Ethylene Scavenging Films Based on Ecofriendly Plastic Materials and Nano-TiO2: Preparation, Characterization, and In Vivo Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
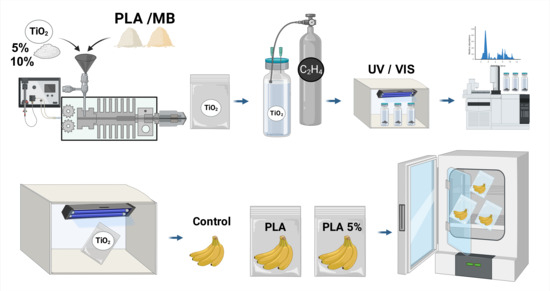
2.2. Fabrication of Active Films
2.3. Characterization of Active Films
2.3.1. Morphological Properties
2.3.2. Thermal Properties
2.3.3. Dynamics Properties
2.3.4. Mechanical Properties
2.4. Ethylene Removal Study
2.5. In Vivo Evaluation in Banana
2.6. Statistical Analysis
3. Results
3.1. Characterization of Active Films
3.1.1. Morphological Properties
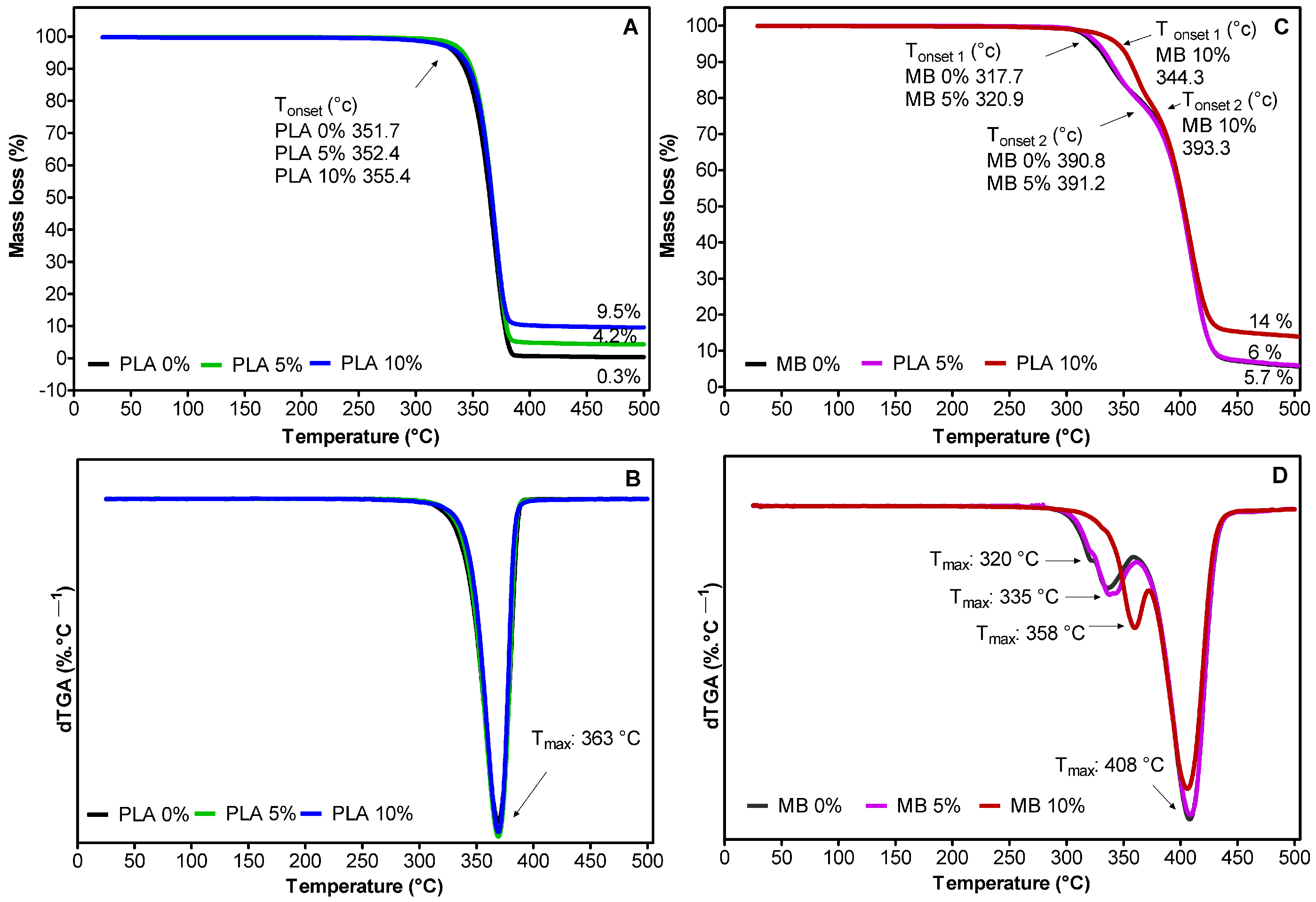
3.1.2. Thermal Properties
3.1.3. Dynamics Properties
3.1.4. Mechanical Properties
3.2. Ethylene Removal Study
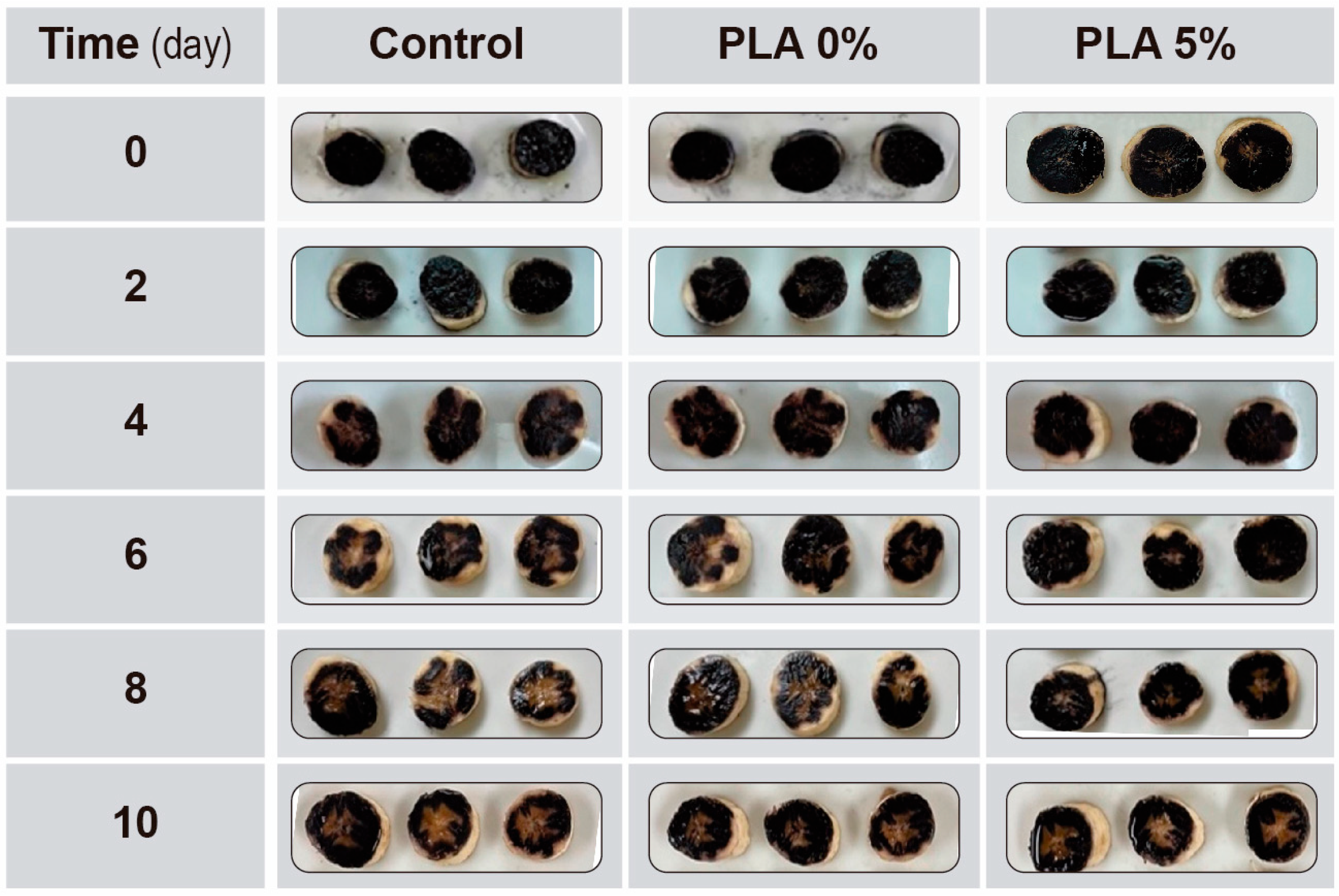
3.3. In Vivo Study in Banana
3.3.1. Ripeness Index and Color of the Peel
3.3.2. Fruit Pulp Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sadeghi, K.; Lee, Y.; Seo, J. Ethylene Scavenging Systems in Packaging of Fresh Produce: A Review. Food Rev. Int. 2019, 37, 155–176. [Google Scholar] [CrossRef]
- Álvarez-Hernández, M.H.; Artés-Hernández, F.; Ávalos-Belmontes, F.; Castillo-Campohermoso, M.A.; Contreras-Esquivel, J.C.; Ventura-Sobrevilla, J.M.; Martínez-Hernández, G.B. Current Scenario of Adsorbent Materials Used in Ethylene Scavenging Systems to Extend Fruit and Vegetable Postharvest Life. Food Bioprocess Technol. 2018, 11, 511–525. [Google Scholar] [CrossRef]
- Payasi, A.; Sanwal, G. Ripening of climacteric fruits and their control. J. Food Biochem. 2010, 34, 679–710. [Google Scholar] [CrossRef]
