Maltodextrin-Based Cross-Linked Electrospun Mats as Sustainable Sorbents for the Removal of Atenolol from Water
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
2.3. Processing and Curing
2.4. Solubility Test
2.5. Thermogravimetric Analysis (TGA)
2.6. Attenuated Total Reflectance Fourier Transform Infrared (FTIR-ATR) Spectroscopy
2.7. Scanning Electron Microscopy (SEM)
2.8. Atenolol Sorption Tests and HPLC-UV/Vis Detection
3. Results and Discussion
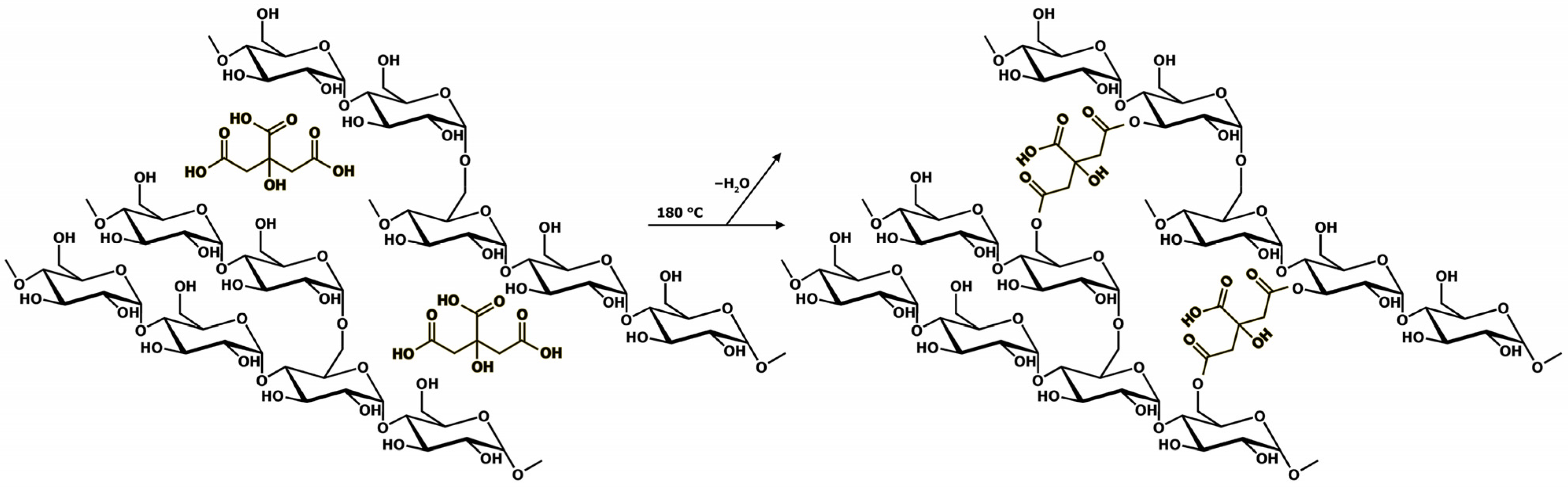
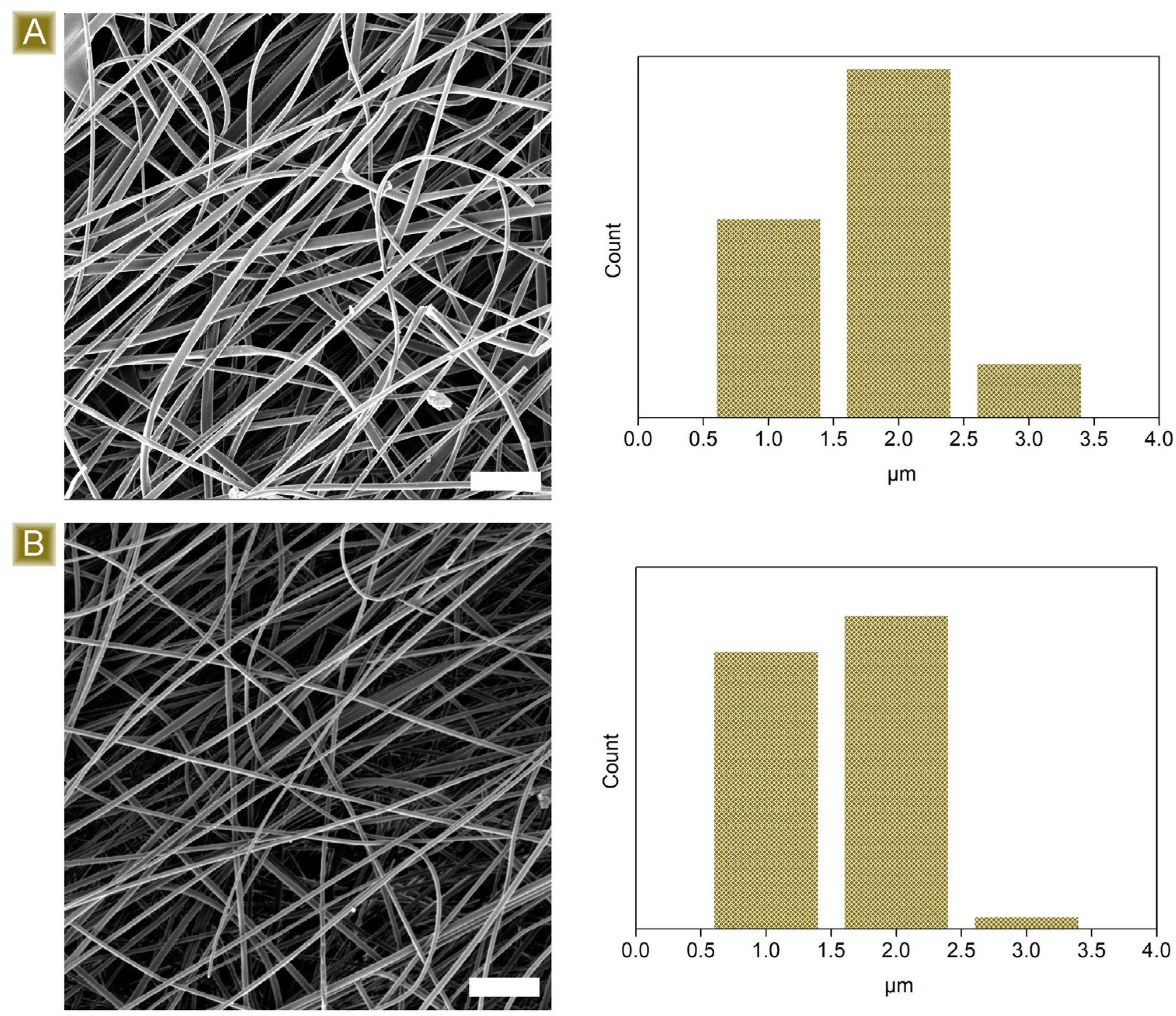
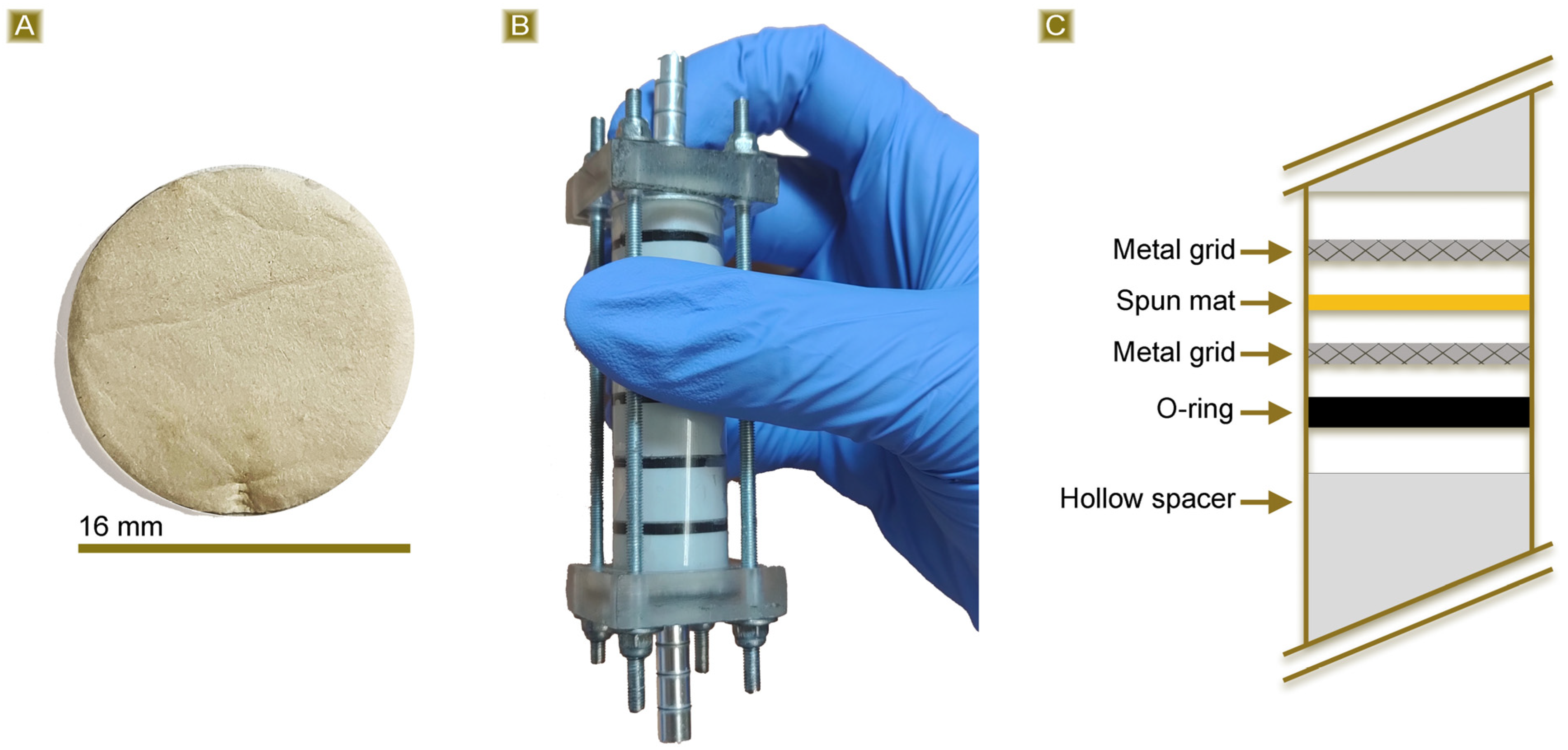
3.1. Deposition and Characterisation of the Mats
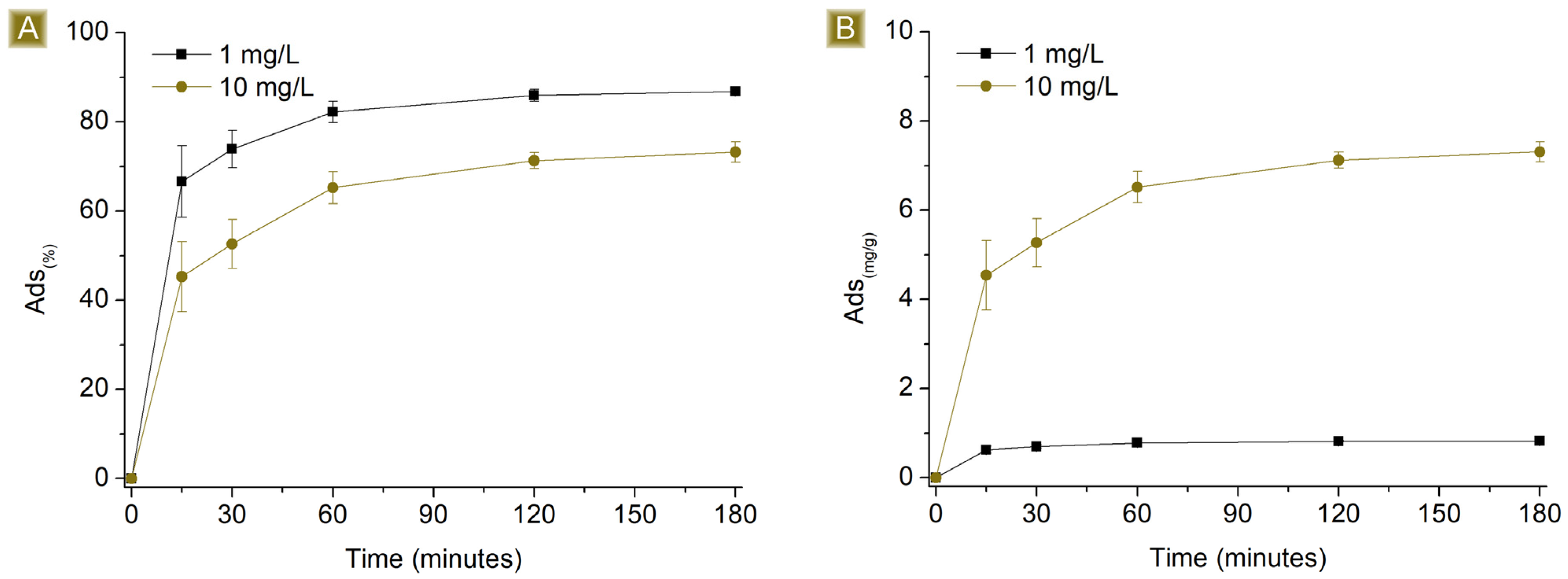
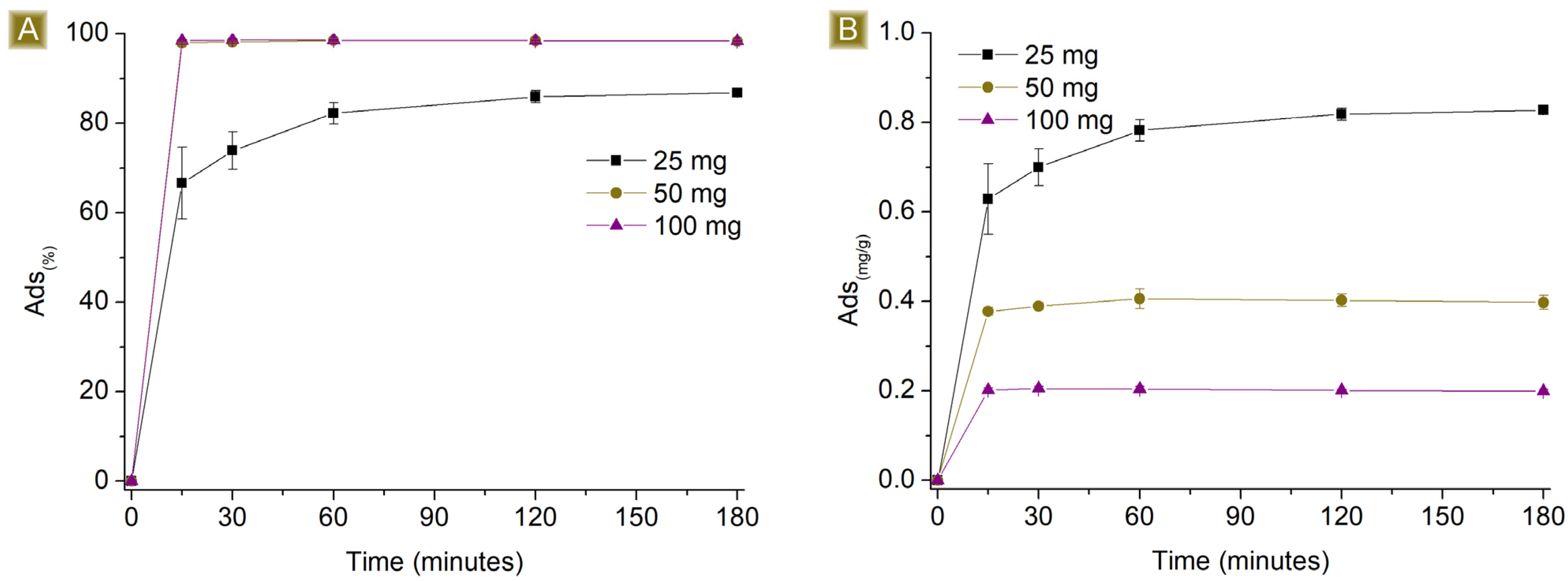
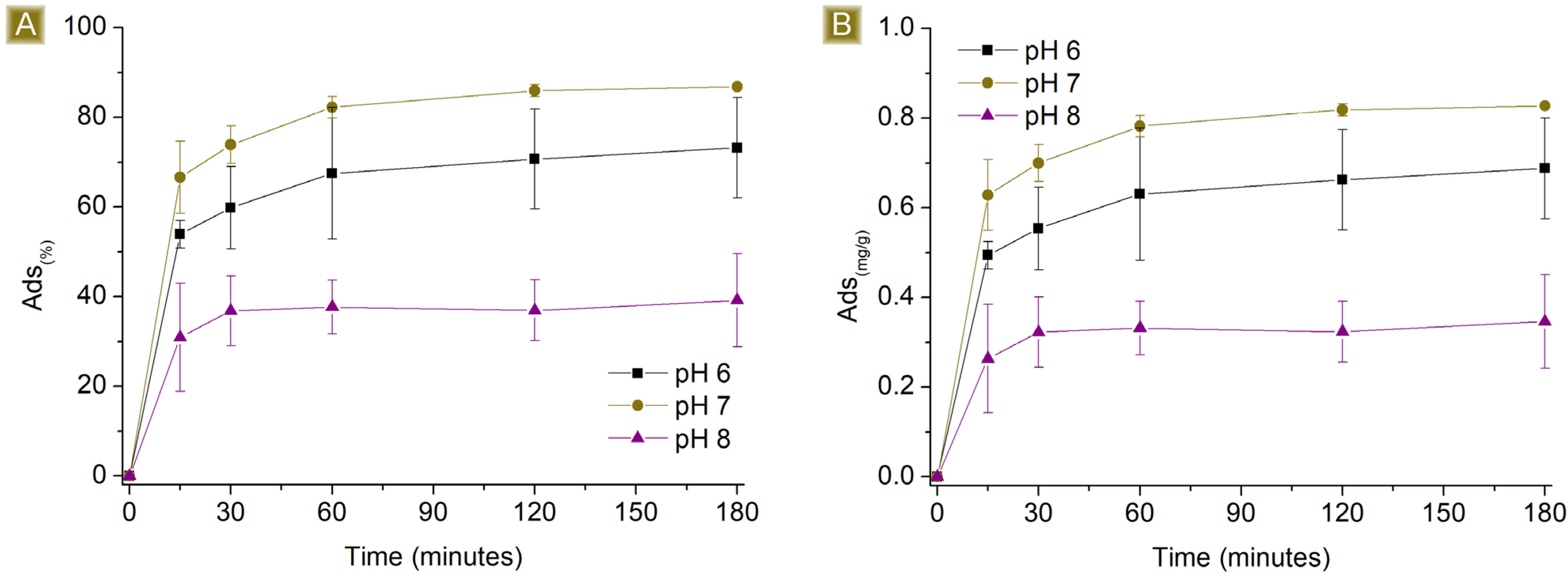
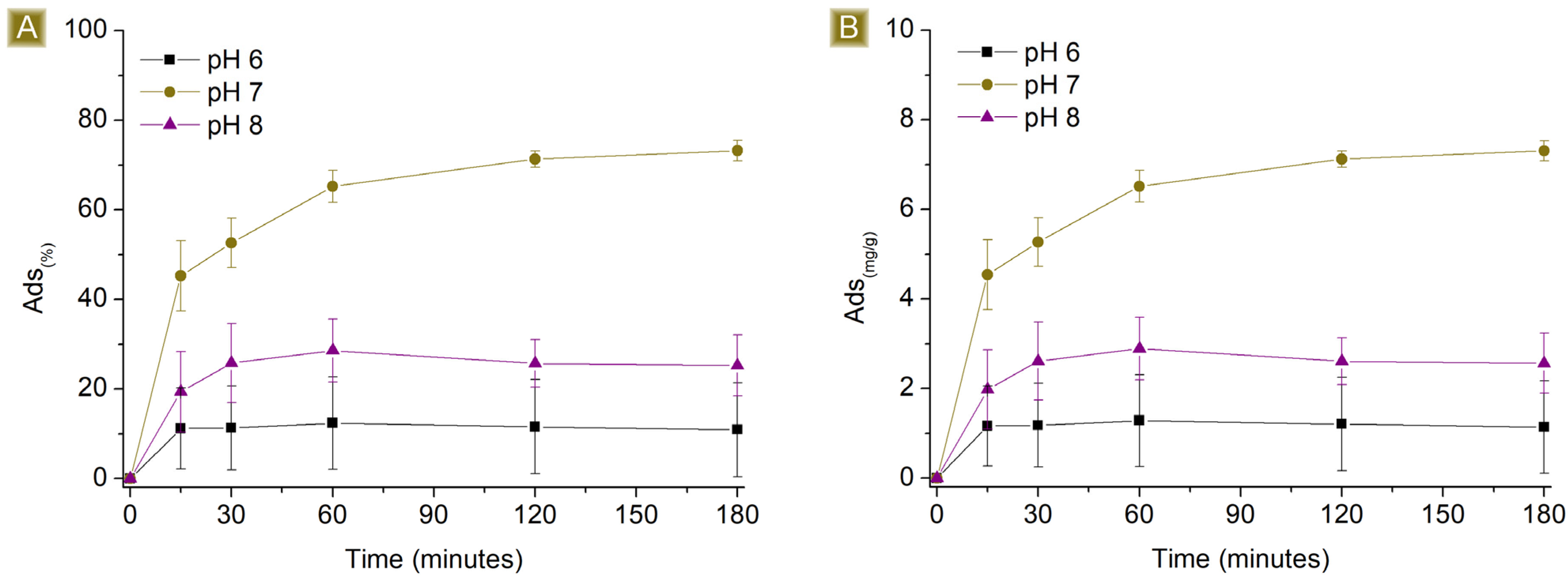
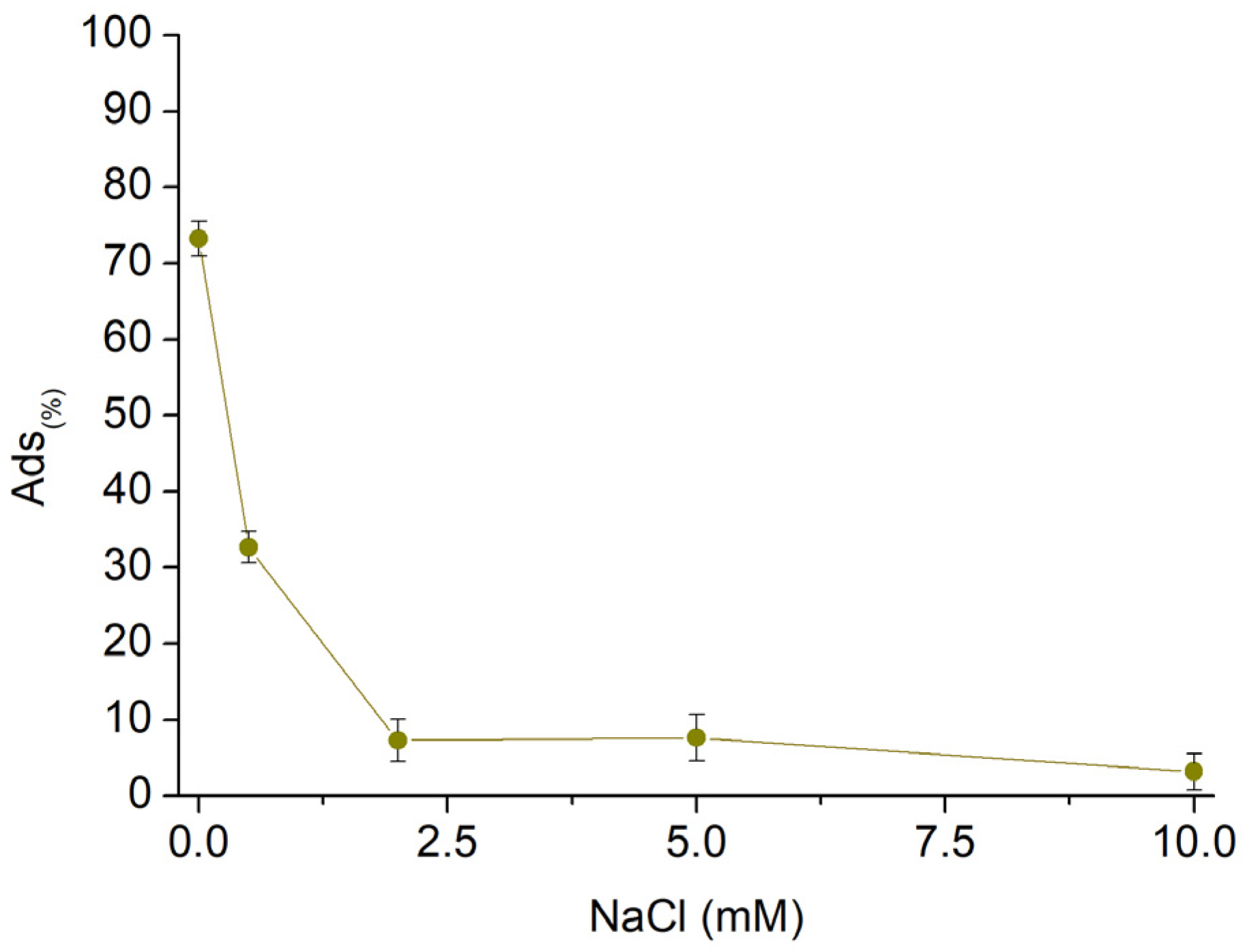
3.2. AT Batch Sorption Test
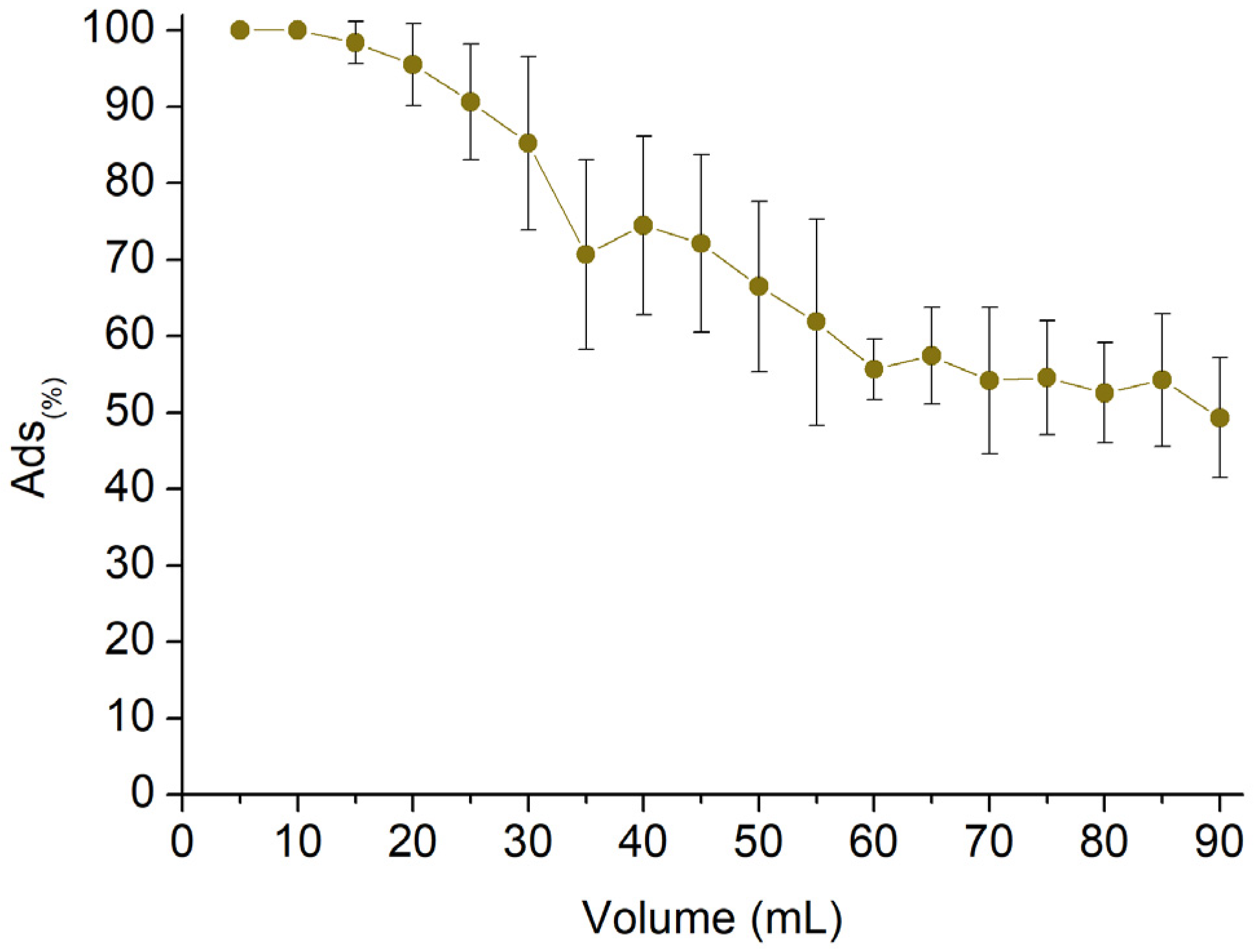
3.3. AT Fixed Bed Sorption Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bishop, G.; Styles, D.; Lens, P.N.L. Environmental performance comparison of bioplastics and petrochemical plastics: A review of life cycle assessment ( LCA ) methodological decisions. Resour. Conserv. Recycl. 2021, 168, 105451. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Soroudi, A.; Jakubowicz, I. Recycling of bioplastics, their blends and biocomposites: A review. Eur. Polym. J. 2013, 49, 2839–2858. [Google Scholar] [CrossRef]
- Yang, J.; Ching, Y.C.; Chuah, C.H. Applications of lignocellulosic fibers and lignin in bioplastics: A review. Polymers 2019, 11, 751. [Google Scholar] [CrossRef] [PubMed]
