OH End-Capped Silicone as an Effective Nucleating Agent for Polylactide—A Robotizing Method for Evaluating the Mechanical Characteristics of PLA/Silicone Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PLA/Silicone Blends
2.3. Preparation of Final Samples
2.4. Experimental Workstation Setup Design for Automated Robotic Flexural Tests
2.5. Characterization Methods
- ∆ HM—melt enthalpy;
- ∆ Hcc—cold crystallization enthalpy;
- ∆ HMPLA—melting enthalpy corresponding to crystalline PLA 93 J/g [57];
- φ—silicone amount in the blend material.
- Xc [%]—degree of crystallinity;
- Ac—crystallized area on the diffractogram;
- Aa—amorphous area on the diffractogram.
3. Results
3.1. Evaluation of PLA/Silicone Blends Morphology
3.1.1. XRD Results
3.1.2. FTIR Spectra Analysis
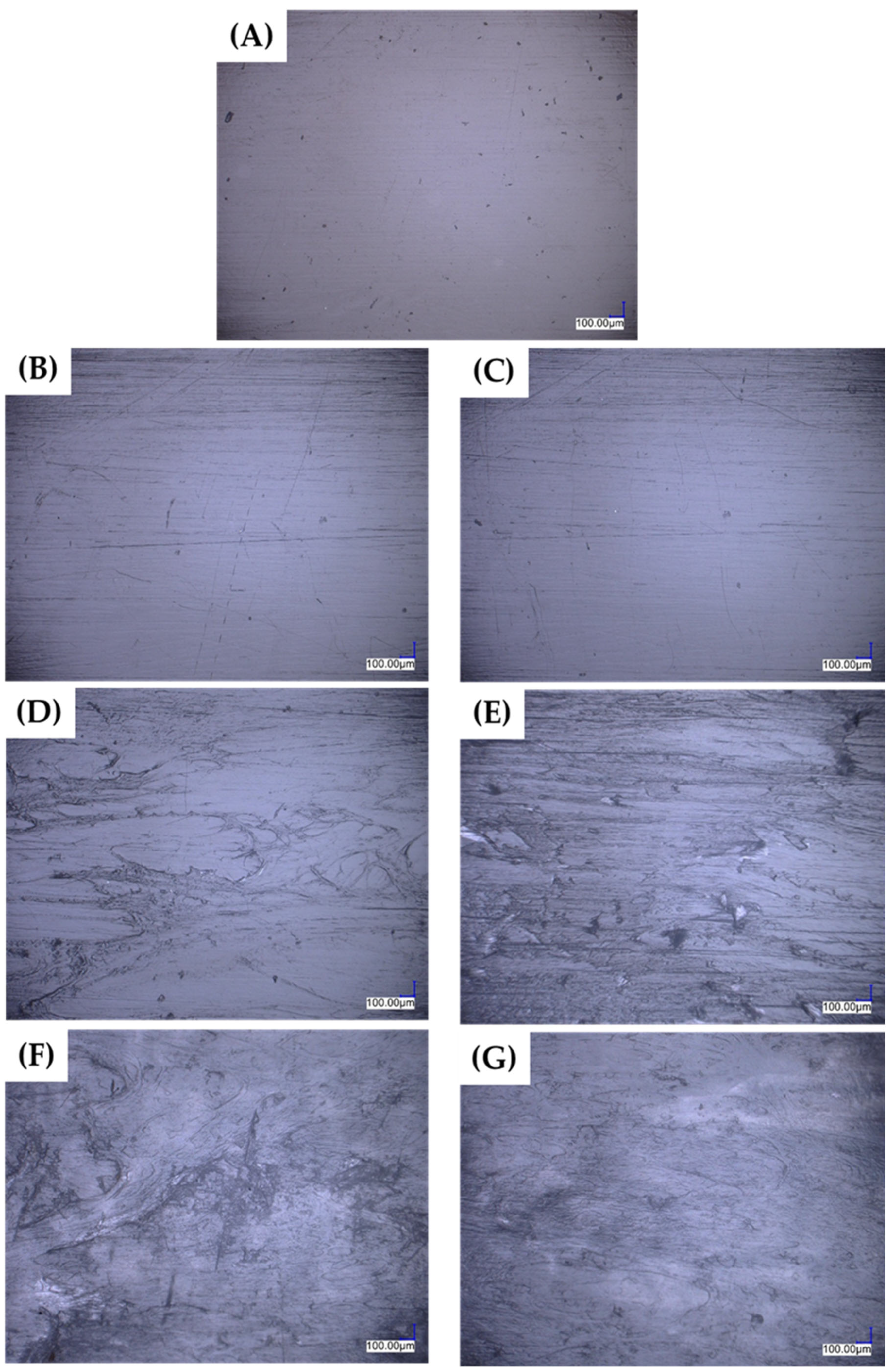
3.2. Composite Structure Evaluation—SEM/MO Observations
3.2.1. Optical Microscopy Observations (MO)
3.2.2. Scanning Electron Microscopy (SEM, SEM–EDS)
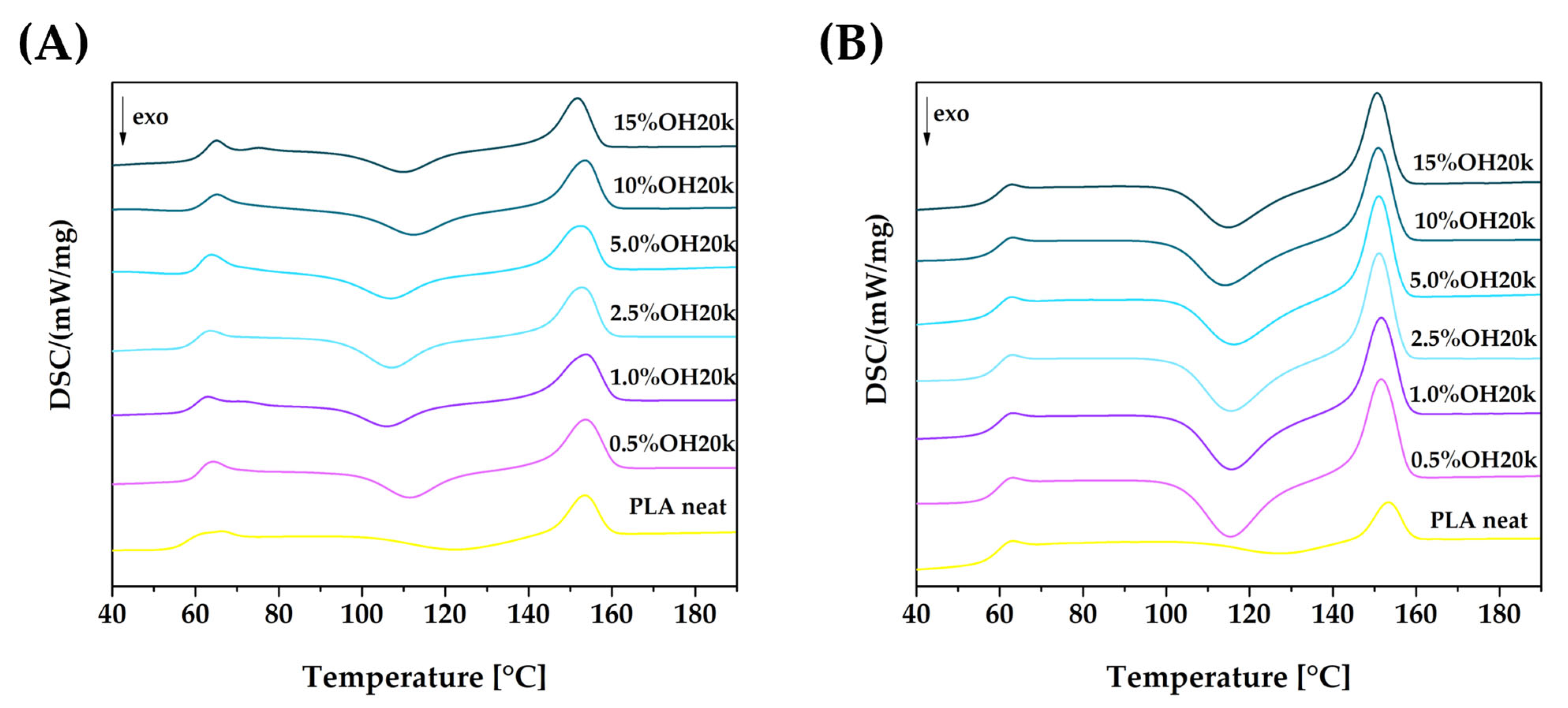
3.3. Thermal Analysis Results
3.4. Rheology
3.5. Mechanical Performance
3.5.1. Flexural Behavior Analysis
3.5.2. Static Tensile Behavior and Impact Resistance Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lay, M.; Thajudin, N.L.N.; Hamid, Z.A.A.; Rusli, A.; Abdullah, M.K.; Shuib, R.K. Comparison of Physical and Mechanical Properties of PLA, ABS and Nylon 6 Fabricated Using Fused Deposition Modeling and Injection Molding. Compos. Part B Eng. 2019, 176, 107341. [Google Scholar] [CrossRef]
- Masood, S.H.; Mau, K.; Song, W.Q. Tensile Properties of Processed FDM Polycarbonate Material. Mater. Sci. Forum 2010, 654–656, 2556–2559. [Google Scholar] [CrossRef]
- Dar, U.A.; Xu, Y.J.; Zakir, S.M.; Saeed, M. The Effect of Injection Molding Process Parameters on Mechanical and Fracture Behavior of Polycarbonate Polymer. J. Appl. Polym. Sci. 2017, 134, app.44474. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Jaafar, M. A Review on Degradation Mechanisms of Polylactic Acid: Hydrolytic, Photodegradative, Microbial, and Enzymatic Degradation. Polym. Eng. Sci. 2020, 60, 2061–2075. [Google Scholar] [CrossRef]
- Xie, F.; Luckman, P.; Milne, J.; McDonald, L.; Young, C.; Tu, C.Y.; Pasquale, T.D.; Faveere, R.; Halley, P.J. Thermoplastic Starch. J. Renew. Mater. 2014, 2, 95–106. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Yusoh, K. A Review on the Recent Research of Polycaprolactone (PCL). Adv. Mater. Res. 2015, 1134, 249–255. [Google Scholar] [CrossRef]
- Taib, N.-A.A.B.; Rahman, M.R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bakri, M.K.B.; Julaihi, M.R.M.B.; Khan, A. A Review on Poly Lactic Acid (PLA) as a Biodegradable Polymer. Polym. Bull. 2023, 80, 1179–1213. [Google Scholar] [CrossRef]
