The Effect of Nonterminal Liquid Crystalline Epoxy Resin Structure and Curing Agents on the Glass Transition of Polymer Networks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- 4,4′-dihydroxybiphenyl, Glentham Life Sciences, Corsham, UK;
- Hydroquinone, Fluka, Buchs, Switzerland;
- DCC; N,N′-dicyclohexylcarbodiimide, Sigma Aldrich, Steinheim, Germany;
- p-hydroxybenzoic acid, Sigma Aldrich, Steinheim, Germany;
- p-TSA; p-toluenesulfonic acid, Sigma Aldrich, Steinheim, Germany;
- m-CPBA; m-chloroperbenzoic acid, Sigma Aldrich, Steinheim, Germany;
- oleic acid, Fischer Chemical, Loughborough, UK;
- ethanol, methanol, ethyl acetate, dichloromethane and acetone, Chempur, Piekary Śląskie, Poland;
- decalin, Fluka, Buchs, Switzerland;
- o-PDA, o-phenylenediamine, Thermo Scientific; Waltham, MA, USA;
- m-PDA; m-phenylenediamine, Sigma Aldrich, Steinheim, Germany;
- p-PDA; p-phenylenediamine, Merck, Darmstadt, Germany;
- 3,3′-DDM; 3,3′-diaminodiphenylmethane, Sigma Aldrich, Steinheim, Germany;
- 4,4′-DDM; 4,4′-diaminodiphenylmethane, Sigma Aldrich, Steinheim, Germany;
- 3,4′-ODA; 3,4′-oxydianiline, Angene Chemical, Telangana, India;
- 4,4′-ODA; 4,4′-oxydianiline, Angene Chemical, Telangana, India.
2.2. Methods
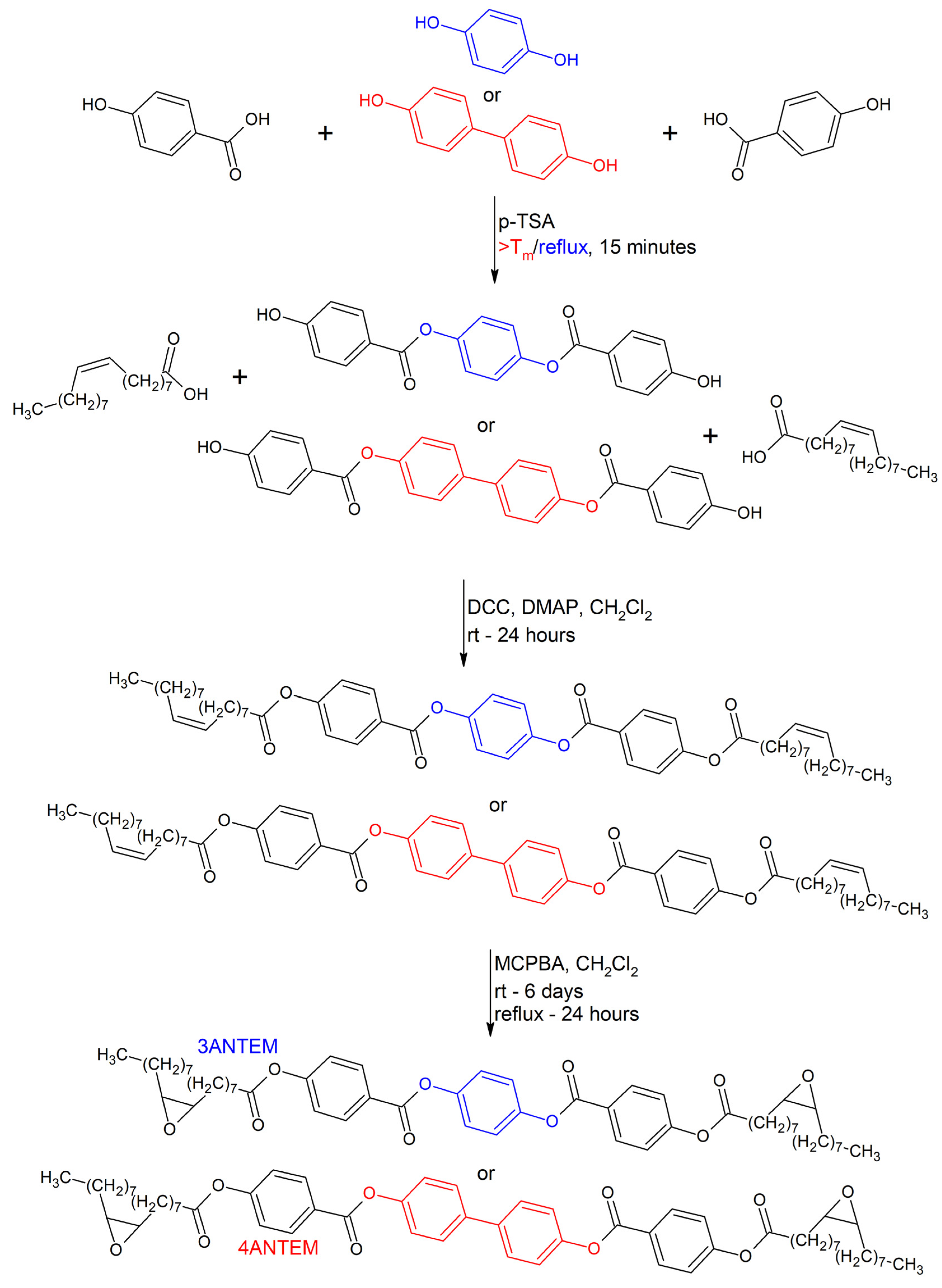
2.2.1. Synthesis of Nonterminal LCERs
2.2.2. Preparation of the Curing Mixture
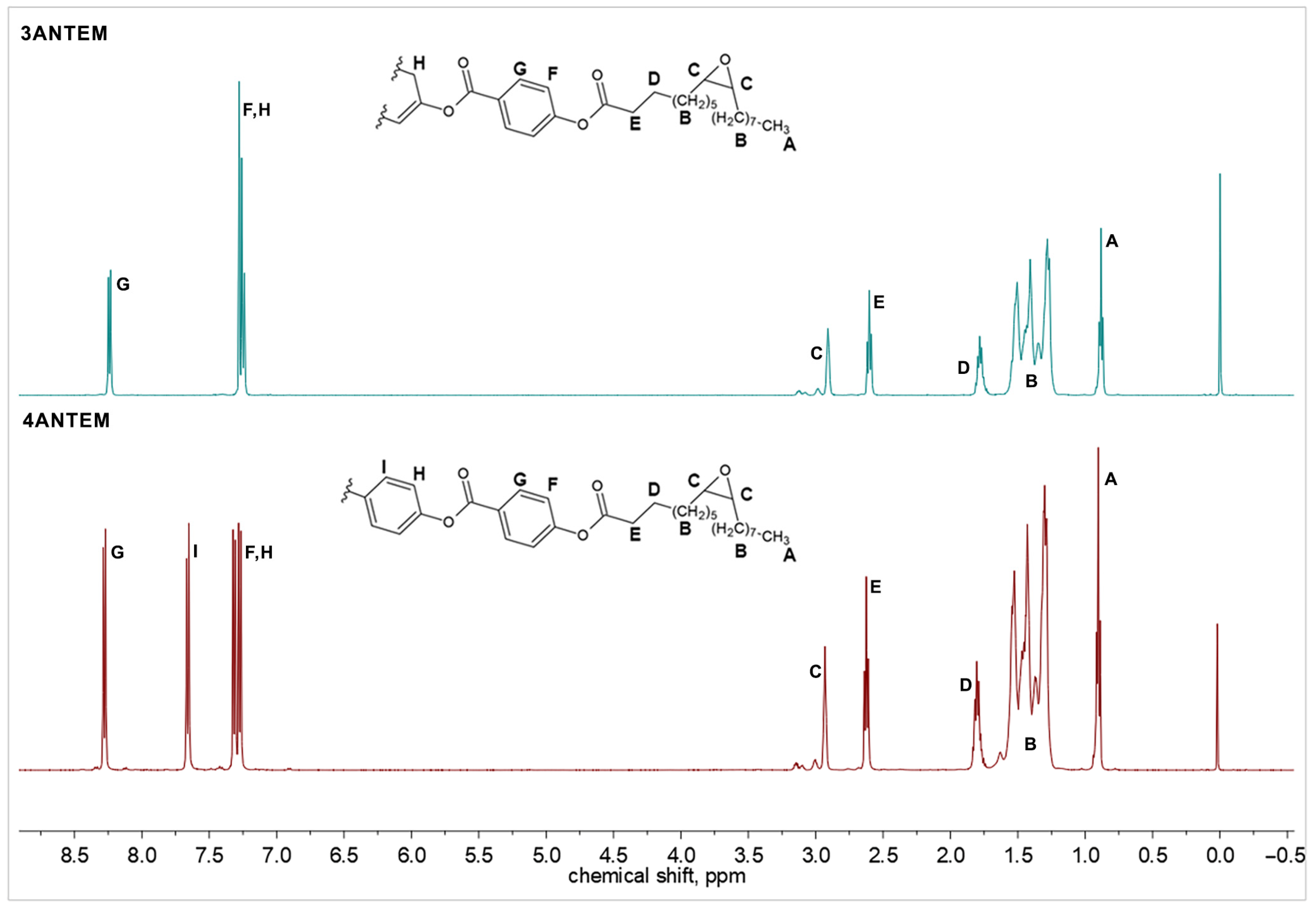
2.2.3. 1H-NMR Analysis
2.2.4. DSC Analysis
2.2.5. POM Analysis
2.2.6. Elemental Analysis
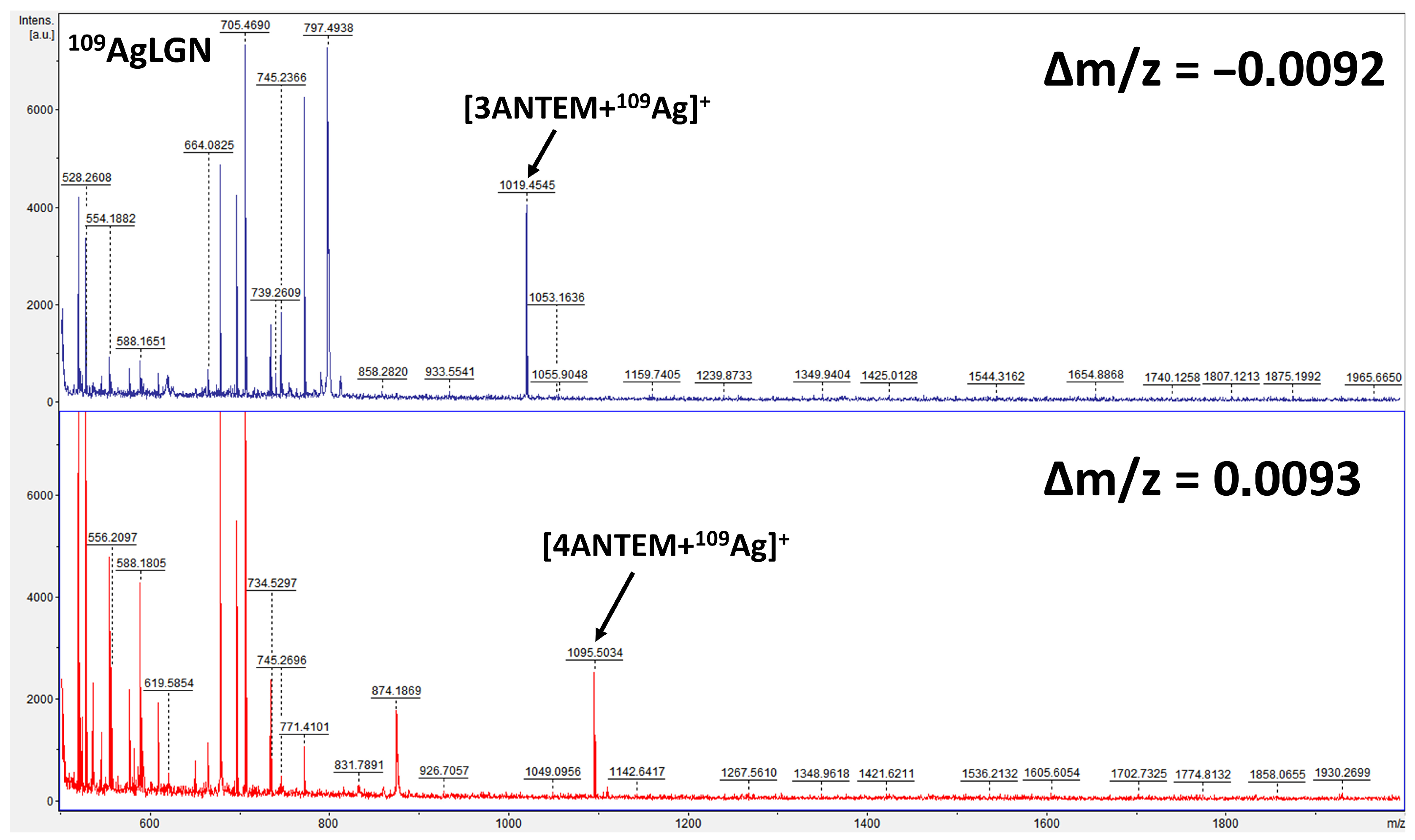
2.2.7. Mass Spectrometry Analysis
2.2.8. TGA Analysis
3. Results and Discussion
3.1. Structural Analysis of the Synthesis Products
3.2. Thermal Characteristics of Resins and Hardeners
3.3. Analysis of the Curing Process
3.4. The Effect of Hardener Type and Resin Structure on the Tg of the Polymer Network
4. Conclusions
- Low-Tg LC polymer networks can be synthesized using the new kind of LCERs—nonterminal compounds;
- The introduction of the fourth aromatic ring into the mesogen core generally increases stiffness and the Tg of the network;
- The curing of LCERs can be constrained by the high viscosity of a mixture at low temperatures, especially when boosted by oligomer formation in the first minutes of the reaction;
- The larger core of resin facilitates intercalation and increases the extent of the cure;
- The prediction of the Tg of the polymers is possible but complicated, and the interpretation of the DSC curve in this regard is necessary;
- Polar interactions between resin and a curing agent may result in an increase in Tg thanks to the interactions themselves and easier penetration between resin scaffolds.