- Ochi, S. Development of high strength biodegradable composites using Manila hemp fiber and starch-based biodegradable resin. Compos. Part. A Appl. Sci. Manuf. 2006, 37, 1879–1883. [Google Scholar] [CrossRef]
- Xie, F.; Pollet, E.; Halley, P.J.; Avérous, L. Starch-based nano-biocomposites. Prog. Polym. Sci. 2013, 38, 1590–1628. [Google Scholar] [CrossRef]
- Brodin, M.; Vallejos, M.; Opedal, M.T.; Area, M.C.; Chinga-Carrasco, G.J. Lignocellulosics as sustainable resources for production of bioplastics – A review. Clean. Prod. 2017, 162, 646–664. [Google Scholar] [CrossRef]
- Prado, H.J.; Matulewicz, M.C. Cationization of polysaccharides: A path to greener derivatives with many industrial applications. Eur. Polym. J. 2014, 52, 53–75. [Google Scholar] [CrossRef]
- Blackburn, R.S. Natural polysaccharides and their interactions with dye molecules: Applications in effluent treatment. Environ. Sci. Technol. 2004, 38, 4905–4909. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Z.Y.; Su, X.P.; Chen, X.; Jiang, L.; Yao, J.M. Adsorption removal of dyes from single and binary solutions using a cellulose-based bioadsorbent. ACS Sustain. Chem. Eng. 2015, 3, 432–442. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.M.; Leong, K.W. Natural polymers for gene delivery and tissue engineering. Adv. Drug Deliv. Rev. 2006, 58, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Bratskaya, S.; Schwarz, S.; Laube, J.; Liebert, T.; Heinze, T.; Krentz, O.; Lohmann, C.; Kulicke, W.M. Effect of poly electrolyte structural features on flocculation behavior: Cationic polysaccharides vs. Synthetic polycations. Macromol. Mater. Eng. 2005, 290, 778–785. [Google Scholar] [CrossRef]
- Desbrières, J.; Guibal, E. Chitosan for wastewater treatment. Polym. Int. 2018, 67, 7–14. [Google Scholar] [CrossRef]
