Degradable Nanogels Based on Poly[Oligo(Ethylene Glycol) Methacrylate] (POEGMA) Derivatives through Thermo-Induced Aggregation of Polymer Chain and Subsequent Chemical Crosslinking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymer Synthesis
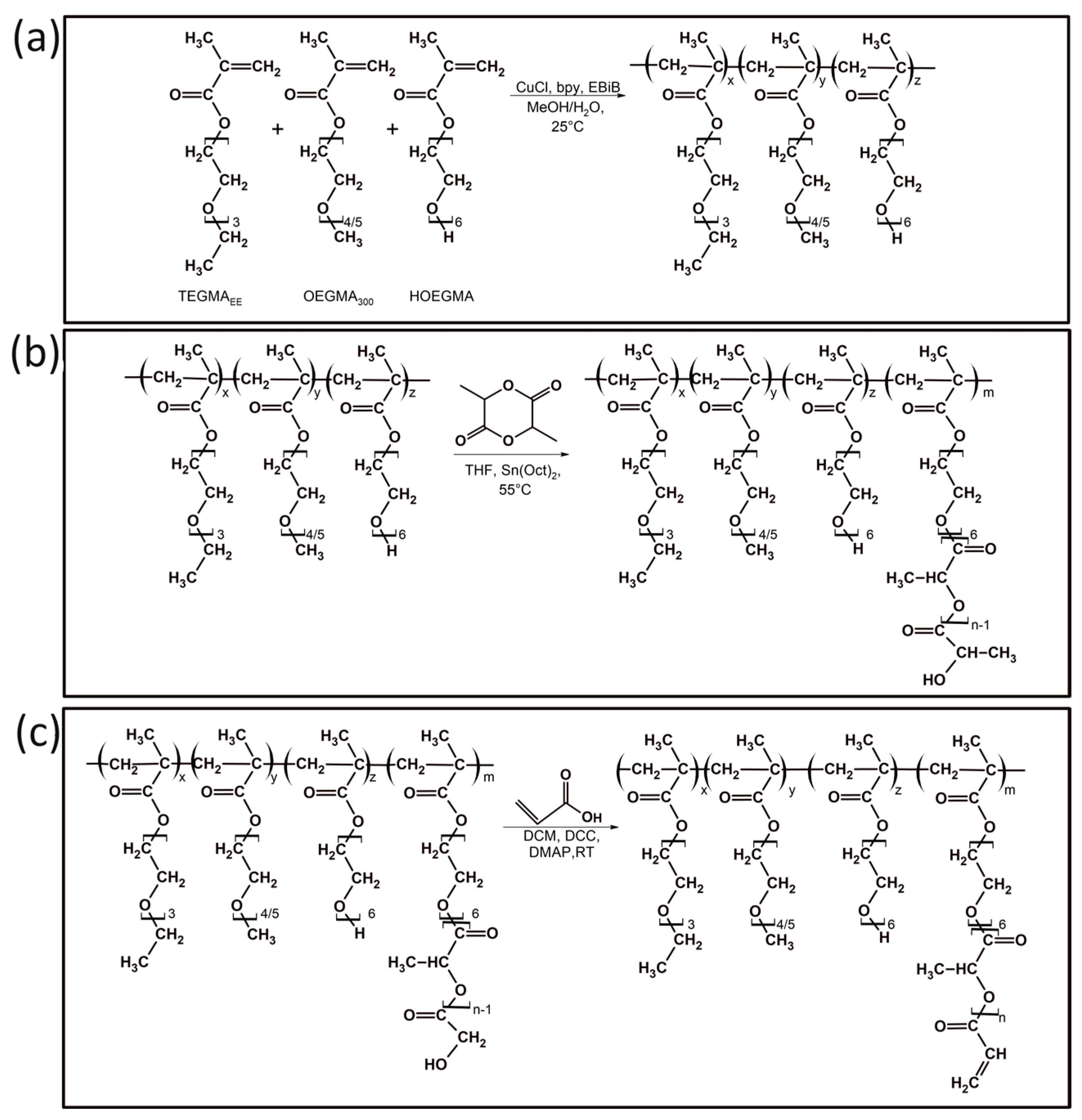
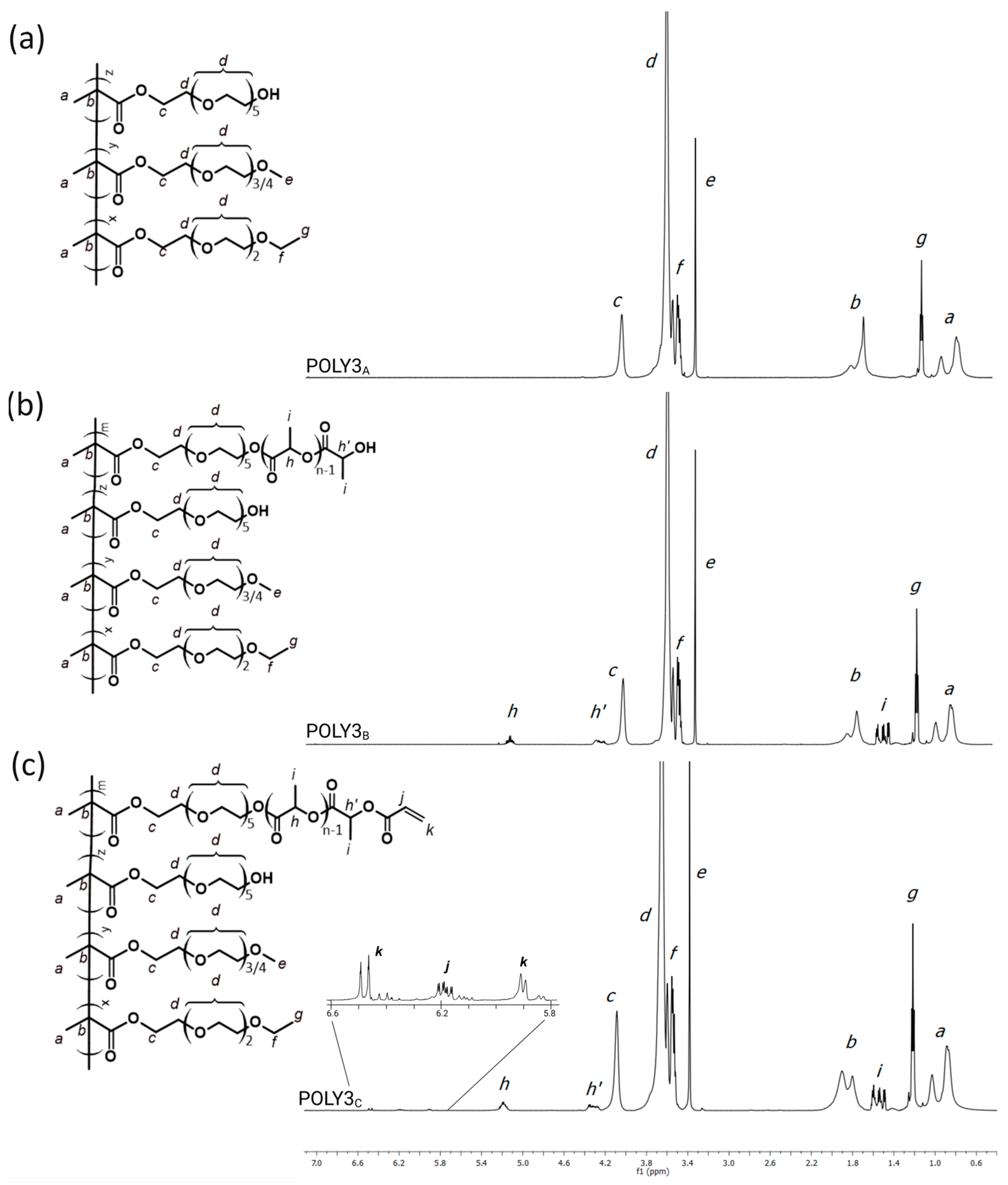
2.2.1. Synthesis of Copolymers of Oligo(Ethylene Glycol) Methacrylates (POLY XA)
2.2.2. Modification of Oligo(Ethylene Glycol) Methacrylate Copolymers with Oligo(Lactic Acid) Units (POLY XB)
2.2.3. Introduction of Acrylate Groups into Oligo(Ethylene Glycol) Methacrylate Copolymers (POLY XC)
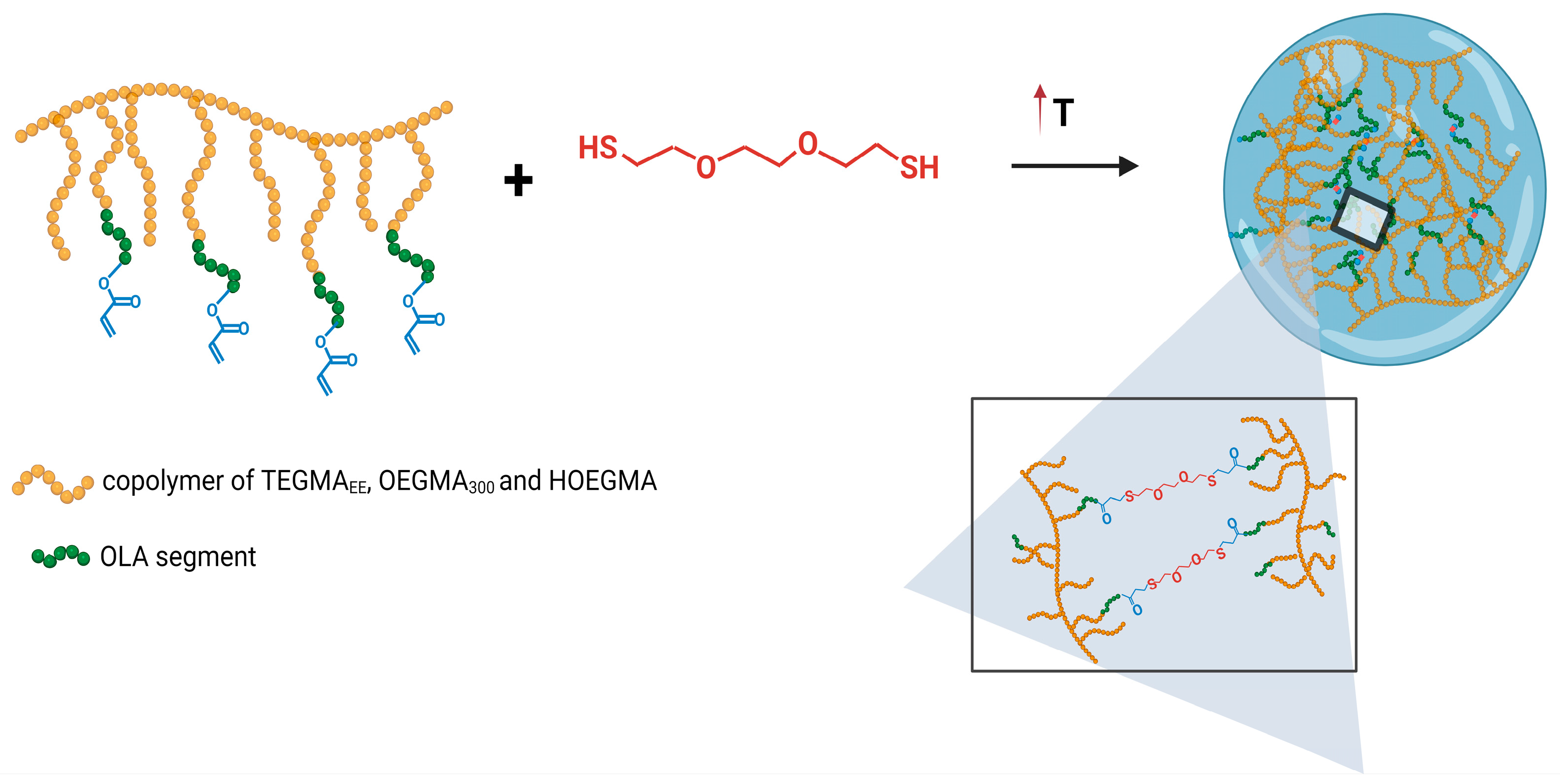
2.3. Preparation of Nanogels
2.4. Degradation of the Nanogels
2.5. Methods
3. Results
3.1. Synthesis and Characterization of Poly[Oligo(Ethylene Glycol)Methacrylate] Derivatives
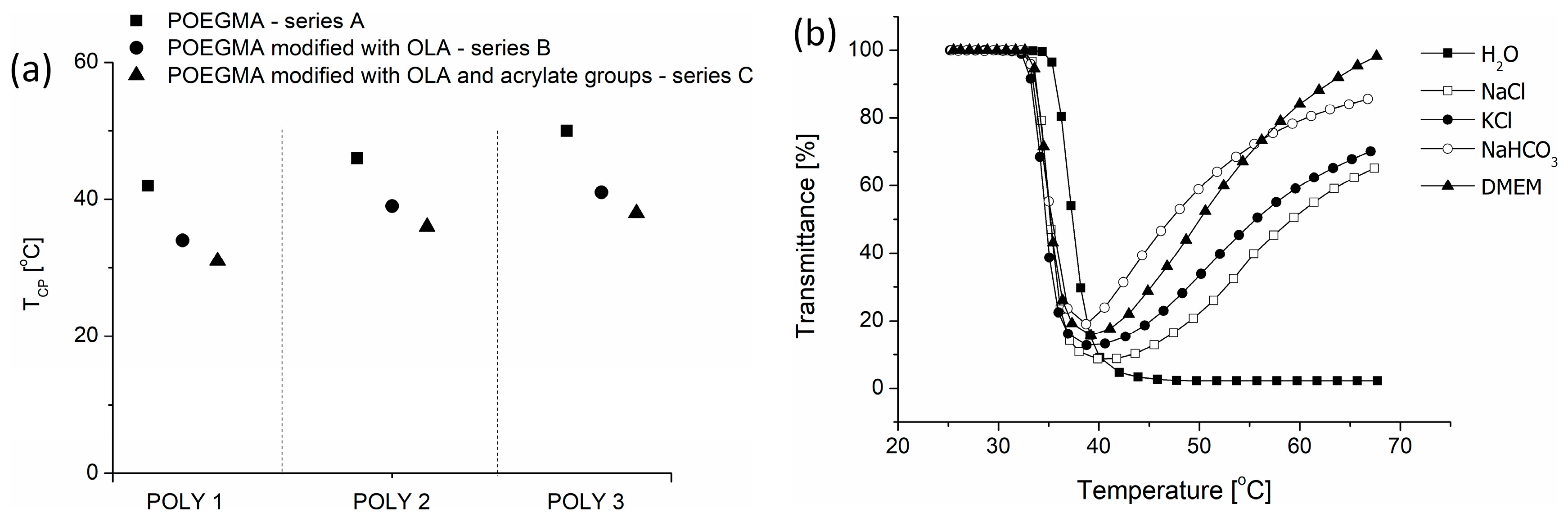
3.2. The Thermoresponsive Behavior of Copolymers
3.3. Preparation of Mesoglobules and Their Crosslinking to Nanogels
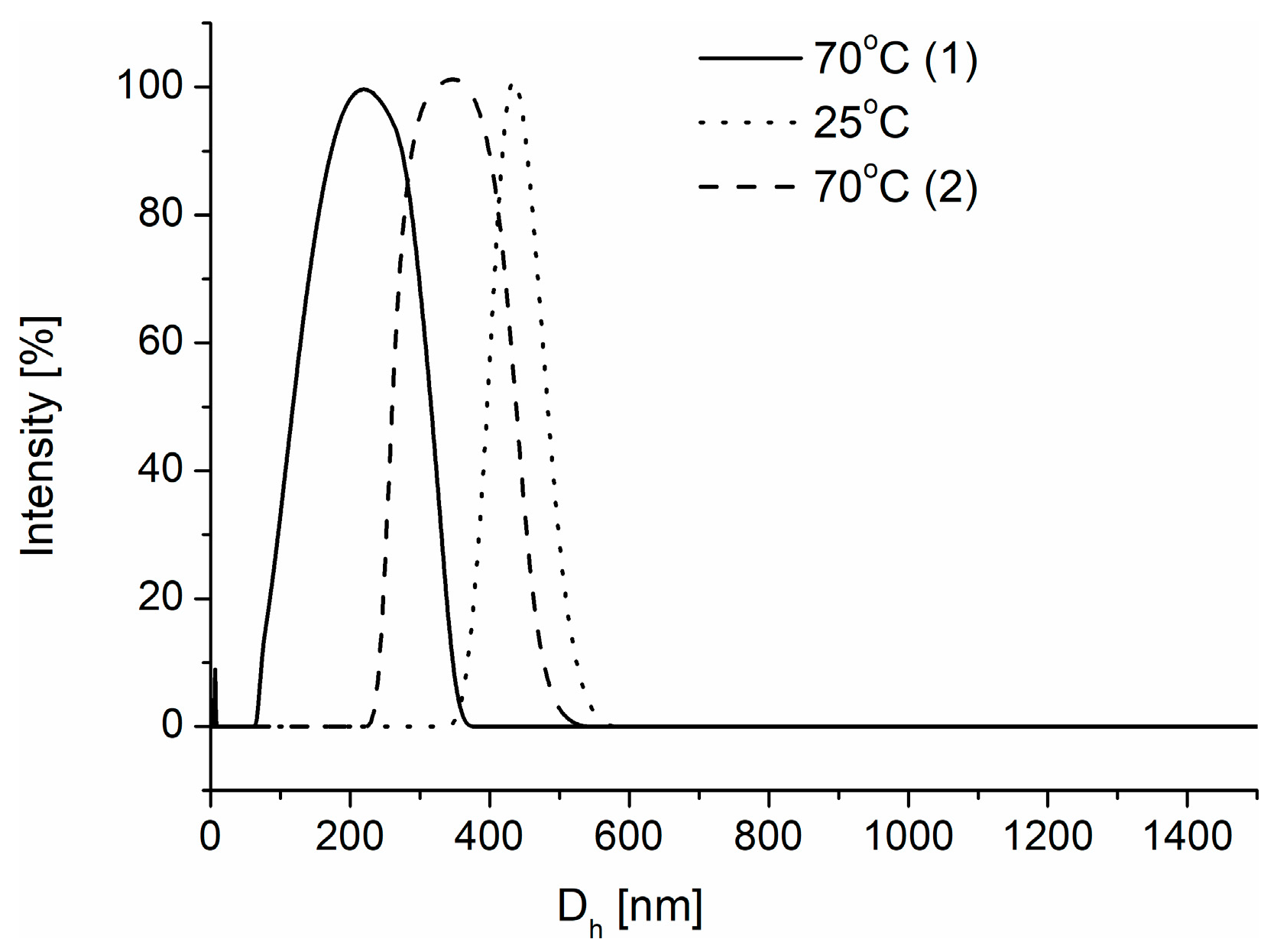
3.4. Degradation of Nanogels
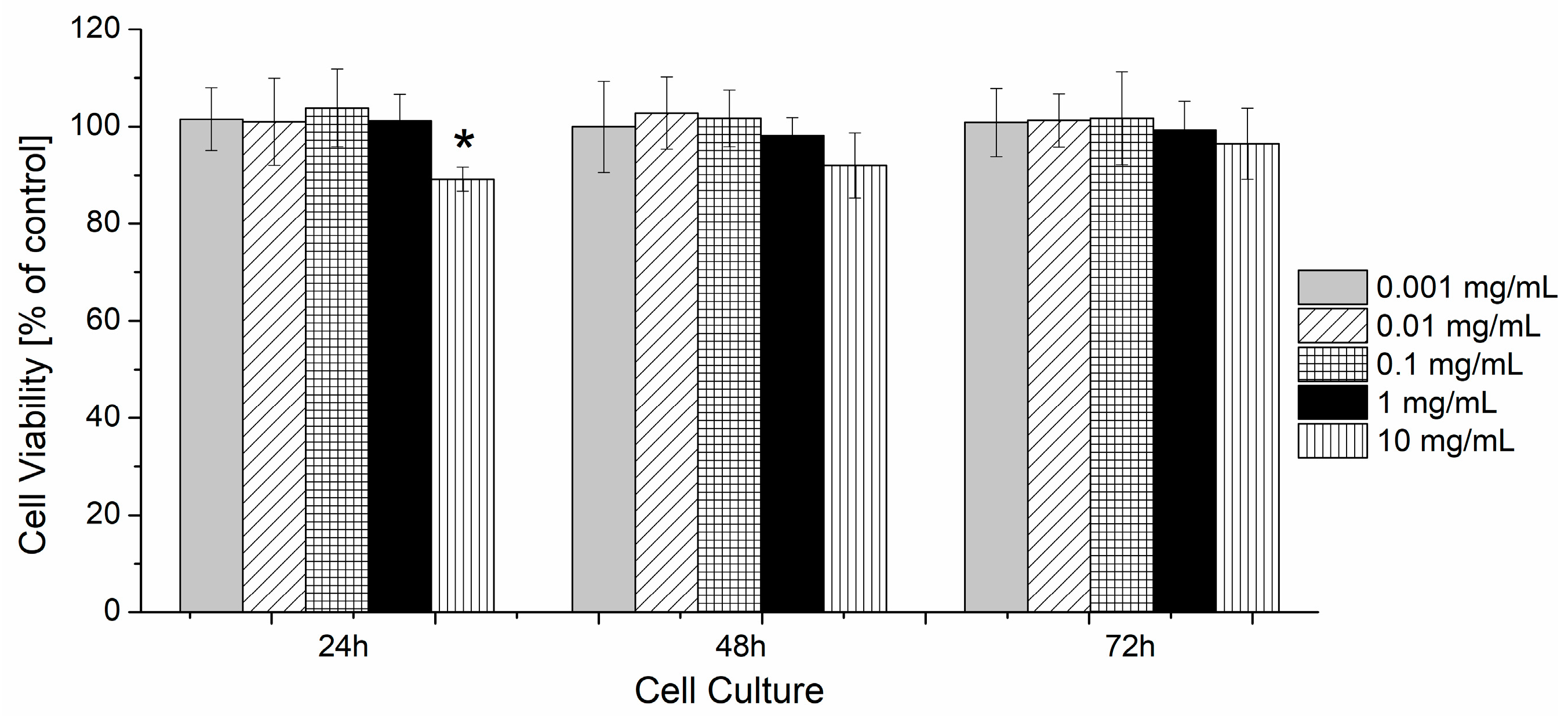
3.5. The Evaluation of the Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Boholm, M.; Arvidsson, R. A Definition Framework for the Terms Nanomaterial and Nanoparticle. Nanoethics 2016, 10, 25–40. [Google Scholar] [CrossRef]
- Zielinska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Nagasamy Venkatesh, D.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, C. Polymer Nanoparticles-Preparations, Applications and Future Insights: A Concise Review. Polym. Technol. Mater. 2021, 60, 1996–2024. [Google Scholar] [CrossRef]
- Lutz, J.F.; Lehn, J.M.; Meijer, E.W.; Matyjaszewski, K. From Precision Polymers to Complex Materials and Systems. Nat. Rev. Mater. 2016, 1, 16024. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Polymer Chemistry: Current Status and Perspective. Chem. Int. 2017, 39, 7–11. [Google Scholar] [CrossRef]
- Aflori, M. Smart Nanomaterials for Biomedical Applications—A Review. Nanomaterials 2021, 11, 396. [Google Scholar] [CrossRef] [PubMed]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Nasir, A.; Kausar, A.; Younus, A. A Review on Preparation, Properties and Applications of Polymeric Nanoparticle-Based Materials. Polym. Plast. Technol. Eng. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Lima, A.L.; Gratieri, T.; Cunha-Filho, M.; Gelfuso, G.M. Polymeric Nanocapsules: A Review on Design and Production Methods for Pharmaceutical Purpose. Methods 2022, 199, 54–66. [Google Scholar] [CrossRef]