- Murariu, M.; Paint, Y.; Murariu, O.; Laoutid, F.; Dubois, P. Tailoring and Long-Term Preservation of the Properties of PLA Composites with “Green” Plasticizers. Polymers 2022, 14, 4836. [Google Scholar] [CrossRef]
- Lee, M.; Jung, B.N.; Kim, G.H.; Kang, D.; Park, H.J.; Shim, J.K.; Hwang, S.W. The Effect of Triethyl Citrate on the Dispersibility and Water Vapor Sorption Behavior of Polylactic Acid/Zeolite Composites. Polym. Test. 2020, 89, 106571. [Google Scholar] [CrossRef]
- Tábi, T.; Ageyeva, T.; Kovács, J.G. The Influence of Nucleating Agents, Plasticizers, and Molding Conditions on the Properties of Injection Molded PLA Products. Mater. Today Commun. 2022, 32, 103936. [Google Scholar] [CrossRef]
- Brząkalski, D.; Sztorch, B.; Frydrych, M.; Pakuła, D.; Dydek, K.; Kozera, R.; Boczkowska, A.; Marciniec, B.; Przekop, R.E. Limonene Derivative of Spherosilicate as a Polylactide Modifier for Applications in 3D Printing Technology. Molecules 2020, 25, 5882. [Google Scholar] [CrossRef] [PubMed]
- Chaos, A.; Sangroniz, A.; Fernández, J.; Del Río, J.; Iriarte, M.; Sarasua, J.R.; Etxeberria, A. Plasticization of Poly(Lactide) with Poly(Ethylene Glycol): Low Weight Plasticizer vs Triblock Copolymers. Effect on Free Volume and Barrier Properties. J. Appl. Polym. Sci. 2020, 137, 48868. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Perdiguero, M.; Fiori, S.; Kenny, J.M.; Peponi, L. Biodegradable Electrospun PLA-PHB Fibers Plasticized with Oligomeric Lactic Acid. Polym. Degrad. Stab. 2020, 179, 109226. [Google Scholar] [CrossRef]
- Simmons, H.; Tiwary, P.; Colwell, J.E.; Kontopoulou, M. Improvements in the Crystallinity and Mechanical Properties of PLA by Nucleation and Annealing. Polym. Degrad. Stab. 2019, 166, 248–257. [Google Scholar] [CrossRef]
- Foglia, F.; De Meo, A.; Iozzino, V.; Volpe, V.; Pantani, R. Isothermal Crystallization of PLA: Nucleation Density and Growth Rates of α and α’ Phases. Can. J. Chem. Eng. 2020, 98, 1998–2007. [Google Scholar] [CrossRef]
- Aliotta, L.; Sciara, L.M.; Cinelli, P.; Canesi, I.; Lazzeri, A. Improvement of the PLA Crystallinity and Heat Distortion Temperature Optimizing the Content of Nucleating Agents and the Injection Molding Cycle Time. Polymers 2022, 14, 977. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Jia, C.; Li, Z.; Li, L.; Zhao, Q.; Wang, J.; Wu, H. Solution-blow Spun PLA/SiO2 Nanofiber Membranes toward High Efficiency Oil/Water Separation. J. Appl. Polym. Sci. 2020, 137, 49103. [Google Scholar] [CrossRef]
- Borkowski, G.; Martyła, A.; Dobrosielska, M.; Marciniak, P.; Gabriel, E.; Głowacka, J.; Jałbrzykowski, M.; Pakuła, D.; Przekop, R.E. Carbonate Lake Sediments in the Plastics Processing-Preliminary Polylactide Composite Case Study: Mechanical and Structural Properties. Materials 2022, 15, 6106. [Google Scholar] [CrossRef] [PubMed]