Author Contributions
Funding

Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Paluvai, N.R.; Mohanty, S.; Nayak, S.K. Synthesis and Modifications of Epoxy Resins and Their Composites: A Review. Polym. Plast. Technol. Eng. 2014, 53, 1723–1758. [Google Scholar] [CrossRef]
- Jin, F.L.; Li, X.; Park, S.J. Synthesis and Application of Epoxy Resins: A Review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Mohan, P. A Critical Review: The Modification, Properties, and Applications of Epoxy Resins. Polym. Plast. Technol. Eng. 2013, 52, 107–125. [Google Scholar] [CrossRef]
- Vadivelu, M.A.; Kumar, C.R.; Joshi, G.M. Polymer Composites for Thermal Management: A Review. Compos. Interfaces 2016, 23, 847–872. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and Chemical Recycling of Solid Plastic Waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Li, Y.; Ambrogi, V.; Cerruti, P.; Goswami, M.; Yang, Z.; Kessler, M.R.; Rios, O. Functional Liquid Crystalline Epoxy Networks and Composites: From Materials Design to Applications. Int. Mater. Rev. 2022, 67, 201–229. [Google Scholar] [CrossRef]
- Thakur, T.; Jaswal, S.; Parihar, S.; Gaur, B.; Singha, A.S. Bio-Based Epoxy Thermosets with Rosin Derived Imidoamine Curing Agents and Their Structure-Property Relationships. Express Polym. Lett. 2020, 14, 512–529. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Zhu, L.; Du, Z.; Zhang, B.; Zhang, Y. Curing Behaviors and Kinetics of Epoxy Resins with a Series of Biphenyl Curing Agents Having Different Methylene Units. Thermochim. Acta 2011, 521, 18–25. [Google Scholar] [CrossRef]