- Crucho, C.I.C.; Barros, M.T. Polymeric Nanoparticles: A Study on the Preparation Variables and Characterization Methods. Mater. Sci. Eng. C 2017, 80, 771–784. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Urban, M.W. Stimuli-Responsive Polymeric Nanoparticles. Macromol. Rapid Commun. 2017, 38, 1700030. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Raheja, K.; Milbrandt, N.B.; Beilharz, S.; Tene, S.; Oshabaheebwa, S.; Gurkan, U.A.; Samia, A.C.S.; Karayilan, M. Thermoresponsive Polymers with LCST Transition: Synthesis, Characterization, and Their Impact on Biomedical Frontiers. RSC Appl. Polym. 2023, 1, 158–189. [Google Scholar] [CrossRef]
- Xia, Y.; Zeng, Y.; Hu, D.; Shen, H.; Deng, J.; Lu, Y.; Xia, X.; Xu, W. Light and PH Dual-Sensitive Biodegradable Polymeric Nanoparticles for Controlled Release of Cargos. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1773–1783. [Google Scholar] [CrossRef]
- Sun, W.; Li, S.; Häupler, B.; Liu, J.; Jin, S.; Steffen, W.; Schubert, U.S.; Butt, H.-J.; Liang, X.-J.; Wu, S.; et al. An Amphiphilic Ruthenium Polymetallodrug for Combined Photodynamic Therapy and Photochemotherapy In Vivo. Adv. Sci. 2017, 29, 1603702. [Google Scholar] [CrossRef] [PubMed]
- Abidian, M.R.; Kim, D.-H.; Martin, D.C. Conducting-Polymer Nanotubes for Controlled Drug Release. Adv. Mater. 2006, 18, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Aseyev, V.O.; Tenhu, H.; Winnik, F.M. Temperature Dependence of the Colloidal Stability of Neutral Amphiphilic Polymers in Water. Adv. Polym. Sci. 2006, 196, 1–85. [Google Scholar] [CrossRef]
- Trzebicka, B.; Szweda, D.; Rangelov, S.; Kowalczuk, A.; Mendrek, B.; Utrata-Wesołek, A.; Dworak, A. (Co) Polymers of Oligo (Ethylene Glycol) Methacrylates—Temperature- Induced Aggregation in Aqueous Solution. Polym. Chem. 2013, 51, 614–623. [Google Scholar] [CrossRef]
- Lipowska-Kur, D.; Szweda, R.; Trzebicka, B.; Dworak, A. Preparation and Characterization of Doxorubicin Nanocarriers Based on Thermoresponsive Oligo(Ethylene Glycol) Methyl Ether Methacrylate Polymer-Drug Conjugates. Eur. Polym. J. 2018, 109, 391–401. [Google Scholar] [CrossRef]
- Zhu, C.; Xiao, J.; Tang, M.; Feng, H.; Chen, W.; Du, M. Platinum Covalent Shell Cross-Linked Micelles Designed to Deliver Doxorubicin for Synergistic Combination Cancer Therapy. Int. J. Nanomed. 2017, 12, 3697–3710. [Google Scholar] [CrossRef]