- Orta, M.D.M.; Martín, J.; Santos, J.L.; Aparicio, I.; Medina-Carrasco, S.; Alonso, E. Biopolymer-Clay Nanocomposites as Novel and Ecofriendly Adsorbents for Environmental Remediation. Appl. Clay Sci. 2020, 198, 105838. [Google Scholar] [CrossRef]
- Sztorch, B.; Pakuła, D.; Kustosz, M.; Romanczuk-Ruszuk, E.; Gabriel, E.; Przekop, R.E. The Influence of Organofunctional Substituents of Spherosilicates on the Functional Properties of PLA/TiO2 Composites Used in 3D Printing (FDM/FFF). Polymers 2022, 14, 5493. [Google Scholar] [CrossRef]
- Dobrosielska, M.; Dobrucka, R.; Kozera, P.; Brząkalski, D.; Gabriel, E.; Głowacka, J.; Jałbrzykowski, M.; Kurzydłowski, K.J.; Przekop, R.E. Beeswax as a Natural Alternative to Synthetic Waxes for Fabrication of PLA/Diatomaceous Earth Composites. Sci. Rep. 2023, 13, 1161. [Google Scholar] [CrossRef] [PubMed]
- Martinez Villadiego, K.; Arias Tapia, M.J.; Useche, J.; Escobar Macías, D. Thermoplastic Starch (TPS)/Polylactic Acid (PLA) Blending Methodologies: A Review. J. Polym. Environ. 2022, 30, 75–91. [Google Scholar] [CrossRef]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M. Mechanical, Physical and Thermal Properties of Sugar Palm Nanocellulose Reinforced Thermoplastic Starch (TPS)/Poly (Lactic Acid) (PLA) Blend Bionanocomposites. Polymers 2020, 12, 2216. [Google Scholar] [CrossRef] [PubMed]
- Rahmatabadi, D.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Baghani, M. 3D Printing of PLA-TPU with Different Component Ratios: Fracture Toughness, Mechanical Properties, and Morphology. J. Mater. Res. Technol. 2022, 21, 3970–3981. [Google Scholar] [CrossRef]
- Kilic, N.T.; Can, B.N.; Kodal, M.; Özkoç, G. Reactive Compatibilization of Biodegradable PLA/TPU Blends via Hybrid Nanoparticle. Prog. Rubber Plast. Recycl. Technol. 2021, 37, 301–326. [Google Scholar] [CrossRef]
- Aliotta, L.; Vannozzi, A.; Canesi, I.; Cinelli, P.; Coltelli, M.-B.; Lazzeri, A. Poly(Lactic Acid) (PLA)/Poly(Butylene Succinate-Co-Adipate) (PBSA) Compatibilized Binary Biobased Blends: Melt Fluidity, Morphological, Thermo-Mechanical and Micromechanical Analysis. Polymers 2021, 13, 218. [Google Scholar] [CrossRef] [PubMed]
- Nofar, M.; Salehiyan, R.; Ciftci, U.; Jalali, A.; Durmuş, A. Ductility Improvements of PLA-Based Binary and Ternary Blends with Controlled Morphology Using PBAT, PBSA, and Nanoclay. Compos. Part B Eng. 2020, 182, 107661. [Google Scholar] [CrossRef]
- Raj, A.; Samuel, C.; Prashantha, K. Role of Compatibilizer in Improving the Properties of PLA/PA12 Blends. Front. Mater. 2020, 7, 193. [Google Scholar] [CrossRef]