- Bodbodak, S.; Rafiee, Z. Recent Trends in Active Packaging in Fruits and Vegetables. In Eco-Friendly Technology for Postharvest Produce Quality; Elsevier: Amsterdam, The Netherlands, 2016; pp. 77–125. [Google Scholar]
- Álvarez-Hernández, M.H.; Martínez-Hernández, G.B.; Avalos-Belmontes, F.; Miranda-Molina, F.D.; Artés-Hernández, F. Postharvest quality retention of apricots by using a novel sepiolite–loaded potassium permanganate ethylene scavenger. Postharvest Biol. Technol. 2020, 160, 111061. [Google Scholar] [CrossRef]
- Bodaghi, H.; Mostofi, Y.; Oromiehie, A.; Zamani, Z.; Ghanbarzadeh, B.; Costa, C.; Conte, A.; Del Nobile, M.A. Evaluation of the photocatalytic antimicrobial effects of a TiO2 nanocomposite food packaging film by in vitro and in vivo tests. LWT 2013, 50, 702–706. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef]
- Wang, Q.; Qiao, Z.; Jiang, P.; Kuang, J.; Liu, W.; Cao, W. Hydrothermal synthesis and enhanced photocatalytic activity of mixed-phase TiO2 powders with controllable anatase/rutile ratio. Solid State Sci. 2018, 77, 14–19. [Google Scholar] [CrossRef]
- Pathak, N.; Caleb, O.J.; Geyer, M.; Herppich, W.B.; Rauh, C.; Mahajan, P.V. Photocatalytic and Photochemical Oxidation of Ethylene: Potential for Storage of Fresh Produce—A Review. Food Bioprocess Technol. 2017, 10, 982–1001. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Singh, S.; Negi, Y.S. Ethylene scavengers for active packaging of fresh food produce. Environ. Chem. Lett. 2020, 18, 269–284. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Kaewklin, P. Fabrication and characterization of chitosan-titanium dioxide nanocomposite film as ethylene scavenging and antimicrobial active food packaging. Food Hydrocoll. 2018, 84, 125–134. [Google Scholar] [CrossRef]
- Kaewklin, P.; Siripatrawan, U.; Suwanagul, A.; Lee, Y.S. Active packaging from chitosan-titanium dioxide nanocomposite film for prolonging storage life of tomato fruit. Int. J. Biol. Macromol. 2018, 112, 523–529. [Google Scholar] [CrossRef]
- Li, G.; Lv, L.; Fan, H.; Ma, J.; Li, Y.; Wan, Y.; Zhao, X. Effect of the agglomeration of TiO2 nanoparticles on their photocatalytic performance in the aqueous phase. J. Colloid Interface Sci. 2010, 348, 342–347. [Google Scholar] [CrossRef]
- Böhmer-Maas, B.W.; Fonseca, L.M.; Otero, D.M.; Zavareze, E.d.R.; Zambiazi, R.C. Photocatalytic zein-TiO2 nanofibers as ethylene absorbers for storage of cherry tomatoes. Food Packag. Shelf Life 2020, 24, 100508. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Shang, Y.; Wen, Y. Electrospun Nanofibers Containing TiO2 for the Photocatalytic Degradation of Ethylene and Delaying Postharvest Ripening of Bananas. Food Bioprocess Technol. 2019, 12, 281–287. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Biodegradable Poly(Butylene Adipate-Co-Terephthalate) and Thermoplastic Starch-Blended TiO2 Nanocomposite Blown Films as Functional Active Packaging of Fresh Fruit. Polymers 2021, 13, 4192. [Google Scholar] [CrossRef] [PubMed]
- ASTM D882-12; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2012.