- Varlas, S.; Neal, T.J.; Armes, S.P. Polymerization-Induced Self-Assembly and Disassembly during the Synthesis of Thermoresponsive ABC Triblock Copolymer Nano-Objects in Aqueous Solution. Chem. Sci. 2022, 13, 7295–7303. [Google Scholar] [CrossRef]
- Simpson, M.J.; Corbett, B.; Arezina, A.; Hoare, T. Narrowly Dispersed, Degradable, and Scalable Poly(Oligoethylene Glycol Methacrylate)-Based Nanogels via Thermal Self-Assembly. Ind. Eng. Chem. Res. 2018, 57, 7495–7506. [Google Scholar] [CrossRef]
- Alejo, T.; Uson, L.; Landa, G.; Prieto, M.; Yus Argón, C.; Garcia-Salinas, S.; De Miguel, R.; Rodríguez-Largo, A.; Irusta, S.; Sebastian, V.; et al. Nanogels with High Loading of Anesthetic Nanocrystals for Extended Duration of Sciatic Nerve Block. ACS Appl. Mater. Interfaces 2021, 13, 17220–17235. [Google Scholar] [CrossRef]
- Wen, Y.; Oh, J.K. Intracellular Delivery Cellulose-Based Bionanogels with Dual Temperature/PH-Response for Cancer Therapy. Colloids Surf. B Biointerfaces 2015, 133, 246–253. [Google Scholar] [CrossRef]
- Liwinska, W.; Waleka-Bagiel, E.; Stojek, Z.; Karbarz, M.; Zabost, E. Enzyme-Triggered- and Tumor-Targeted Delivery with Tunable, Methacrylated Poly(Ethylene Glycols) and Hyaluronic Acid Hybrid Nanogels. Drug Deliv. 2022, 29, 2561–2578. [Google Scholar] [CrossRef]
- Tian, Y.; Bian, S.; Yang, W. A Redox-Labile Poly(Oligo(Ethylene Glycol)Methacrylate)-Based Nanogel with Tunable Thermosensitivity for Drug Delivery. Polym. Chem. 2016, 7, 1913–1921. [Google Scholar] [CrossRef]
- Lutz, J.F. Thermo-Switchable Materials Prepared Using the OEGMA-Platform. Adv. Mater. 2011, 23, 2237–2243. [Google Scholar] [CrossRef]
- Kalelkar, P.P.; Collard, D.M. Tricomponent Amphiphilic Poly(Oligo(Ethylene Glycol) Methacrylate) Brush-Grafted Poly(Lactic Acid): Synthesis, Nanoparticle Formation, and in Vitro Uptake and Release of Hydrophobic Dyes. Macromolecules 2020, 53, 4274–4283. [Google Scholar] [CrossRef]
- Mousavi, M.; Ghaleh, H.; Jalili, K.; Abbasi, F. Multi-Layer PDMS Films Having Antifouling Property for Biomedical Applications. J. Biomater. Sci. Polym. Ed. 2020, 32, 678–693. [Google Scholar] [CrossRef]
- Ozer, I.; Slezak, A.; Sirohi, P.; Li, X.; Zakharov, N.; Yao, Y.; Everitt, J.I.; Spasojevic, I.; Craig, S.L.; Collier, J.H.; et al. An Injectable PEG-like Conjugate Forms a Subcutaneous Depot and Enables Sustained Delivery of a Peptide Drug. Biomaterials 2023, 294, 121985. [Google Scholar] [CrossRef] [PubMed]
