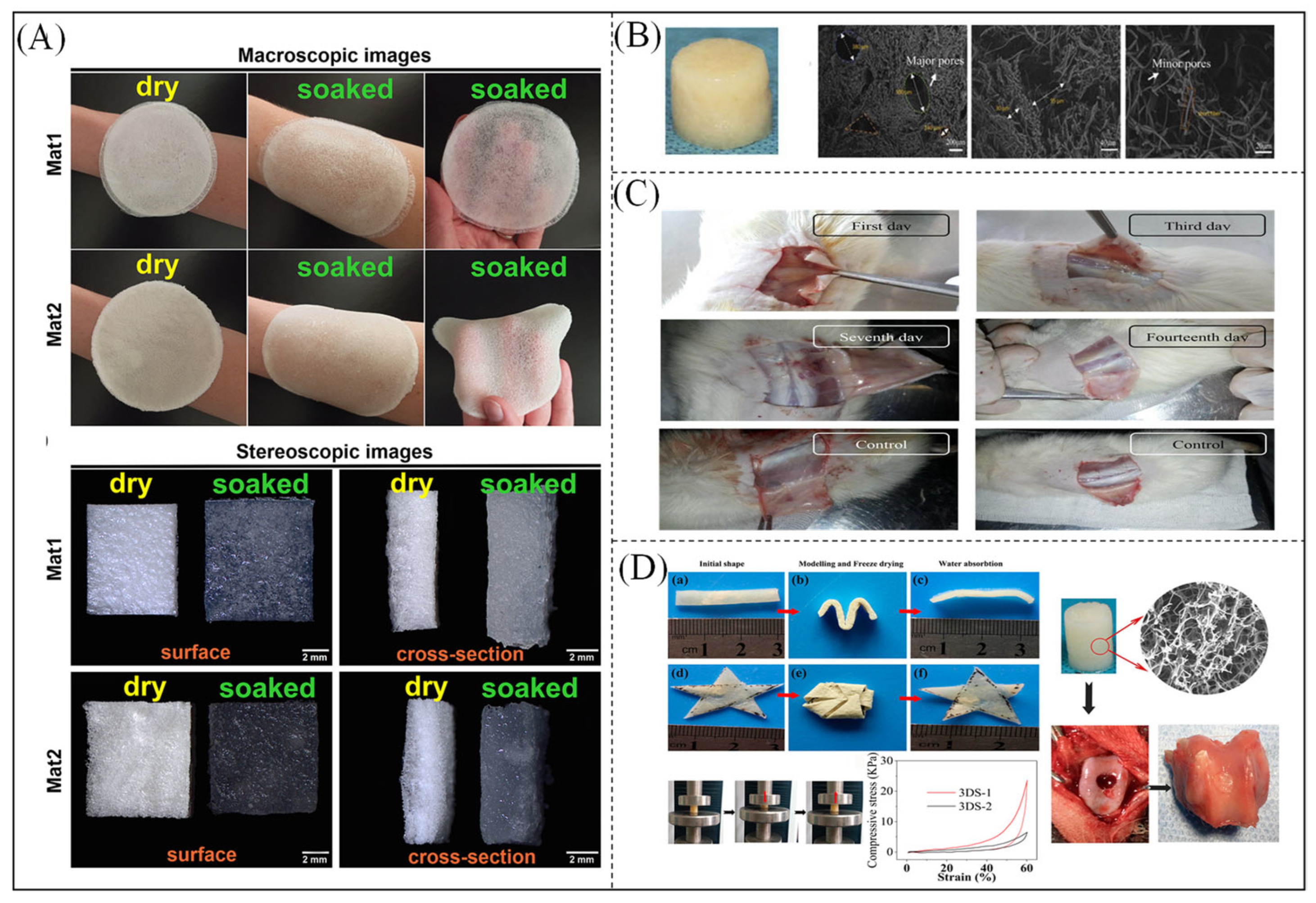
| Material | Molecular Weight (Monomer) | Physical Property | Water Absorbency (g/g) | Water Holding Rate | Application | Hydrophilic Group | Reference |
|---|---|---|---|---|---|---|---|
| Polyacrylic acid | 72 | Colorless/light yellow liquid | 59 | >90% | Horticulture, agriculture, drug delivery | –COOH | [39,40] |
| Polyacrylonitrile | 53 | White opaque powder | 95 | 85% | Drug delivery, construction work, oil and water separation | –CH3NO,–COOR | [41,42,43] |
| Polyvinyl alcohol | 44 | Flocculent, granular, powdery white solid | 74 | 93% | Cement, adsorption of alkali metals, cardiac tissue engineering | –COOH | [44,45,46,47] |
| Cellulose | 162 | Macromolecular polysaccharide | 119 | 61% | Soil water retention conditioning | –COOH | [48,49,50] |
| Chitosan | 161 | White or off-white, semi-transparent, flaky or powdery solid | 670 | 56% | Soil water retention, isolated lysozyme, wound dressings | –SO3H | [51,52,53] |
| Starch | 162 | White powdery solid | 343 | 80% | Soil water retention, flexible batteries, controlled release fertilizers | –OH, –COOH | [54,55,56,57] |
| Protein | 75–240 | A macromolecule consisting of n peptide chains | 860 | 30% | Hygiene products, hemostatic agents | –CO–NH | [58,59,60] |
| Amino acid | 75–240 | White crystals | 590 | 55% | Viral diagnostic monitoring, wound dressing, drug delivery | –CO–NH | [61,62,63] |
| Alginate | 176 | White or light yellow powder | 1700 | 70% | Dye adsorption, water-absorbent fabrics, concrete anti-cracking | –COONa,–OH | [64,65,66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Liang, Z.; Zhang, R.; Zhou, S.; Yang, H.; Chen, Y.; Zhang, J.; Yin, H.; Yu, D. Research Advances in Superabsorbent Polymers. Polymers 2024, 16, 501. https://doi.org/10.3390/polym16040501
Yang Y, Liang Z, Zhang R, Zhou S, Yang H, Chen Y, Zhang J, Yin H, Yu D. Research Advances in Superabsorbent Polymers. Polymers. 2024; 16(4):501. https://doi.org/10.3390/polym16040501
Chicago/Turabian StyleYang, Yaoyao, Zhiyuan Liang, Rui Zhang, Shengwei Zhou, Haobo Yang, Yanyu Chen, Jiahui Zhang, Hongyi Yin, and Dengguang Yu. 2024. "Research Advances in Superabsorbent Polymers" Polymers 16, no. 4: 501. https://doi.org/10.3390/polym16040501