- Hu, J.; Shan, J.; Zhao, J.; Tong, Z. Water Resistance and Curing Kinetics of Epoxy Resins with a Novel Curing Agent of Biphenyl-Containing Amine Synthesized by One-Pot Method. Thermochim. Acta 2015, 606, 58–65. [Google Scholar] [CrossRef]
- Carfagna, C.; Amendola, E.; Giamberini, M. Liquid Crystalline Epoxy Based Thermosetting Polymers. Prog. Polym. Sci. 1997, 22, 1607–1647. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Li, N.; Jie, S.; Luo, Y.; Gao, X. Branched Thermotropic Liquid Crystal Polymer with Favourable Processability and Dielectric Properties. Eur. Polym. J. 2023, 196, 112302. [Google Scholar] [CrossRef]
- Pei, Z.; Yang, Y.; Chen, Q.; Terentjev, E.M.; Wei, Y.; Ji, Y. Mouldable Liquid-Crystalline Elastomer Actuators with Exchangeable Covalent Bonds. Nat. Mater. 2014, 13, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Fitriyani, S.; Liu, C.Y.; Hung, Y.H.; Zhang, Y.S.; Liu, J.H. Bending Control of Liquid-Crystal Elastomers Based on Doped Azo Derivatives Synthesized via Controlled Gradient Polymerization. Express Polym. Lett. 2020, 14, 566–575. [Google Scholar] [CrossRef]