- Ozer, I.; Kelly, G.; Gu, R.; Li, X.; Zakharov, N.; Sirohi, P.; Nair, S.K.; Collier, J.H.; Hershfield, M.S.; Hucknall, A.M.; et al. Polyethylene Glycol-Like Brush Polymer Conjugate of a Protein Drug Does Not Induce an Antipolymer Immune Response and Has Enhanced Pharmacokinetics than Its Polyethylene Glycol Counterpart. Adv. Sci. 2022, 9, 2103672. [Google Scholar] [CrossRef]
- Ozer, I.; Pitoc, G.A.; Layzer, J.M.; Moreno, A.; Olson, L.B.; Layzer, K.D.; Hucknall, A.M.; Sullenger, B.A.; Chilkoti, A. PEG-Like Brush Polymer Conjugate of RNA Aptamer That Shows Reversible Anticoagulant Activity and Minimal Immune Response. Adv. Mater. 2022, 34, 2107852. [Google Scholar] [CrossRef]
- Padín-González, E.; Lancaster, P.; Bottini, M.; Gasco, P.; Tran, L.; Fadeel, B.; Wilkins, T.; Monopoli, M.P. Understanding the Role and Impact of Poly (Ethylene Glycol) (PEG) on Nanoparticle Formulation: Implications for COVID-19 Vaccines. Front. Bioeng. Biotechnol. 2022, 10, 882363. [Google Scholar] [CrossRef]
- Li, Q.; Constantinou, A.P.; Georgiou, T.K. A Library of Thermoresponsive PEG-Based Methacrylate Homopolymers: How Do the Molar Mass and Number of Ethylene Glycol Groups Affect the Cloud Point? J. Polym. Sci. 2021, 59, 230–239. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, L.; Huang, W.; Xue, X.; Yang, H.; Jiang, Q.; Jiang, B. Ultrasonic Responsive Nanostructures Prepared by Self-Assembly of Polymeric Single-Chain Nanoparticles. Mater. Res. Innov. 2022, 26, 182–188. [Google Scholar] [CrossRef]
- Banerjee, R.; Maiti, S.; Dhara, D. Water-Soluble Nanoparticles from Poly(Ethylene Glycol)-Based Cationic Random Copolymers and Double-Tail Surfactant. Colloids Surfaces A Physicochem. Eng. Asp. 2012, 395, 255–261. [Google Scholar] [CrossRef]
- Lutz, J.F.; Akdemir, Ö.; Hoth, A. Point by Point Comparison of Two Thermosensitive Polymers Exhibiting a Similar LCST: Is the Age of Poly(NIPAM) Over? J. Am. Chem. Soc. 2006, 128, 13046–13047. [Google Scholar] [CrossRef]
- Otulakowski, Ł.; Kasprów, M.; Strzelecka, A.; Dworak, A.; Trzebicka, B. Thermal Behaviour of Common Thermoresponsive Polymers in Phosphate Buffer and in Its Salt Solutions. Polymers 2021, 13, 90. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, H.; Whittaker, A.K. NMR Investigation of Effect of Dissolved Salts on the Thermoresponsive Behavior of Oligo(Ethylene Glycol)-Methacrylate-Based Polymers. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 2375–2385. [Google Scholar] [CrossRef]