- Maldonado, A.; Cheuquepan, P.; Gutiérrez, S.; Gallegos, N.; Donoso, M.; Hauser, C.; Arrieta, M.P.; Torres, A.; Bruna, J.; Valenzuela, X.; et al. Study of Ethylene-Removing Materials Based on Eco-Friendly Composites with Nano-TiO2. Polymers 2023, 15, 3369. [Google Scholar] [CrossRef]
- Gomes, J.F.S.; Vieira, R.R.; Leta, F.R. Colorimetric indicator for classification of bananas during ripening. Sci. Hortic. 2013, 150, 201–205. [Google Scholar] [CrossRef]
- Salah, L.S.; Ouslimani, N.; Danlée, Y.; Beltrán, F.R.; Huynen, I.; de la Orden, M.U. Investigation of mechanical recycling effect on electromagnetic properties of polylactic acid (PLA)–nanoclay nanocomposites: Towards a valorization of recycled PLA nanocomposites. Compos. Part C Open Access 2023, 10, 100339. [Google Scholar] [CrossRef]
- Deghiche, A.; Haddaoui, N.; Zerriouh, A.; Fenni, S.E.; Cavallo, D.; Erto, A.; Benguerba, Y. Effect of the stearic acid-modified TiO2 on PLA nanocomposites: Morphological and thermal properties at the microscopic scale. J. Environ. Chem. Eng. 2021, 9, 106541. [Google Scholar] [CrossRef]
- Ortiz-Bustos, J.; Fajardo, M.; del Hierro, I.; Pérez, Y. Versatile titanium dioxide nanoparticles prepared by surface-grown polymerization of polyethylenimine for photodegradation and catalytic C C bond forming reactions. Mol. Catal. 2019, 475, 110501. [Google Scholar] [CrossRef]
- Donoso, M.; Gallegos, N. Fabricación y Caracterización de Películas Eco Amigables Con Capacidad de Remoción de Etileno Basadas En Mater-Bi®/Nano-TiO2. Bachelor’s Thesis, Universidad de Santiago de Chile, Santiago, Chile, 2022. [Google Scholar]
- Borchani, K.E.; Carrot, C.; Jaziri, M. Biocomposites of Alfa fibers dispersed in the Mater-Bi® type bioplastic: Morphology, mechanical and thermal properties. Compos. Part A Appl. Sci. Manuf. 2015, 78, 371–379. [Google Scholar] [CrossRef]
- Esmaeili, H.; Patino-Guerrero, A.; Hasany, M.; Aansari, M.O.; Memic, A.; Dolatshahi-Pirouz, A.; Nikkhah, M. Electroconductive biomaterials for cardiac tissue engineering. Acta Biomater. 2021, 139, 118–140. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.; Nisar, M.; Palza, H.; Yazdani-Pedram, M.; Aguilar-Bolados, H.; Quijada, R. Development of bio degradable nanocomposites based on PLA and functionalized graphene oxide. Polym. Test. 2023, 124, 108066. [Google Scholar] [CrossRef]
- Buzarovska, A. PLA Nanocomposites with Functionalized TiO2Nanoparticles. Polym. Plast. Technol. Eng. 2013, 52, 280–286. [Google Scholar] [CrossRef]