- Kweon, M.R.; Sosulski, F.W.; Bhirud, P.R. Cationization of waxy and normal corn and barley starches by an aqueous alcohol process. Starch/Staerke 1997, 49, 59–66. [Google Scholar] [CrossRef]
- Mignon, A.; De Belie, N.; Dubruel, P.; Van Vlierberghe, S. Superabsorbent polymers: A review on the characteristics and applications of synthetic, polysaccharide-based, semi-synthetic and ‘smart’ derivatives. Eur. Polym. J. 2019, 117, 165–178. [Google Scholar] [CrossRef]
- Dokic-Baucal, L.; Dokic, P.; Jakovljevic, J. Influence of different maltodextrins on properties of O/W emulsions. Food Hydrocoll. 2004, 18, 233–239. [Google Scholar] [CrossRef]
- Qi, X.; Tester, R.F. Is Starch or Maltodextrin “Glucose?”. Starch/Staerke 2018, 70, 10–14. [Google Scholar] [CrossRef]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef]
- O’Connor, N.A.; Abugharbieh, A.; Yasmeen, F.; Buabeng, E.; Mathew, S.; Samaroo, D.; Cheng, H.-P. The crosslinking of polysaccharides with polyamines and dextran-polyallylamine antibacterial hydrogels. Int. J. Biol. Macromol. 2015, 72, 88–93. [Google Scholar] [CrossRef]
- Castro-Cabado, M.; Parra-Ruiz, F.J.; Casado, A.L.; Román, J.S. Thermal crosslinking of maltodextrin and citric acid. Methodology to control polycondensation reaction under processing conditions. Polym. Polym. Compos. 2016, 24, 643–654. [Google Scholar] [CrossRef]
- Gyawali, D.; Nair, P.; Zhang, Y.; Tran, R.T.; Zhang, C.; Samchukov, M.; Makarov, M.; Kim, H.K.W.; Yang, J. Citric acid-derived in situ crosslinkable biodegradable polymers for cell delivery. Biomaterials 2010, 31, 9092–9105. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Hovgaard, L.; Brøndsted, H.J. Dextran hydrogels for colon-specific drug delivery. Control. Release 1995, 36, 159–166. [Google Scholar] [CrossRef]