- Luzon, M.; Boyer, C.; Peinado, C.; Corrales, T.; Whittaker, M.; Tao, L.E.I.; Davis, T.P. Water-Soluble, Thermoresponsive, Hyperbranched Copolymers Based on PEG-Methacrylates: Synthesis, Characterization, and LCST Behavior. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2783–2792. [Google Scholar] [CrossRef]
- Otulakowski, Ł.; Trzebicka, B. Aggregation of Thermoresponsive Polymethacrylates in a Dulbecco’s Modified Eagle Medium and Its Salts. Polymers 2023, 15, 3587. [Google Scholar] [CrossRef] [PubMed]
- Dworak, A.; Utrata-Wesołek, A.; Szweda, D.; Kowalczuk, A.; Trzebicka, B.; Anioł, J.; Sieroń, A.L.; Klama-Baryła, A.; Kawecki, M. Poly[Tri (Ethylene Glycol) Ethyl Ether Methacrylate ] -Coated Surfaces for Controlled Fibroblasts Culturing. ACS Appl. Mater. Interfaces 2013, 5, 2197–2207. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Li, Y.; Li, T.; Yang, Y.; Huang, Z.; de la Fuente, J.M.; Ni, J.; Cui, D. Long-Circulating Drug-Dye-Based Micelles with Ultrahigh PH-Sensitivity for Deep Tumor Penetration and Superior Chemo-Photothermal Therapy. Adv. Funct. Mater. 2020, 30, 1906309. [Google Scholar] [CrossRef]
- Song, S.; Zhou, H.; Puzhitsky, M.; Zhang, Y.; Hicks, G.; Lu, Y.; Manners, I.; Winnik, M.A. Crystallization-Driven Self-Assembly of a Block Copolymer with Amphiphilic Pendant Groups. Macromolecules 2021, 54, 930–940. [Google Scholar] [CrossRef]
- De Morais Zanata, D.; Felisberti, M.I. Self-Assembly of Dual-Responsive Amphiphilic POEGMA-b-P4VP-b-POEGMA Triblock Copolymers: Effect of Temperature, PH, and Complexation with Cu2+. Polym. Chem. 2021, 12, 4668–4679. [Google Scholar] [CrossRef]
- Osawa, S.; Kashiwakura, M.; Yamaguchi, H.; Otsuka, H. Influence of Anchoring Segments on Methacrylate-Based Comb-Type Hydrophilic Polymer Segments Coated on Silica Surfaces Using the Grafting-to Method. Colloids Interface Sci. Commun. 2020, 36, 100258. [Google Scholar] [CrossRef]
- Zhang, C.; Luan, H.; Wang, G. A Novel Thermosensitive Triblock Copolymer from 100% Renewably Sourced Poly(Trimethylene Ether) Glycol. J. Appl. Polym. Sci. 2018, 135, 46112. [Google Scholar] [CrossRef]
- Yang, Z.; Zou, H.; Liu, H.; Xu, W.; Zhang, L. Self-Assembly and Drug Release Control of Dual-Responsive Copolymers Based on Oligo(Ethylene Glycol)Methyl Ether Methacrylate and Spiropyran. Iran. Polym. J. (Engl. Ed.) 2019, 28, 39–49. [Google Scholar] [CrossRef]
- Lipowska-Kur, D.; Otulakowski, Ł.; Trzebicka, B.; Utrata-Wesołek, A.; Dworak, A. Thermoresponsive Nanogels of Modified Poly(Di(Ethylene Glycol) Methyl Ether Methacrylate)-Co-(2-Aminoethyl Methacrylate)S. Polymers 2020, 12, 1645. [Google Scholar] [CrossRef] [PubMed]