- Cardoso, E.C.L.; Oliveira, R.R.; Machado, G.A.F.; Moura, E.A.B. Study of Flexible Films Prepared from PLA/PBAT Blend and PLA E-Beam Irradiated as Compatibilizing Agent. In Characterization of Minerals, Metals, and Materials; Ikhmayies, S., Li, B., Carpenter, J.S., Li, J., Hwang, J.-Y., Monteiro, S.N., Firrao, D., Zhang, M., Peng, Z., Escobedo-Diaz, J.P., et al., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 121–129. [Google Scholar]
- Delgado, P.A.; Brutman, J.P.; Masica, K.; Molde, J.; Wood, B.; Hillmyer, M.A. High surface area carbon black (BP-2000) as a reinforcing agent for poly[(−)-lactide]. J. Appl. Polym. Sci. 2016, 133, 43926. [Google Scholar] [CrossRef]
- Zhang, Y.; Rempel, C.; Liu, Q. Thermoplastic Starch Processing and Characteristics—A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1353–1370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, J.; Yang, L.; Chen, R.; Zou, W.; Lin, X.; Qu, J. Preparation, characterization and properties of PLA/TiO2 nanocomposites based on a novel vane extruder. RSC Adv. 2014, 5, 4639–4647. [Google Scholar] [CrossRef]
- Neuba, L.d.M.; Junio, R.F.P.; Souza, A.T.; Carvalho, M.T.; Ribeiro, M.E.A.; Lazarus, B.S.; Pereira, A.C.; Monteiro, S.N. Dynamic mechanical and thermal mechanical analysis of Cyperus malaccensis sedge fiber reinforced GO-incorporated epoxy nanocomposites: A short communication. J. Mater. Res. Technol. 2023, 24, 1653–1662. [Google Scholar] [CrossRef]
- Marrakchi, Z.; Oueslati, H.; Belgacem, M.; Mhenni, F.; Mauret, E. Biocomposites based on polycaprolactone reinforced with alfa fibre mats. Compos. Part A Appl. Sci. Manuf. 2012, 43, 742–747. [Google Scholar] [CrossRef]
- Krouit, M.; Belgacem, M.N.; Bras, J. Chemical versus solvent extraction treatment: Comparison and influence on polyester based bio-composite mechanical properties. Compos. Part A Appl. Sci. Manuf. 2010, 41, 703–708. [Google Scholar] [CrossRef]
- Fonseca, C.; Ochoa, A.; Ulloa, M.T.; Alvarez, E.; Canales, D.; Zapata, P.A. Poly(lactic acid)/TiO2 nanocomposites as alternative biocidal and antifungal materials. Mater. Sci. Eng. C 2015, 57, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Prakash, M.; Ghosh, A.K. Rheology-cell structure correlation for foam processing of polypropylene-titanium dioxide and polypropylene-graphene nanocomposites. Polym. Eng. Sci. 2023, 63, 4174–4185. [Google Scholar] [CrossRef]
- Campaña, O.; Guerrero, V.H.; Hugo, V. Caracterización Mecánica y Térmica de Ácido Poliláctico (PLA) Reforzado Con Polvo de Bambú (PB) Mechanical and Thermal Characterization of Poly Lactic Acid (PLA) Reinforced with Bamboo Powder (PB). Rev. Politécnica 2018, 42, 17–24. [Google Scholar]