- Kuijpers, A.J.; Van Wachem, P.B.; Van Luyn, M.J.A.; Engbers, G.H.M.; Krijgsveld, J.; Zaat, S.A.J.; Dankert, J.; Feijen, J. In vivo and in vitro release of lysozyme from cross-linked gelatin hydrogels: A model system for the delivery of antibacterial proteins from prosthetic heart valves. J. Control. Release 2000, 67, 323–336. [Google Scholar] [CrossRef]
- Cecone, C.; Costamagna, G.; Ginepro, M.; Trotta, F. One-step sustainable synthesis of cationic high- swelling polymers obtained from starch-derived maltodextrins. RSC Adv. 2021, 11, 7653–7662. [Google Scholar] [CrossRef]
- Stijnman, A.C.; Bodnar, I.; Tromp, R.H. Electrospinning of food-grade polysaccharides. Food Hydrocoll. 2011, 25, 1393–1398. [Google Scholar] [CrossRef]
- Bhushani, J.A.; Anandharamakrishnan, C. Electrospinning and electrospraying techniques: Potential food based applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Cecone, C.; Zanetti, M.; Anceschi, A.; Caldera, F.; Trotta, F.; Bracco, P. Microfibers of microporous carbon obtained from the pyrolysis of electrospun β-cyclodextrin/pyromellitic dianhydride nanosponges. Polym. Degrad. Stab. 2019, 161, 277–282. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; Von Gunten, U.; Wehrli, B. The Challenge of Micropollutants in Aquatic Systems. Science (1979) 2006, 313, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Watkinson, A.J.; Murby, E.J.; Kolpin, D.W.; Costanzo, S.D. The occurrence of antibiotics in an urban watershed: From wastewater to drinking water. Sci. Total Environ. 2009, 407, 2711–2723. [Google Scholar] [CrossRef] [PubMed]