- Jeong, I.; Kim, C.B.; Kang, D.G.; Jeong, K.U.; Jang, S.G.; You, N.H.; Ahn, S.; Lee, D.S.; Goh, M. Liquid Crystalline Epoxy Resin with Improved Thermal Conductivity by Intermolecular Dipole–Dipole Interactions. J. Polym. Sci. A Polym. Chem. 2019, 57, 708–715. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Y.; Yang, Y.; Xu, Y.; Qian, X.; Wei, Y.; Ji, Y. Durable Liquid-Crystalline Vitrimer Actuators. Chem. Sci. 2019, 10, 3025–3030. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, M.; Shen, W.; Chen, G.; Zhang, L.; Yang, H. A Study on the Polymer Structures and Electro-Optical Properties of Epoxy-Mercaptan-Based Polymer Dispersed Liquid Crystal Films. Liq. Cryst. 2019, 46, 1718–1726. [Google Scholar] [CrossRef]
- Shen, W.; Wang, L.; Zhong, T.; Chen, G.; Li, C.; Chen, M.; Zhang, C.; Zhang, L.; Li, K.; Yang, Z.; et al. Electrically Switchable Light Transmittance of Epoxy-Mercaptan Polymer/Nematic Liquid Crystal Composites with Controllable Microstructures. Polymer 2019, 160, 53–64. [Google Scholar] [CrossRef]
- Lin, Z.; Cong, Y.; Zhang, B.; Huang, H. Synthesis and Characterisation of a Novel Y-Shaped Liquid Crystalline Epoxy and Its Effect on Isotropic Epoxy Resin. Liq. Cryst. 2019, 46, 1467–1477. [Google Scholar] [CrossRef]
- Mossety-Leszczak, B.; Pilch-Pitera, B.; Karaś, J.; Kisiel, M.; Zając, W.; Włodarska, M. The Application of Liquid Crystalline Epoxy Resin for Forming Hybrid Powder Coatings. Prog. Org. Coat. 2022, 168, 106873. [Google Scholar] [CrossRef]