- Sani, M.A.; Maleki, M.; Eghbaljoo-Gharehgheshlaghi, H.; Khezerlou, A.; Mohammadian, E.; Liu, Q.; Jafari, S.M. Titanium dioxide nanoparticles as multifunctional surface-active materials for smart/active nanocomposite packaging films. Adv. Colloid Interface Sci. 2022, 300, 102593. [Google Scholar] [CrossRef]
- Keller, N.; Ducamp, M.-N.; Robert, D.; Keller, V. Ethylene Removal and Fresh Product Storage: A Challenge at the Frontiers of Chemistry. Toward an Approach by Photocatalytic Oxidation. Chem. Rev. 2013, 113, 5029–5070. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Barreto, F.F.; Rafael-Delgado, D.A.; Ramírez-Tixe, E.E. Efecto del tiempo y temperatura de almacenamiento en los parámetros físico-químicos y de color de frutos de aguaymanto (Physalis peruviana). Rev. Investig. Agropecu. Sci. Biotechnol. 2022, 2, 29–38. [Google Scholar] [CrossRef]





| Film | Tg (°C) | Tcc (°C) | ΔHcc (J·g−1) | Tm1 (°C) | ∆Hm1 (J·g−1) | X (%) |
|---|---|---|---|---|---|---|
| PLA 0% | 58.6 ± 0.1 A | 128.8 ± 0.9 A | 6.4 ± 0.3 B | 150.8 ± 0.5 A | 7.9 ± 0.1 B | 1.6 ± 0.2 A |
| PLA 5% | 58.7 ± 0.1 A | 118.3 ± 0.1 B | 18.1 ± 1.1 A | 148.2 ± 0.2 B | 20.2 ± 0.9 A | 2.3 ± 0.2 A |
| PLA 10% | 58.4 ± 0.3 A | 126.2 ± 1.7 A | 6.2 ± 0.9 B | 150.2 ± 0.6 A | 6.9 ± 0.9 B | 0.7 ± 0.1 B |
| Film | Tg (°C) | Tm1 (°C) | ∆Hm1 (J·g−1) | Tm2 (°C) | ∆Hm2 (J·g−1) |
|---|---|---|---|---|---|
| MB 0% | 63.2 ± 0.1 A | 122.7 ± 0.1 A | 4.6 ± 0.1 A | 166.2 ± 0.1 A | 0.8 ± 0.2 A |
| MB 5% | 63.1 ± 0.1 A | 123.1 ± 0.2 A | 4.7 ± 0.3 A | 166.1 ± 0.1 A | 1.0 ± 0.1 A |
| MB 10% | 63.0 ± 0.2 A | 127.1 ± 0.3 A | 4.6 ± 0.3 A | 166.1 ± 0.2 A | 1.3 ± 0.2 A |
| Film | TiO2 Content (%) | Thickness (µm) | Young’s Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|---|
| PLA | 0 | 65.6 ± 2.2 Ba | 2697.3 ± 256.9 Aa | 50.5 ± 4.4 Aa | 2.2 ± 0.1 Bb |
| 5 | 46.1 ± 4.9 Bb | 3073.4 ± 199.1 Aa | 52.6 ± 3.5 Aa | 2.1 ± 0.1 Ba | |
| 10 | 14.1 ± 0.8 Bc | 1247.5 ± 71.7 Ab | 16.6 ± 1.6 Ab | 1.8 ± 0.1 Bc | |
| MB | 0 | 81.1 ± 1.8 Aa | 375.3 ± 12.3 Ba | 19.0 ± 3.7 Ba | 546.9 ± 84.3 Ab |
| 5 | 86.2 ± 7.6 Ab | 114.5 ± 3.2 Ba | 16.1 ± 0.7 Ba | 744.1 ± 19.4 Aa | |
| 10 | 82.7 ± 4.9 Ac | 95.2 ± 4.7 Bb | 6.3 ± 0.7 Bb | 439.7 ± 43.7 Ac |
| Matrix | TiO2 Content (%) | Final C2H4 Removal (%) | |
|---|---|---|---|
| UV | VIS | ||
| PLA | 0 | 48.2 ± 5.3 | 45.1 ± 0.8 |
| 5 | 68.3 ± 5.0 | 55.4 ± 3.9 | |