- Golet, E.M.; Xifra, I.; Siegrist, H.; Alder, A.C.; Giger, W. Environmental exposure assessment of fluoroquinolone antibacterial agents from sewage to soil. Environ. Sci. Technol. 2003, 37, 3243–3249. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, E.; Barquero, A.; Muñoz-sanchez, B.; Paulis, M.; Leiza, J.R. Green electrospinning of polymer latexes: A systematic study of the effect of latex properties on fiber morphology. Nanomaterials 2021, 11, 706. [Google Scholar] [CrossRef]
- Stackelberg, P.E.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Henderson, A.K.; Reissman, D.B. Persistence of pharmaceutical compounds and other organic wastewater contaminants in a conventional drinking-water-treatment plant. Sci. Total Environ. 2004, 329, 99–113. [Google Scholar] [CrossRef]
- Loos, R.; Carvalho, R.; António, D.C.; Comero, S.; Locoro, G.; Tavazzi, S.; Paracchini, B.; Ghiani, M.; Lettieri, T.; Blaha, L.; et al. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 2013, 47, 6475–6487. [Google Scholar] [CrossRef]
- Anastas, P.T.; Kirchhoff, M.M. Origins, Current Status, and Future Challenges of Green Chemistry. Acc. Chem. Res. 2002, 35, 686–694. [Google Scholar] [CrossRef]
- Anastas, P.T.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Vargas-Campos, L.; Figueroa-Cárdenas, J.d.D.; Tochihuitl-Vázquez, D.; Ramírez-Bon, R.; Yáñez-Limón, J.M.; Pérez-Robles, J.F. Study of the dextrose equivalent of maltodextrins in electrospinning using an ethanol/water mixture as the electrospinning solvent. Food Hydrocoll. 2023, 139, 108498. [Google Scholar] [CrossRef]