- Rasselet, D.; Caro-Bretelle, A.-S.; Taguet, A.; Lopez-Cuesta, J.-M. Reactive Compatibilization of PLA/PA11 Blends and Their Application in Additive Manufacturing. Materials 2019, 12, 485. [Google Scholar] [CrossRef]
- Sathornluck, S.; Choochottiros, C. Modification of Epoxidized Natural Rubber as a PLA Toughening Agent. J. Appl. Polym. Sci. 2019, 136, 48267. [Google Scholar] [CrossRef]
- Juntuek, P.; Ruksakulpiwat, C.; Chumsamrong, P.; Ruksakulpiwat, Y. Effect of Glycidyl Methacrylate-Grafted Natural Rubber on Physical Properties of Polylactic Acid and Natural Rubber Blends. J. Appl. Polym. Sci. 2012, 125, 745–754. [Google Scholar] [CrossRef]
- Mark, J.E. Some Interesting Things about Polysiloxanes. Acc. Chem. Res. 2004, 37, 946–953. [Google Scholar] [CrossRef]
- Abdilla, A.; D’Ambra, C.A.; Geng, Z.; Shin, J.J.; Czuczola, M.; Goldfeld, D.J.; Biswas, S.; Mecca, J.M.; Swier, S.; Bekemeier, T.D.; et al. Silicone-Based Polymer Blends: Enhancing Properties through Compatibilization. J. Polym. Sci. 2021, 59, 2114–2128. [Google Scholar] [CrossRef]
- Alam, M.S.; Ansari, A.; Ahsan, I.; Shafiq-un-Nabi, S.; Md, S.; Shaik, R.A.; Eid, B.G.; Ahmad, M.Z.; Ahmad, J. Topical Gel Containing Polysiloxanes and Hyaluronic Acid for Skin Scar: Formulation Design, Characterization, and In Vivo Activity. J. Cosmet. Dermatol. 2023, 22, 1220–1232. [Google Scholar] [CrossRef]
- Singh, N.; Sinha, S.K. Tribological and Mechanical Analysis of Hybrid Epoxy Based Polymer Composites with Different in Situ Liquid Lubricants (Silicone Oil, PAO and SN150 Base Oil). Wear 2022, 504–505, 204404. [Google Scholar] [CrossRef]
- Meekum, U.; Khiansanoi, A. PLA and Two Components Silicon Rubber Blends Aiming for Frozen Foods Packaging Applications. Results Phys. 2018, 8, 79–88. [Google Scholar] [CrossRef]
- Meekum, U.; Khiansanoi, A. PLA and Single Component Silicone Rubber Blends for Sub-Zero Temperature Blown Film Packaging Applications. Results Phys. 2018, 9, 1127–1135. [Google Scholar] [CrossRef]
- Yıldız, S.; Karaağaç, B.; Ozkoc, G. Thoughening of Poly(Lactic Acid) with Silicone Rubber. Polym. Eng. Sci. 2014, 54, 2029–2036. [Google Scholar] [CrossRef]
- Khuenkeao, T.; Petchwattana, N.; Covavisaruch, S. Thermal and Mechanical Properties of Bioplastic Poly(Lactic Acid) Compounded with Silicone Rubber and Talc; AIP Publishing: Jeju Island, Republic of Korea, 2016; p. 080005. [Google Scholar]
- Maier, W.F.; Stöwe, K.; Sieg, S. Combinatorial and High-Throughput Materials Science. Angew. Chem. Int. Ed. 2007, 46, 6016–6067. [Google Scholar] [CrossRef]
- Hook, A.L.; Anderson, D.G.; Langer, R.; Williams, P.; Davies, M.C.; Alexander, M.R. High Throughput Methods Applied in Biomaterial Development and Discovery. Biomaterials 2010, 31, 187–198. [Google Scholar] [CrossRef]