- Macchione, M.A.; Sacarelli, M.F.; Racca, A.C.; Biglione, C.; Panzetta-Dutari, G.M.; Strumia, M.C. Dual-Responsive Nanogels Based on Oligo(Ethylene Glycol) Methacrylates and Acidic Co-Monomers. Soft Matter 2019, 15, 9700–9709. [Google Scholar] [CrossRef]
- Wilms, D.; Adler, Y.; Schröer, F.; Bunnemann, L.; Schmidt, S. Elastic Modulus Distribution in Poly(N-Isopopylacrylamide) and Oligo(Ethylene Glycol Methacrylate)-Based Microgels Studied by AFM. Soft Matter 2021, 17, 5711–5717. [Google Scholar] [CrossRef]
- Agnihotri, P.; Raj, R.; Kumar, D.; Dan, A. Short Oligo(Ethylene Glycol) Chain Incorporated Thermoresponsive Microgels: From Structural Analysis to Modulation of Solution Properties. Soft Matter 2020, 16, 7845–7859. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Jackson, R.A.; Lascelles, S.F.; Armes, S.P. Facile Synthesis of Well-Defined Water-Soluble Polymers via Atom Transfer Radical Polymerization in Aqueous Media at Ambient Temperature. Chem. Commun. 1999, 1817–1818. [Google Scholar] [CrossRef]
- Fu, W.; Luo, C.; Morin, E.A.; He, W.; Li, Z.; Zhao, B. UCST-Type Thermosensitive Hairy Nanogels Synthesized by RAFT Polymerization-Induced Self-Assembly. ACS Macro Lett. 2017, 6, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Gawlitza, K.; Radulescu, A.; Von Klitzing, R.; Wellert, S. On the Structure of Biocompatible, Thermoresponsive Poly(Ethylene Glycol) Microgels. Polymer 2014, 55, 6717–6724. [Google Scholar] [CrossRef]
- Wellert, S.; Radulescu, A.; Carl, A.; Klitzing, R.V.; Gawlitza, K. Evolution of Size and Structure during the Polymerization Process: A SANS Study on EG-Based Microgels. Macromolecules 2015, 48, 4901–4909. [Google Scholar] [CrossRef]
- Lincha, V.R.; Zhao, J.; Wen, X.; Xiong, C.; Chow, D.S.; Li, C. A Polymeric Micellar Drug Delivery System Developed through a Design of Experiment Approach Improves Pancreatic Tumor Accumulation of Calcipotriol and Paclitaxel. Int. J. Pharm. 2021, 601, 120523. [Google Scholar] [CrossRef]
- Lutz, J.-F.; Andrieu, J.; Uzgun, S.; Rudolph, C.; Agarwal, S. Biodegradable: Simple Preparation of “All-in-One” Biorelevant Polymers. Macromolecules 2007, 40, 8540–8543. [Google Scholar] [CrossRef]
- Riachi, C.; Schüwer, N.; Klok, H.A. Degradable Polymer Brushes Prepared via Surface-Initiated Controlled Radical Polymerization. Macromolecules 2009, 42, 8076–8081. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, P.; Jérôme, V.; Freitag, R.; Agarwal, S. Enzymatically Degradable Polyester-Based Adhesives. ACS Biomater. Sci. Eng. 2015, 1, 971–977. [Google Scholar] [CrossRef]