- Kutzli, I.; Gibis, M.; Baier, S.K.; Weiss, J. Electrospinning of whey and soy protein mixed with maltodextrin – Influence of protein type and ratio on the production and morphology of fibers. Food Hydrocoll. 2019, 93, 206–214. [Google Scholar] [CrossRef]
- Kutzli, I.; Griener, D.; Gibis, M.; Schmid, C.; Dawid, C.; Baier, S.K.; Hofmann, T.; Weiss, J. Influence of Maillard reaction conditions on the formation and solubility of pea protein isolate-maltodextrin conjugates in electrospun fibers. Food Hydrocoll. 2020, 101, 105535. [Google Scholar] [CrossRef]
- Gibis, M.; Pribek, F.; Weiss, J. Effects of Electrospun Potato Protein–Maltodextrin Mixtures and Thermal Glycation on Trypsin Inhibitor Activity. Foods 2022, 11, 918. [Google Scholar] [CrossRef] [PubMed]
- Gibis, M.; Pribek, F.; Kutzli, I.; Weiss, J. Influence of the protein content on fiber morphology and heat treatment of electrospun potato protein–maltodextrin fibers. Appl. Sci. 2021, 11, 7896. [Google Scholar] [CrossRef]
- Ruggeri, M.; Bianchi, E.; Rossi, S.; Boselli, C.; Cornaglia, A.I.; Malavasi, L.; Carzino, R.; Suarato, G.; Sánchez-Espejo, R.; Athanassiou, A.; et al. Maltodextrin-amino acids electrospun scaffolds cross-linked with Maillard-type reaction for skin tissue engineering. Biomater. Adv. 2022, 133, 112593. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Hsieh, T.H.; Chiu, W.Y. Evaluation of thermally crosslinkable chitosan-based nanofibrous mats for the removal of metal ions. Carbohydr. Polym. 2015, 116, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Nthunya, L.N.; Masheane, M.L.; Malinga, S.P.; Nxumalo, E.N.; Mhlanga, S.D. Electrospun chitosan-based nanofibres for removal of phenols from drinking water. Water SA 2018, 44, 377–386. [Google Scholar] [CrossRef]