- Mennen, S.M.; Alhambra, C.; Allen, C.L.; Barberis, M.; Berritt, S.; Brandt, T.A.; Campbell, A.D.; Castañón, J.; Cherney, A.H.; Christensen, M.; et al. The Evolution of High-Throughput Experimentation in Pharmaceutical Development and Perspectives on the Future. Org. Process Res. Dev. 2019, 23, 1213–1242. [Google Scholar] [CrossRef]
- Yang, L.; Pijuan-Galito, S.; Rho, H.S.; Vasilevich, A.S.; Eren, A.D.; Ge, L.; Habibović, P.; Alexander, M.R.; De Boer, J.; Carlier, A.; et al. High-Throughput Methods in the Discovery and Study of Biomaterials and Materiobiology. Chem. Rev. 2021, 121, 4561–4677. [Google Scholar] [CrossRef] [PubMed]
- Duncombe, T.A.; Dittrich, P.S. Droplet Barcoding: Tracking Mobile Micro-Reactors for High-Throughput Biology. Curr. Opin. Biotechnol. 2019, 60, 205–212. [Google Scholar] [CrossRef]
- Zeng, W.; Guo, L.; Xu, S.; Chen, J.; Zhou, J. High-Throughput Screening Technology in Industrial Biotechnology. Trends Biotechnol. 2020, 38, 888–906. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, Z.; Wang, Z.; Zhong, J.; Zhao, L.; Jiang, L.; Zhou, R.; Liu, Y.; Huang, L.; Tan, L.; et al. High-Throughput Method-Accelerated Design of Ni-Based Superalloys. Adv. Funct. Mater. 2022, 32, 2109367. [Google Scholar] [CrossRef]
- Sztorch, B.; Brząkalski, D.; Głowacka, J.; Pakuła, D.; Frydrych, M.; Przekop, R.E. Trimming Flow, Plasticity, and Mechanical Properties by Cubic Silsesquioxane Chemistry. Sci. Rep. 2023, 13, 14156. [Google Scholar] [CrossRef] [PubMed]
- Brząkalski, D.; Przekop, R.E.; Sztorch, B.; Frydrych, M.; Pakuła, D.; Jałbrzykowski, M.; Markiewicz, G.; Marciniec, B. Why POSS-Type Compounds Should Be Considered Nanomodifiers, Not Nanofillers—A Polypropylene Blends Case Study. Polymers 2021, 13, 2124. [Google Scholar] [CrossRef] [PubMed]
- Przybyszewski, B.; Kozera, R.; Krawczyk, Z.D.; Boczkowska, A.; Dolatabadi, A.; Amer, A.; Sztorch, B.; Przekop, R.E. A Wind Tunnel Experimental Study of Icing on NACA0012 Aircraft Airfoil with Silicon Compounds Modified Polyurethane Coatings. Materials 2021, 14, 5687. [Google Scholar] [CrossRef]
- PN-EN ISO 20753:2019; Plastics—Test Specimens. ISO: Geneva, Switzerland, 2019.
- Głowacka, J.; Derpeński, Ł.; Frydrych, M.; Sztorch, B.; Bartoszewicz, B.; Przekop, R.E. Robotization of Three-Point Bending Mechanical Tests Using PLA/TPU Blends as an Example in the 0–100% Range. Materials 2023, 16, 6927. [Google Scholar] [CrossRef]
- PN-EN ISO 178:2019; Plastics—Determination of Flexural Properties. ISO: Geneva, Switzerland, 2019.
- PN-EN ISO 527:2019; Plastics—Determination of Tensile Properties. ISO: Geneva, Switzerland, 2019.
- PN-EN ISO 179-1:2023; Plastics—Charpy Impact Determination—Part 1: Non-Instrumental Impact Testing. ISO: Geneva, Switzerland, 2023.