| 10 | 46.7 ± 6.7 | 43.7 ± 6.7 | |
| MB | 0 | 43.6 ± 4.9 | 42.8 ± 4.0 |
| 5 | 46.7 ± 0.8 | 48.0 ± 5.3 | |
| 10 | 60.9 ± 2.0 | 37.5 ± 6.4 | |
| Parm. | Film | Time (Day) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | ||
| Control | 67.4 ± 1.9 Aa | 70.2 ± 1.7 Aa | 65.2 ± 0.3 Ab | 66.0 ± 1.0 Aab | 64.3 ± 1.9 Ab | 64.2 ± 1.0 Ab | |
| PLA 0% | 67.4 ± 1.9 Aa | 66.2 ± 1.2 Aa | 65.3 ± 0.5 Ab | 67.0 ± 1.6 Aab | 66.7 ± 0.8 Ab | 66.2 ± 1.4 Ab | |
| PLA 5% | 67.4 ± 1.9 Aa | 69.0 ± 1.3 Aa | 67.1 ± 0.6 Ab | 66.8 ± 0.7 Aab | 66.7 ± 0.8 Ab | 65.5 ± 0.7 Ab | |
| a* | Control | −10.7 ± 2.8 Aa | −5.8 ± 0.9 Ab | −2.7 ± 1.1 Ac | 1.1 ± 0.5 Ad | 1.2 ± 0.8 Ad | 3.2 ± 0.7 Ad |
| PLA 0% | −10.7 ± 2.8 Ba | −10.4 ± 1.4 Bb | −6.8 ± 0.4 Bc | −1.2 ± 0.5 Bd | 0.8 ± 1.6 Bd | 0.0 ± 0.6 Bd | |
| PLA 5% | −10.7 ± 2.8 Ba | −10.0 ± 1.9 Bb | −6.3 ± 1.7 Bc | −1.0 ± 1.6 Bd | −0.7 ± 1.0 Cd | −0.2 ± 0.4 Bd | |
| b* | Control | 43.8 ± 3.0 Aa | 42.2 ± 3.3 Ab | 35.7 ± 1.7 Ac | 33.1 ± 0.6 Ad | 34.6 ± 0.8 Ac | 39.6 ± 1.2 Ad |
| PLA 0% | 43.8 ± 3.0 Ba | 40.2 ± 0.4 Bb | 36.2 ± 0.8 Bc | 34.0 ± 1.1 Bd | 35.8 ± 2.2 Bc | 34.6 ± 2.1 Bd | |
| PLA 5% | 43.8 ± 3.0 Ba | 41.0 ± 1.1 Bb | 37.9 ± 1.1 Bc | 33.6 ± 1.0 Bd | 36.8 ± 0.9 Bc | 31.6 ± 1.2 Bd | |
| ∆E | PLA 0% | - | 27.3 ± 1.1 Ad | 19.3 ± 0.1 Ac | 10.3 ± 1.7 Ab | 8.0 ± 1.1 Aa | 6.2 ± 1.7 Ac |
| PLA 5% | - | 17.9 ± 1.5 Bd | 6.9 ± 0.1 Bc | 9.8 ± 1.0 Bb | 3.9 ± 0.6 Ba | 2.0 ± 0.3 Bc | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldonado, A.; Aguilar, T.; Hauser, C.; Wehnert, G.; Söthje, D.; Schlachter, H.; Torres, A.; Bruna, J.; Valenzuela, X.; Rodríguez-Mercado, F. Ethylene Scavenging Films Based on Ecofriendly Plastic Materials and Nano-TiO2: Preparation, Characterization, and In Vivo Evaluation. Polymers 2024, 16, 853. https://doi.org/10.3390/polym16060853
Maldonado A, Aguilar T, Hauser C, Wehnert G, Söthje D, Schlachter H, Torres A, Bruna J, Valenzuela X, Rodríguez-Mercado F. Ethylene Scavenging Films Based on Ecofriendly Plastic Materials and Nano-TiO2: Preparation, Characterization, and In Vivo Evaluation. Polymers. 2024; 16(6):853. https://doi.org/10.3390/polym16060853
Chicago/Turabian StyleMaldonado, Alba, Tomas Aguilar, Carolin Hauser, Gerd Wehnert, Dominik Söthje, Herbert Schlachter, Alejandra Torres, Julio Bruna, Ximena Valenzuela, and Francisco Rodríguez-Mercado. 2024. "Ethylene Scavenging Films Based on Ecofriendly Plastic Materials and Nano-TiO2: Preparation, Characterization, and In Vivo Evaluation" Polymers 16, no. 6: 853. https://doi.org/10.3390/polym16060853