- Włodarska, M.; Mossety-Leszczak, B.; Bąk, G.W.; Kisiel, M.; Dłużniewski, M.; Okrasa, L. Epoxy Matrix with Triaromatic Mesogenic Unit in Dielectric Spectroscopy Observation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 194, 102–110. [Google Scholar] [CrossRef]
- Kisiel, M.; Mossety-Leszczak, B.; Strachota, B.; Strachota, A. Achieving Structural Anisotropy of Liquid Crystalline Epoxy by Manipulation with Crosslinking Parameters. Express Polym. Lett. 2021, 15, 274–287. [Google Scholar] [CrossRef]
- Włodarska, M.; Mossety-Leszczak, B.; Kisiel, M.; Zając, W.; Okrasa, L. Changes in Molecular Relaxations and Network Properties of a Triaromatic Liquid Crystal Epoxy Resin with Nonterminal Functional Groups. J. Polym. Sci. 2023, 61, 3244–3255. [Google Scholar] [CrossRef]
- Kisiel, M.; Zając, W.; Włodarska, M.; Byczyński, Ł.; Czachor-Jadacka, D.; Mossety-Leszczak, B.; Pietruszewska, G.; Droździel-Jurkiewicz, M.; Bieniaś, J. Nonterminal Liquid Crystalline Epoxy Resins as Structurally Ordered Low Tg Thermosets with Potential as Smart Polymers. Express Polym. Lett. 2024, 18, 516–532. [Google Scholar] [CrossRef]
- Dierking, I. Textures of Liquid Crystals; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
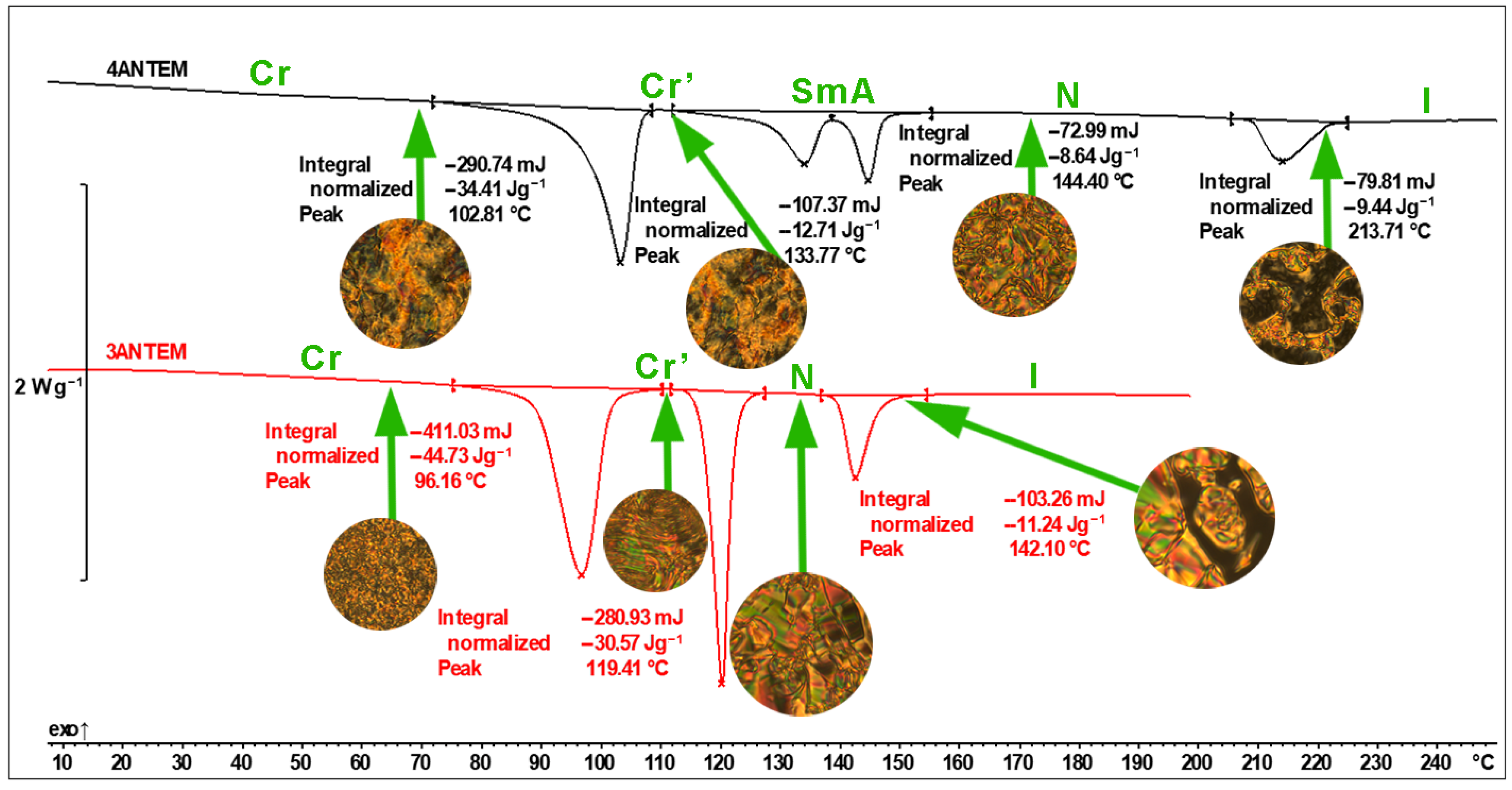
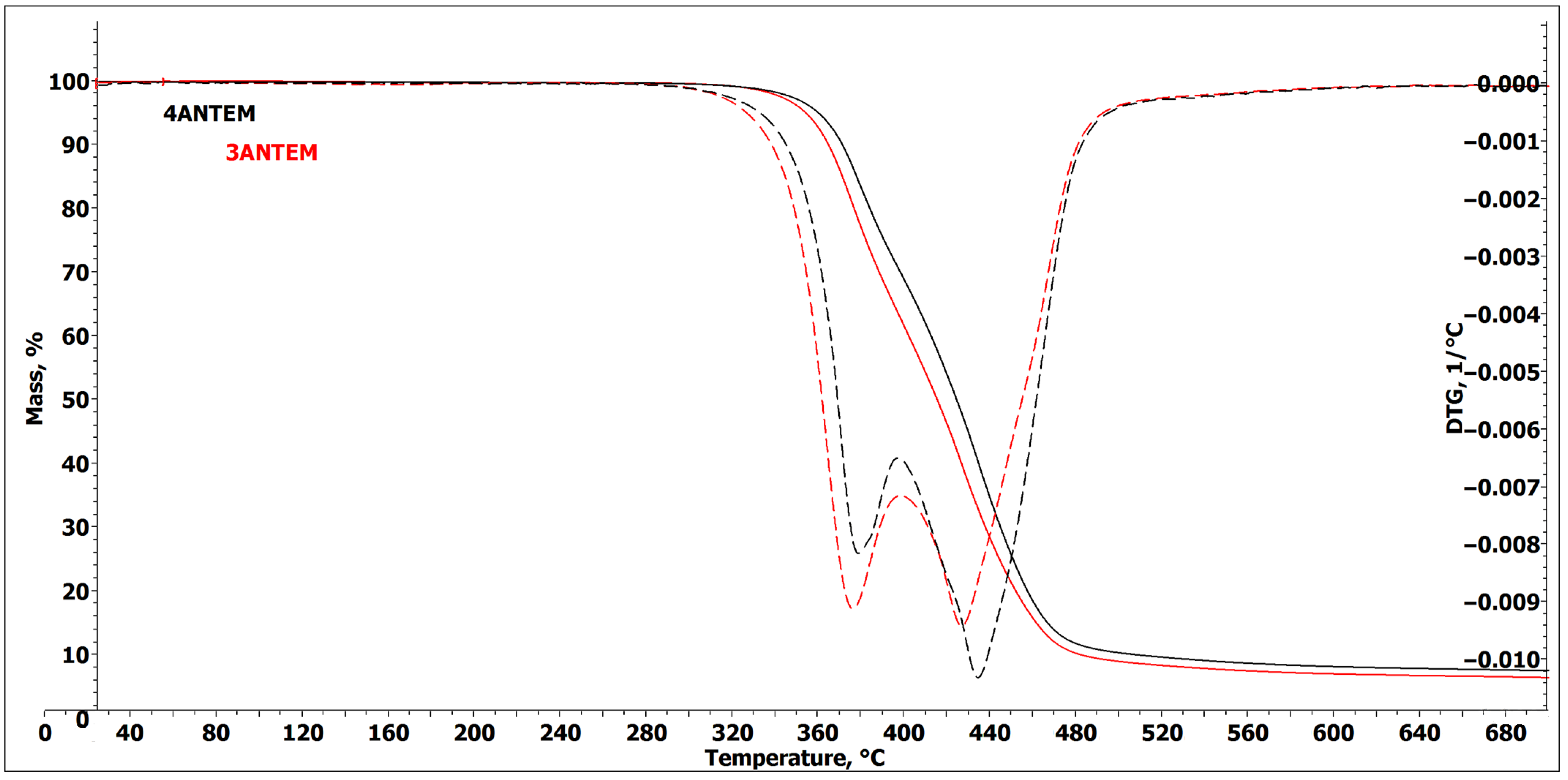

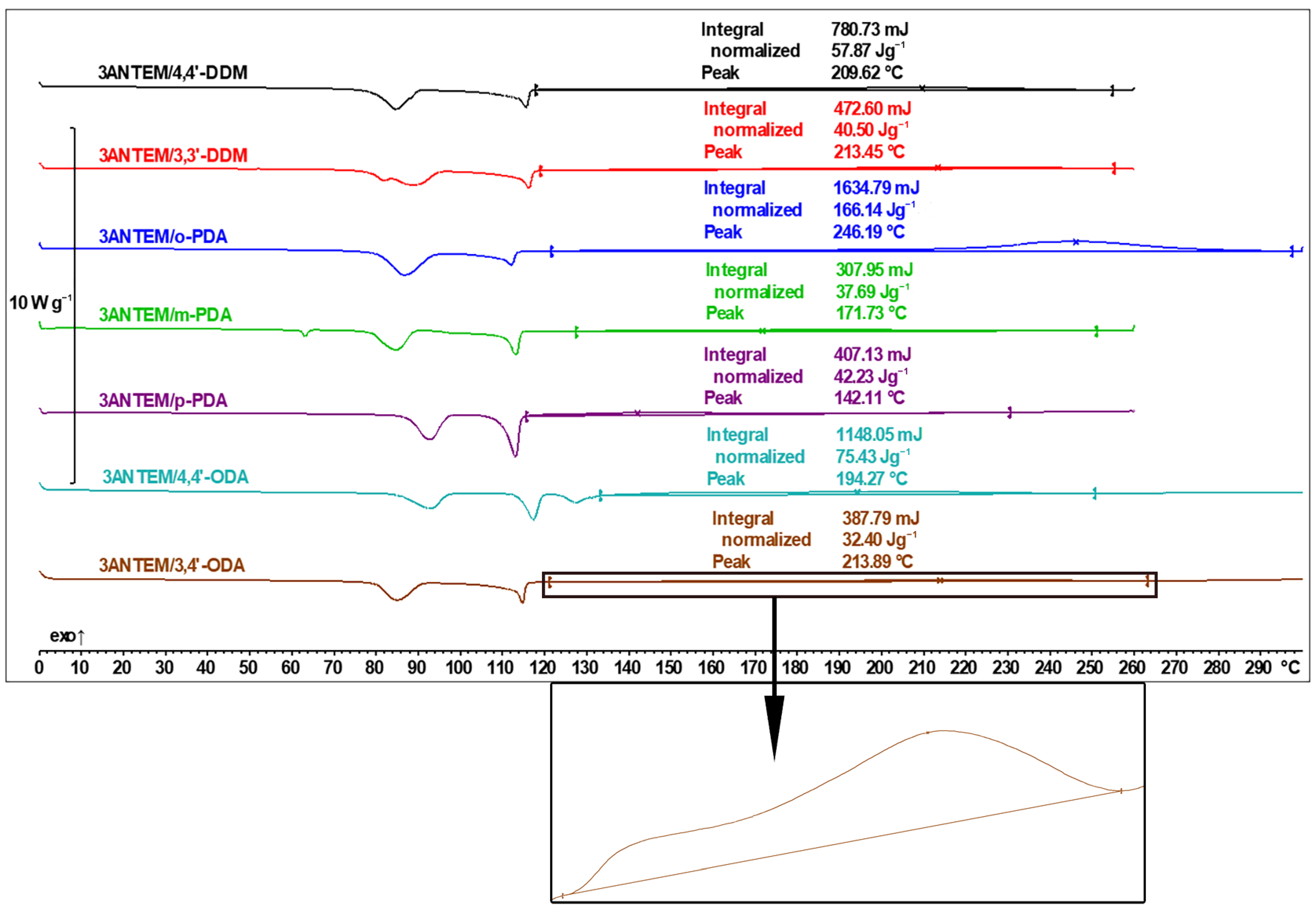


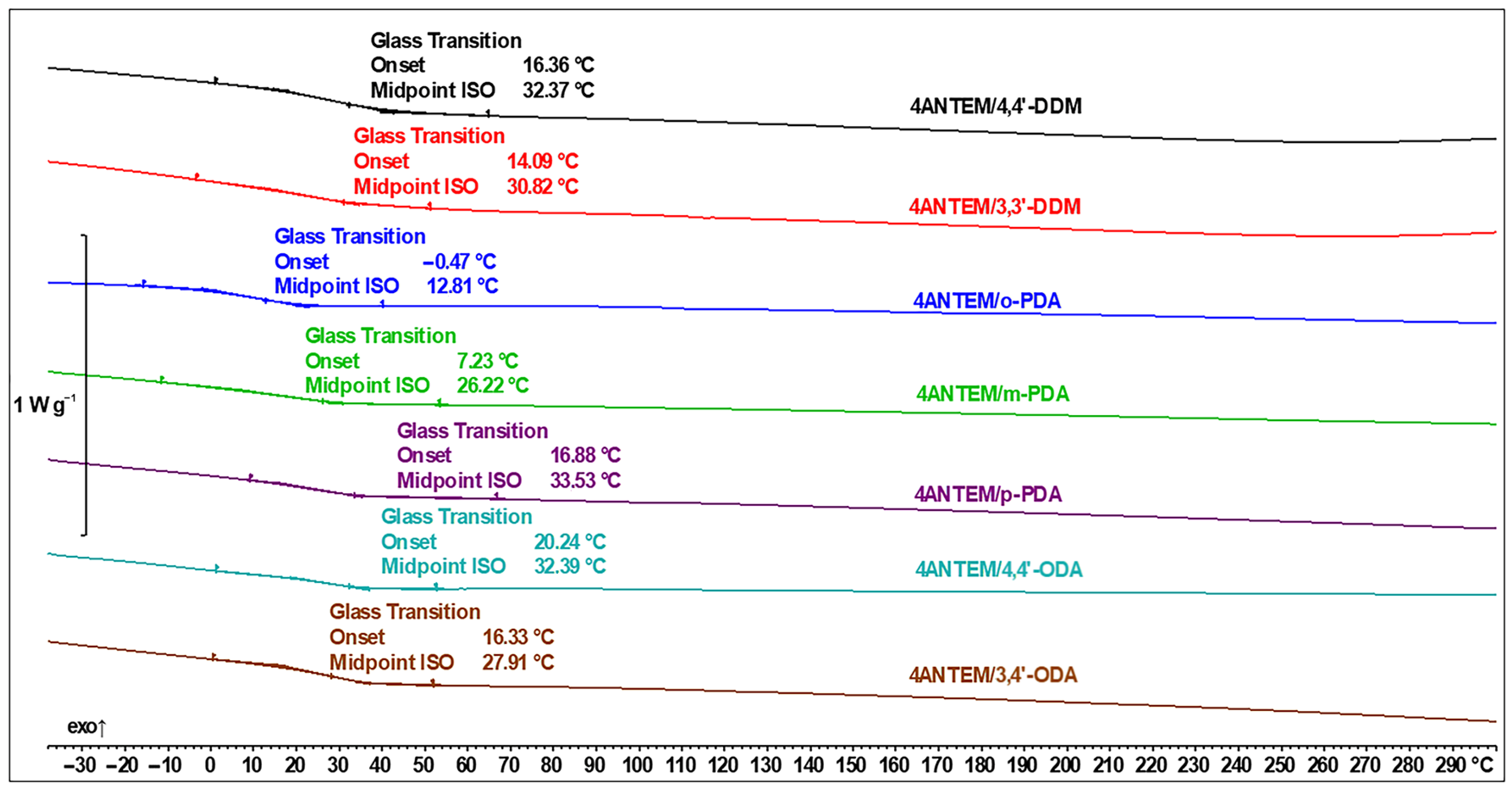
| Transition Type | ΔH, kJ/mol | Tpeak, °C |