- PN-EN ISO 1133-1:2022; Plastics—Determination of the Melt Mass-Flow Rate (MFR) and Melt Volume-Flow Rate (MVR) of Thermoplastics Part 1: Standard Method. ISO: Geneva, Switzerland, 2022.
- Mazur, K.E.; Jakubowska, P.; Gaweł, A.; Kuciel, S. Mechanical, Thermal and Hydrodegradation Behavior of Poly (3-Hydroxybutyrate-Co-3-Hydroxyvalerate) (PHBV) Composites with Agricultural Fibers as Reinforcing Fillers. Sustain. Mater. Technol. 2022, 31, e00390. [Google Scholar] [CrossRef]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the Structure of Solution Grown Crystals of Lactide Copolymers by Means of Chemical Reactions. Kolloid-Z. Z. Polym. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- PN-EN ISO 75-1:2020; Plastics—Determination of Temperature of Deflection under Load Part 1: General Test Method. ISO: Geneva, Switzerland, 2020.
- Farid, T.; Herrera, V.N.; Kristiina, O. Investigation of Crystalline Structure of Plasticized Poly (Lactic Acid)/Banana Nanofibers Composites. IOP Conf. Ser. Mater. Sci. Eng. 2018, 369, 012031. [Google Scholar] [CrossRef]
- Sperling, L.H. Introduction to Physical Polymer Science, 1st ed.; Wiley: New York, NY, USA, 2005; ISBN 978-0-471-70606-9. [Google Scholar]
- Chen, B.-K.; Shih, C.-C.; Chen, A.F. Ductile PLA Nanocomposites with Improved Thermal Stability. Compos. Part Appl. Sci. Manuf. 2012, 43, 2289–2295. [Google Scholar] [CrossRef]
- Echeverría, C.; Limón, I.; Muñoz-Bonilla, A.; Fernández-García, M.; López, D. Development of Highly Crystalline Polylactic Acid with β-Crystalline Phase from the Induced Alignment of Electrospun Fibers. Polymers 2021, 13, 2860. [Google Scholar] [CrossRef] [PubMed]
- Vadas, D.; Nagy, Z.K.; Csontos, I.; Marosi, G.; Bocz, K. Effects of Thermal Annealing and Solvent-Induced Crystallization on the Structure and Properties of Poly(Lactic Acid) Microfibres Produced by High-Speed Electrospinning. J. Therm. Anal. Calorim. 2020, 142, 581–594. [Google Scholar] [CrossRef]
- Pan, P.; Kai, W.; Zhu, B.; Dong, T.; Inoue, Y. Polymorphous Crystallization and Multiple Melting Behavior of Poly(l -Lactide): Molecular Weight Dependence. Macromolecules 2007, 40, 6898–6905. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, Y.; Sato, H.; Tsuji, H.; Noda, I.; Yan, S.; Ozaki, Y. Crystal Modifications and Thermal Behavior of Poly(l -Lactic Acid) Revealed by Infrared Spectroscopy. Macromolecules 2005, 38, 8012–8021. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Disorder-to-Order Phase Transition and Multiple Melting Behavior of Poly(l -Lactide) Investigated by Simultaneous Measurements of WAXD and DSC. Macromolecules 2008, 41, 1352–1357. [Google Scholar] [CrossRef]
- Yee, Y.Y.; Ching, Y.C.; Rozali, S.; Awanis Hashim, N.; Singh, R. Preparation and Characterization of Poly(Lactic Acid)-Based Composite Reinforced with Oil Palm Empty Fruit Bunch Fiber and Nanosilica. BioResources 2016, 11, 2269–2286. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Li, N.; Liao, J.; Zhao, S.; Wang, J.; Wang, S. Relationship between Polymer–Filler Interfaces in Separation Layers and Gas Transport Properties of Mixed Matrix Composite Membranes. J. Membr. Sci. 2015, 495, 252–268. [Google Scholar] [CrossRef]
- Yan, S.; Yin, J.; Yang, Y.; Dai, Z.; Ma, J.; Chen, X. Surface-Grafted Silica Linked with l-Lactic Acid Oligomer: A Novel Nanofiller to Improve the Performance of Biodegradable Poly(l-Lactide). Polymer 2007, 48, 1688–1694. [Google Scholar] [CrossRef]
- Reddy, C.S.; Das, C.K. Propylene-Ethylene Copolymer Filled Nanocomposites: Influence of Zn-Ion Coating upon Nano-SiO 2 on Structural, Thermal, and Dynamic Mechanical Properties. Polym.-Plast. Technol. Eng. 2006, 45, 815–820. [Google Scholar] [CrossRef]