- Mantripragada, S.; Dong, M.; Zhang, L. Sustainable filter/adsorbent materials from cellulose-based electrospun nanofibrous membranes with soy protein coating for high-efficiency GenX fluorocarbon remediation from water. Cellulose 2023, 30, 7063–7078. [Google Scholar] [CrossRef]
- Lapworth, D.J.; Baran, N.; Stuart, M.E.; Ward, R.S. Emerging organic contaminants in groundwater: A review of sources, fate and occurrence. Environ. Pollut. 2012, 163, 287–303. [Google Scholar] [CrossRef]
- Lee, B.C.Y.; Lim, F.Y.; Loh, W.H.; Ong, S.L.; Hu, J. Emerging contaminants: An overview of recent trends for their treatment and management using light-driven processes. Water 2021, 13, 2340. [Google Scholar] [CrossRef]
- Stuart, M.; Lapworth, D.; Crane, E.; Hart, A. Review of risk from potential emerging contaminants in UK groundwater. Sci. Total Environ. 2012, 416, 1–21. [Google Scholar] [CrossRef]
- Verlicchi, P.; Galletti, A.; Petrovic, M.; Barceló, D. Hospital effluents as a source of emerging pollutants: An overview of micropollutants and sustainable treatment options. J. Hydrol. 2010, 389, 416–428. [Google Scholar] [CrossRef]
- Fernandes, J.P.; Almeida, C.M.R.; Salgado, M.A.; Carvalho, M.F.; Mucha, A.P. Pharmaceutical compounds in aquatic environments— occurrence, fate and bioremediation prospective. Toxics 2021, 9, 257. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- Manzetti, S.; Ghisi, R. The environmental release and fate of antibiotics. Mar. Pollut. Bull. 2014, 79, 7–15. [Google Scholar] [CrossRef]