- Sadowski, L.P.; Singh, A.; Luo, D.H.; Majcher, M.J.; Urosev, I.; Rothenbroker, M.; Kapishon, V.; Smeets, N.M.B.; Hoare, T. Functionalized Poly(Oligo(Lactic Acid) Methacrylate)-Block-Poly(Oligo(Ethylene Glycol) Methacrylate) Block Copolymers: A Synthetically Tunable Analogue to PLA-PEG for Fabricating Drug-Loaded Nanoparticles. Eur. Polym. J. 2022, 177, 111443. [Google Scholar] [CrossRef]
- Qiao, Z.Y.; Zhang, R.; Du, F.S.; Liang, D.H.; Li, Z.C. Multi-Responsive Nanogels Containing Motifs of Ortho Ester, Oligo(Ethylene Glycol) and Disulfide Linkage as Carriers of Hydrophobic Anti-Cancer Drugs. J. Control. Release 2011, 152, 57–66. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Q.; Chen, W.; Zhao, Y.; Yong, T.; Gan, L.; Xu, H.; Yang, X. Hydrophilicity/Hydrophobicity Reversable and Redox-Sensitive Nanogels for Anticancer Drug Delivery. Mol. Pharm. 2015, 12, 1636–1647. [Google Scholar] [CrossRef] [PubMed]
- Nuhn, L.; Vanparijs, N.; De Beuckelaer, A.; Lybaert, L.; Verstraete, G.; Deswarte, K.; Lienenklaus, S.; Shukla, N.M.; Salyer, A.C.D.; Lambrecht, B.N.; et al. PH-Degradable Imidazoquinoline-Ligated Nanogels for Lymph Node-Focused Immune Activation. Proc. Natl. Acad. Sci. USA 2016, 113, 8098–8103. [Google Scholar] [CrossRef] [PubMed]
- Dworak, A.; Lipowska, D.; Szweda, D.; Suwinski, J.; Trzebicka, B.; Szweda, R. Degradable Polymeric Nanoparticles by Aggregation of Thermoresponsive Polymers and “Click” Chemistry. Nanoscale 2015, 7, 16823–16833. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Sto, H.D.H. Well-Defined Amphiphilic Thermosensitive Copolymers Based on Poly(Ethylene Glycol Monomethacrylate) and Methyl Methacrylate Prepared by Atom Transfer Radical Polymerization Mir. Macromolecules 2004, 37, 5219–5227. [Google Scholar] [CrossRef]
- Brzeziński, M.; Biela, T. Supramolecular Polylactides by the Cooperative Interaction of the End Groups and Stereocomplexation. Macromolecules 2015, 48, 2994–3004. [Google Scholar] [CrossRef]
- Toncheva, N.; Tsvetanov, C.; Rangelov, S.; Trzebicka, B.; Dworak, A. Hydroxyl End-Functionalized Poly(2-Isopropyl Oxazoline)s Used as Nano-Sized Colloidal Templates for Preparation of Hollow Polymeric Nanocapsules. Polymer 2013, 54, 5166–5173. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization ISO: Geneva, Switzerland, 2009.