- Alexander, L.E. X-ray Diffraction Methods in Polymer Science; Krieger: Huntington, NY, USA, 1979; ISBN 978-0-88275-801-5. [Google Scholar]
- Carrasco, F.; Pagès, P.; Gámez-Pérez, J.; Santana, O.O.; Maspoch, M.L. Kinetics of the Thermal Decomposition of Processed Poly(Lactic Acid). Polym. Degrad. Stab. 2010, 95, 2508–2514. [Google Scholar] [CrossRef]
- Kaavessina, M.; Distantina, S.; Chafidz, A.; Fadilah; Al-Zahrani, S.M. The Influences of Elastomer toward Degradability of Poly (Lactic Acid); AIP Publishing: Bandung, Indonesia, 2016; p. 030031. [Google Scholar]
- Gao, Y.; Wang, J.; Liang, X.; Yan, Z.; Liu, Y.; Cai, Y. Investigation on Permeation Properties of Liquids into HTV Silicone Rubber Materials. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 2428–2437. [Google Scholar] [CrossRef]
- Atta, A.; Abdeltwab, E. Influence of Ion Irradiation on the Surface Properties of Silver-Coated Flexible PDMS Polymeric Films. Braz. J. Phys. 2022, 52, 3. [Google Scholar] [CrossRef]
- Mofokeng, J.P.; Luyt, A.S.; Tábi, T.; Kovács, J. Comparison of Injection Moulded, Natural Fibre-Reinforced Composites with PP and PLA as Matrices. J. Thermoplast. Compos. Mater. 2012, 25, 927–948. [Google Scholar] [CrossRef]
- Bhiogade, A.; Kannan, M. Studies on Thermal and Degradation Kinetics of Cellulose Micro/Nanoparticle Filled Polylactic Acid (PLA) Based Nanocomposites. Polym. Polym. Compos. 2021, 29, S85–S98. [Google Scholar] [CrossRef]
- Jeong, J.; Ayyoob, M.; Kim, J.-H.; Nam, S.W.; Kim, Y.J. In Situ Formation of PLA-Grafted Alkoxysilanes for Toughening a Biodegradable PLA Stereocomplex Thin Film. RSC Adv. 2019, 9, 21748–21759. [Google Scholar] [CrossRef]
- Li, Z.; Muiruri, J.K.; Thitsartarn, W.; Zhang, X.; Tan, B.H.; He, C. Biodegradable Silica Rubber Core-Shell Nanoparticles and Their Stereocomplex for Efficient PLA Toughening. Compos. Sci. Technol. 2018, 159, 11–17. [Google Scholar] [CrossRef]
- Meng, X.; Nguyen, N.A.; Tekinalp, H.; Lara-Curzio, E.; Ozcan, S. Supertough PLA-Silane Nanohybrids by in Situ Condensation and Grafting. ACS Sustain. Chem. Eng. 2018, 6, 1289–1298. [Google Scholar] [CrossRef]
- Marcilla, A.; Beltrán, M. Mechanisms of Plasticizers Action. In Handbook of Plasticizers; Elsevier: Amsterdam, The Netherlands, 2012; pp. 119–133. ISBN 978-1-895198-50-8. [Google Scholar]
- Pakuła, D.; Sztorch, B.; Romańczuk-Ruszuk, E.; Marciniec, B.; Przekop, R.E. High Impact Polylactide Based on Organosilicon Nucleation Agent. Chin. J. Polym. Sci. 2024, 1–11. [Google Scholar] [CrossRef]
- Shinyama, K.; Fujita, S. The Effects of Plasticizer on the Mechanical and Electrical Characteristics of PLA. In Proceedings of the 2008 International Symposium on Electrical Insulating Materials (ISEIM 2008), Yokkaichi, Japan, 7–11 September 2008; pp. 267–270. [Google Scholar]
- Pillin, I.; Montrelay, N.; Grohens, Y. Thermo-Mechanical Characterization of Plasticized PLA: Is the Miscibility the Only Significant Factor? Polymer 2006, 47, 4676–4682. [Google Scholar] [CrossRef]
- Shirai, M.A.; Grossmann, M.V.E.; Mali, S.; Yamashita, F.; Garcia, P.S.; Müller, C.M.O. Development of Biodegradable Flexible Films of Starch and Poly(Lactic Acid) Plasticized with Adipate or Citrate Esters. Carbohydr. Polym. 2013, 92, 19–22. [Google Scholar] [CrossRef]
- Nagarajan, V.; Zhang, K.; Misra, M.; Mohanty, A.K. Overcoming the Fundamental Challenges in Improving the Impact Strength and Crystallinity of PLA Biocomposites: Influence of Nucleating Agent and Mold Temperature. ACS Appl. Mater. Interfaces 2015, 7, 11203–11214. [Google Scholar] [CrossRef]