- Ivantsova, E.; Martyniuk, C.J. A synthesis on the sub-lethal toxicity of atenolol, a beta-blocker, in teleost fish. Environ. Toxicol. Pharmacol. 2023, 102, 104236. [Google Scholar] [CrossRef]
- Tabacova, S.A.; Kimmel, C.A. Atenolol: Pharmacokinetic/dynamic aspects of comparative developmental toxicity. Reprod. Toxicol. 2002, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ulvi, A.; Aydın, S.; Aydın, M.E. Fate of selected pharmaceuticals in hospital and municipal wastewater effluent: Occurrence, removal, and environmental risk assessment. Environ. Sci. Pollut. Res. 2022, 29, 75609–75625. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Sheng, Q.; Sui, Q.; Lu, H. β-blockers in the environment: Distribution, transformation, and ecotoxicity. Environ. Pollut. 2020, 266, 115269. [Google Scholar] [CrossRef] [PubMed]
- Cecone, C.; Hoti, G.; Zanetti, M.; Trotta, F.; Bracco, P. Sustainable production of curable maltodextrin-based electrospun microfibers. RSC Adv. 2021, 12, 762–771. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cecone, C.; Fiume, V.; Bracco, P.; Zanetti, M. Maltodextrin-Based Cross-Linked Electrospun Mats as Sustainable Sorbents for the Removal of Atenolol from Water. Polymers 2024, 16, 752. https://doi.org/10.3390/polym16060752
Cecone C, Fiume V, Bracco P, Zanetti M. Maltodextrin-Based Cross-Linked Electrospun Mats as Sustainable Sorbents for the Removal of Atenolol from Water. Polymers. 2024; 16(6):752. https://doi.org/10.3390/polym16060752
Chicago/Turabian StyleCecone, Claudio, Valentina Fiume, Pierangiola Bracco, and Marco Zanetti. 2024. "Maltodextrin-Based Cross-Linked Electrospun Mats as Sustainable Sorbents for the Removal of Atenolol from Water" Polymers 16, no. 6: 752. https://doi.org/10.3390/polym16060752