|---|---|---|
| 3ANTEM | ||
| Cr → Cr’ | 40.76 | 96.2 |
| Cr’ → N | 27.86 | 119.4 |
| N → I | 10.24 | 142.1 |
| 4ANTEM | ||
| Cr → Cr’ | 33.97 | 102.8 |
| Cr’ → SmA | 12.55 | 133.8 |
| SmA → N | 8.53 | 144.4 |
| N → I | 9.32 | 213.7 |
| Curing Agent | 3ANTEM | 4ANTEM | ||
|---|---|---|---|---|
| ΔH, J/g | Tg, °C | ΔH, J/g | Tg, °C | |
| 4,4′-DDM | 57.9 | 7.5 | 20.3 | 32.4 |
| 3,3′-DDM | 40.5 | 5.5 | 17.6 | 30.8 |
| o-PDA | 166.1 | 37.0 | 110.2 | 12.8 |
| m-PDA | 37.7 | −7.5 | 115.3 | 26.2 |
| p-PDA | 42.2 | 23.4 | 121.6 | 33.5 |
| 4,4′-ODA | 75.4 | 32.0 | 87.7 | 32.4 |
| 3,4′-ODA | 32.4 | 24.1 | 63.0 | 27.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kisiel, M.; Mossety-Leszczak, B. The Effect of Nonterminal Liquid Crystalline Epoxy Resin Structure and Curing Agents on the Glass Transition of Polymer Networks. Polymers 2024, 16, 857. https://doi.org/10.3390/polym16060857
Kisiel M, Mossety-Leszczak B. The Effect of Nonterminal Liquid Crystalline Epoxy Resin Structure and Curing Agents on the Glass Transition of Polymer Networks. Polymers. 2024; 16(6):857. https://doi.org/10.3390/polym16060857
Chicago/Turabian StyleKisiel, Maciej, and Beata Mossety-Leszczak. 2024. "The Effect of Nonterminal Liquid Crystalline Epoxy Resin Structure and Curing Agents on the Glass Transition of Polymer Networks" Polymers 16, no. 6: 857. https://doi.org/10.3390/polym16060857