- Nair, D.P.; Podgórski, M.; Chatani, S.; Gong, T.; Xi, W.; Fenoli, C.R.; Bowman, C.N. The Thiol-Michael Addition Click Reaction: A Powerful and Widely Used Tool in Materials Chemistry. Chem. Mater. 2014, 26, 724–744. [Google Scholar] [CrossRef]
- Akar, I.; Keogh, R.; Blackman, L.D.; Foster, J.C.; Mathers, R.T.; O’Reilly, R.K. Grafting Density Governs the Thermoresponsive Behavior of P(OEGMA-Co-RMA) Statistical Copolymers. ACS Macro Lett. 2020, 9, 1149–1154. [Google Scholar] [CrossRef]
- Karjalainen, E.; Suvarli, N.; Tenhu, H. Thermoresponsive Behavior of Poly[Trialkyl-(4-Vinylbenzyl)Ammonium] Based Polyelectrolytes in Aqueous Salt Solutions. Polym. Chem. 2020, 11, 5870–5883. [Google Scholar] [CrossRef]
- Tokuyama, H.; Mori, H.; Hamaguchi, R.; Kato, G. Prediction of the Lower Critical Solution Temperature of Poly(N-Isopropylacrylamide-Co-Methoxy Triethyleneglycol Acrylate) in Aqueous Salt Solutions Using Support Vector Regression. Chem. Eng. Sci. 2021, 231, 116325. [Google Scholar] [CrossRef]
- Trzebicka, B.; Weda, P.; Utrata-Wesołek, A.; Dworak, A.; Tsvetanov, C. Mesoglobules of Random Copolyethers as Templates for Nanoparticles. J. Polym. Sci. 2010, 48, 4074–4083. [Google Scholar] [CrossRef]
- Toncheva-Moncheva, N.; Dimitrov, P.; Tsvetanov, C.B.; Robak, B.; Trzebicka, B.; Dworak, A.; Rangelov, S. Formation of Mesoglobules in Aqueous Media from Thermo-Sensitive Poly(Ethoxytriethyleneglycol Acrylate). Polym. Bull. 2011, 67, 1335–1346. [Google Scholar] [CrossRef]
- Trzebicka, B.; Haladjova, E.; Otulakowski, Ł.; Oleszko, N.; Wałach, W.; Libera, M.; Rangelov, S.; Dworak, A. Hybrid Nanoparticles Obtained from Mixed Mesoglobules. Polymer 2015, 68, 65–73. [Google Scholar] [CrossRef]
- Rijcken, C.J.; Snel, C.J.; Schiffelers, R.M.; van Nostrum, C.F.; Hennink, W.E. Hydrolysable Core-Crosslinked Thermosensitive Polymeric Micelles: Synthesis, Characterisation and in Vivo Studies. Biomaterials 2007, 28, 5581–5593. [Google Scholar] [CrossRef]
- Van Nostrum, C.F.; Veldhuis, T.F.J.; Bos, G.W.; Hennink, W.E. Hydrolytic Degradation of Oligo(Lactic Acid): A Kinetic and Mechanistic Study. Polymer 2004, 45, 6779–6787. [Google Scholar] [CrossRef]




| A—POEGMA Copolymer | B—POEGMA Substituted with OLA | C—POEGMA Substituted with OLA and Acrylic Acid | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TEGMAEE/ OEGMA300/ HOEGMA 1 | Mn theoretical [g/mol] | TEGMAEE/ OEGMA300/ HOEGMA 2 | Mn [g/mol] and Ð 3 | TCP [°C] 4 | Lactide/OH [mol %] 2 | DPOLA2 | TCP [°C] 4 | Acrylic Bond/OH [mol %] 1 | TCP [°C] 4 | |
| POLY1 | 75/25/10 | 29 600 | 69/22/8 | 37,000 1.49 | 42 | 45 | 12 | 34 | 47 | 31 |
| POLY2 | 65/35/10 | 30 000 | 63/32/8 | 40,000 1.44 | 46 | 45 | 6 | 39 | 20 | 36 |
| POLY3 | 55/45/10 | 30 600 | 54/41/9 | 41,000 1.50 | 50 | 44 | 9 | 41 | 17 | 38 |
| Solvent | Nanoprecipitation | Abrupt Heating |
|---|---|---|
| Dh [nm] | Dh [nm] | |
| H2O | 165 | 140 |
| KCl | 270 | 980 |
| NaHCO3 | 450 | 1000 |
| NaCl | 620 | 980 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipek, K.; Otulakowski, Ł.; Jelonek, K.; Utrata-Wesołek, A. Degradable Nanogels Based on Poly[Oligo(Ethylene Glycol) Methacrylate] (POEGMA) Derivatives through Thermo-Induced Aggregation of Polymer Chain and Subsequent Chemical Crosslinking. Polymers 2024, 16, 1163. https://doi.org/10.3390/polym16081163
Filipek K, Otulakowski Ł, Jelonek K, Utrata-Wesołek A. Degradable Nanogels Based on Poly[Oligo(Ethylene Glycol) Methacrylate] (POEGMA) Derivatives through Thermo-Induced Aggregation of Polymer Chain and Subsequent Chemical Crosslinking. Polymers. 2024; 16(8):1163. https://doi.org/10.3390/polym16081163
Chicago/Turabian StyleFilipek, Katarzyna, Łukasz Otulakowski, Katarzyna Jelonek, and Alicja Utrata-Wesołek. 2024. "Degradable Nanogels Based on Poly[Oligo(Ethylene Glycol) Methacrylate] (POEGMA) Derivatives through Thermo-Induced Aggregation of Polymer Chain and Subsequent Chemical Crosslinking" Polymers 16, no. 8: 1163. https://doi.org/10.3390/polym16081163