| Sample Abbreviation | PLA | Silicone |
|---|---|---|
| PLA neat | 100 | - |
| 0.5% OH20k | 99.5 | 0.5 |
| 1.0% OH20k | 99 | 1.0 |
| 2.5% OH20k | 97.5 | 2.5 |
| 5.0% OH20k | 95 | 5.0 |
| 10% OH20k | 90 | 10 |
| 15% OH20k | 75 | 15 |
| Sample | Xc, % |
|---|---|
| PLA neat | 30, 49 |
| 0.5% OH20k | 31, 23 |
| 1.0% OH20k | 32, 37 |
| 2.5% OH20k | 31, 68 |
| 5.0% OH20k | 32, 15 |
| 10% OH20k | 30, 58 |
| 15% OH20k | 27, 88 |
| Glass Transition Temperature, Tg [°C] | Cold Crystallization Temperature, Tcc [°C] | Melting Temperature, Tm [°C] | |
|---|---|---|---|
| PLA neat | 63.2 | 127.2 | 155.4 |
| 0.5% OH20k | 62.7 | 115.4 | 151.7 |
| 1.0% OH20k | 62.9 | 115.9 | 151.7 |
| 2.5% OH20k | 62.7 | 115.6 | 151.2 |
| 5.0% OH20k | 62.6 | 115.7 | 151.1 |
| 10% OH20k | 62.8 | 114.0 | 151.8 |
| 15% OH20k | 62.2 | 114.7 | 150.8 |
| 5% Mass Loss Temperature, T5% [°C] | Onset Temperature, Tonset [°C] | Temperature of Maximum Mass Loss Rate, Tmax [°C] | |
|---|---|---|---|
| PLA neat | 324.2 | 342.2 | 362.1 |
| 0.5% OH20k | 328.6 | 343.8 | 362.7 |
| 1.0% OH20k | 329.2 | 344.2 | 363.4 |
| 2.5% OH20k | 330.5 | 347.4 | 364.6 |
| 5.0% OH20k | 332.0 | 349.4 | 361.9 |
| 10% OH20k | 332.4 | 345.3, 520.6 | 364.7, 550.4 |
| 15% OH20k | 327.9 | 343.8, 478.0 | 361.2, 507.40 |
| Sample Abbreviation | HDT [°C] | Xc [%] |
|---|---|---|
| PLA neat | 55.80 ± 0.10 | 16.44 |
| 0.5% OH20k | 57.16 ± 0.06 | 34.24 |
| 1.0% OH20k | 57.23 ± 0.06 | 34.04 |
| 2.5% OH20k | 57.17 ± 0.06 | 34.62 |
| 5.0% OH20k | 57.10 ± 0.00 | 32.27 |
| 10% OH20k | 52.73 ± 0.06 | 36.51 |
| 15% OH20k | 52.60 ± 0.10 | 32.48 |
| Sample Abbreviation | Flexural Strength [MPa] | Flexural Modulus [GPa] | Tensile Strength [MPa] | Young’s Modulus [GPa] | Elongation at Break [%] | Impact Resistance [kJ/m2] |
|---|---|---|---|---|---|---|
| PLA neat | 95.60 ± 0.94 | 3.52 ± 0.03 | 60.61 ± 0.42 | 2.69 ± 0.22 | 6.25 ± 0.54 | 17.36 ± 0.47 |
| 0.5% OH20k | 95.87 ± 0.85 | 3.75 ± 0.05 | 55.29 ± 0.41 | 3.36 ± 0.03 | 10.09 ± 1.69 | 17.81 ± 1.20 |
| 1.0% OH20k | 92.33 ± 1.23 | 3.62 ± 0.09 | 53.03 ± 3.62 | 3.28 ± 0.04 | 13.07 ± 3.19 | 20.36 ± 2.81 |
| 2.5% OH20k | 85.85 ± 2.07 | 3.75 ± 0.06 | 52.43 ± 0.80 | 3.33 ± 0.03 | 19.40 ± 3.60 | 27.97 ± 3.52 |
| 5.0% OH20k | 74.40 ± 0.33 | 3.58 ± 0.03 | 45.93 ± 0.72 | 3.05 ± 0.08 | 10.29 ± 2.69 | 24.23 ± 2.88 |
| 10% OH20k | 54.19 ± 3.81 | 3.27 ± 0.05 | 41.24 ± 0.22 | 2.79 ± 0.02 | 2.57 ± 0.22 | 12.46 ± 1.66 |
| 15% OH20k | 40.59 ± 2.85 | 2.78 ± 0.04 | 33.91 ± 0.37 | 2.42 ± 0.02 | 2.18 ± 0.20 | 10.87 ± 2.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przekop, R.E.; Sztorch, B.; Głowacka, J.; Martyła, A.; Romańczuk-Ruszuk, E.; Jałbrzykowski, M.; Derpeński, Ł. OH End-Capped Silicone as an Effective Nucleating Agent for Polylactide—A Robotizing Method for Evaluating the Mechanical Characteristics of PLA/Silicone Blends. Polymers 2024, 16, 1142. https://doi.org/10.3390/polym16081142
Przekop RE, Sztorch B, Głowacka J, Martyła A, Romańczuk-Ruszuk E, Jałbrzykowski M, Derpeński Ł. OH End-Capped Silicone as an Effective Nucleating Agent for Polylactide—A Robotizing Method for Evaluating the Mechanical Characteristics of PLA/Silicone Blends. Polymers. 2024; 16(8):1142. https://doi.org/10.3390/polym16081142
Chicago/Turabian StylePrzekop, Robert E., Bogna Sztorch, Julia Głowacka, Agnieszka Martyła, Eliza Romańczuk-Ruszuk, Marek Jałbrzykowski, and Łukasz Derpeński. 2024. "OH End-Capped Silicone as an Effective Nucleating Agent for Polylactide—A Robotizing Method for Evaluating the Mechanical Characteristics of PLA/Silicone Blends" Polymers 16, no. 8: 1142. https://doi.org/10.3390/polym16081142







